Multimodal Treatment of Advanced Mucosal Melanoma in the Era of Modern Immunotherapy
Simple Summary
Abstract
1. Introduction
2. Results
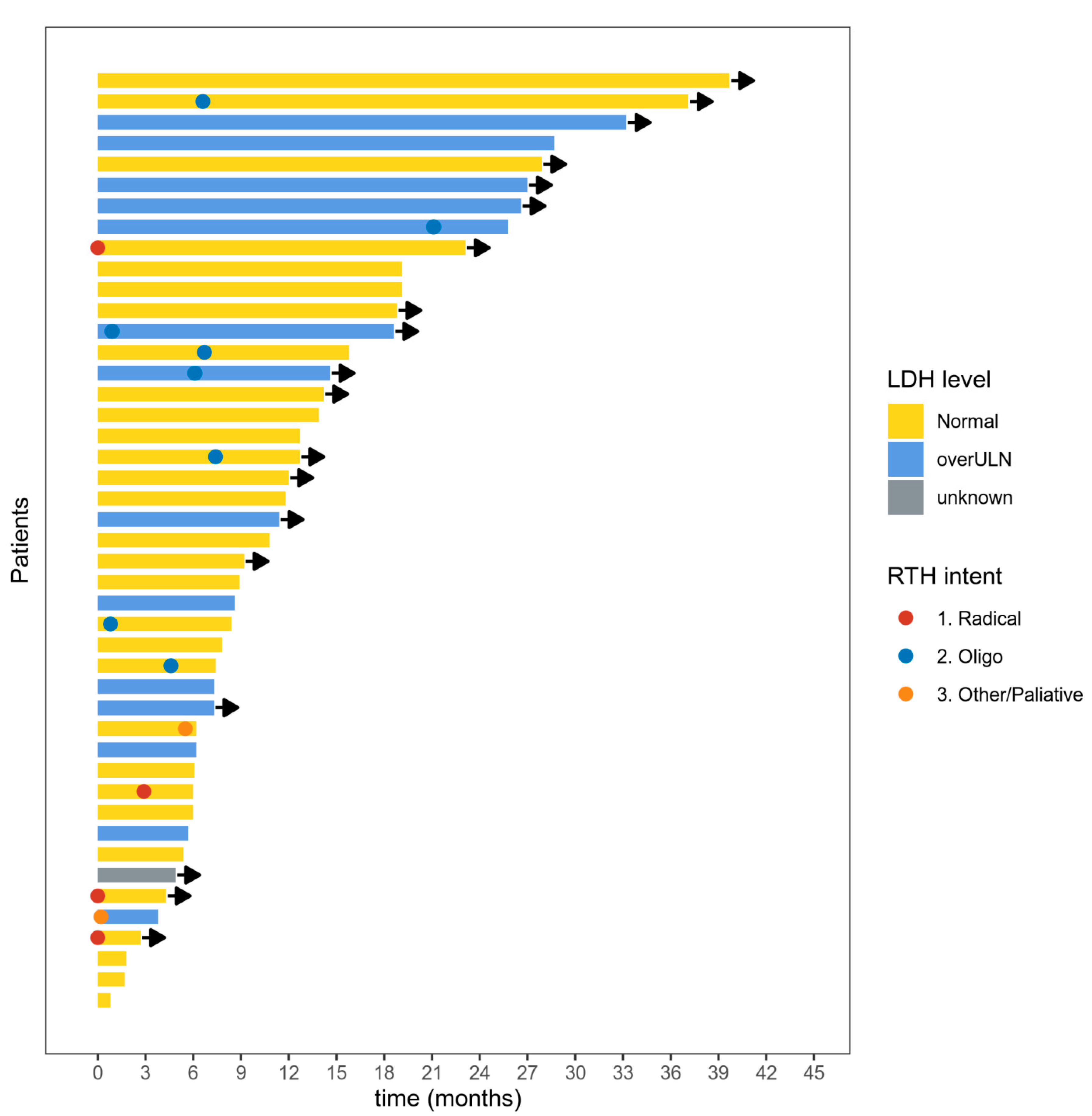
2.1. Cohort
2.2. Treatment
2.3. Response
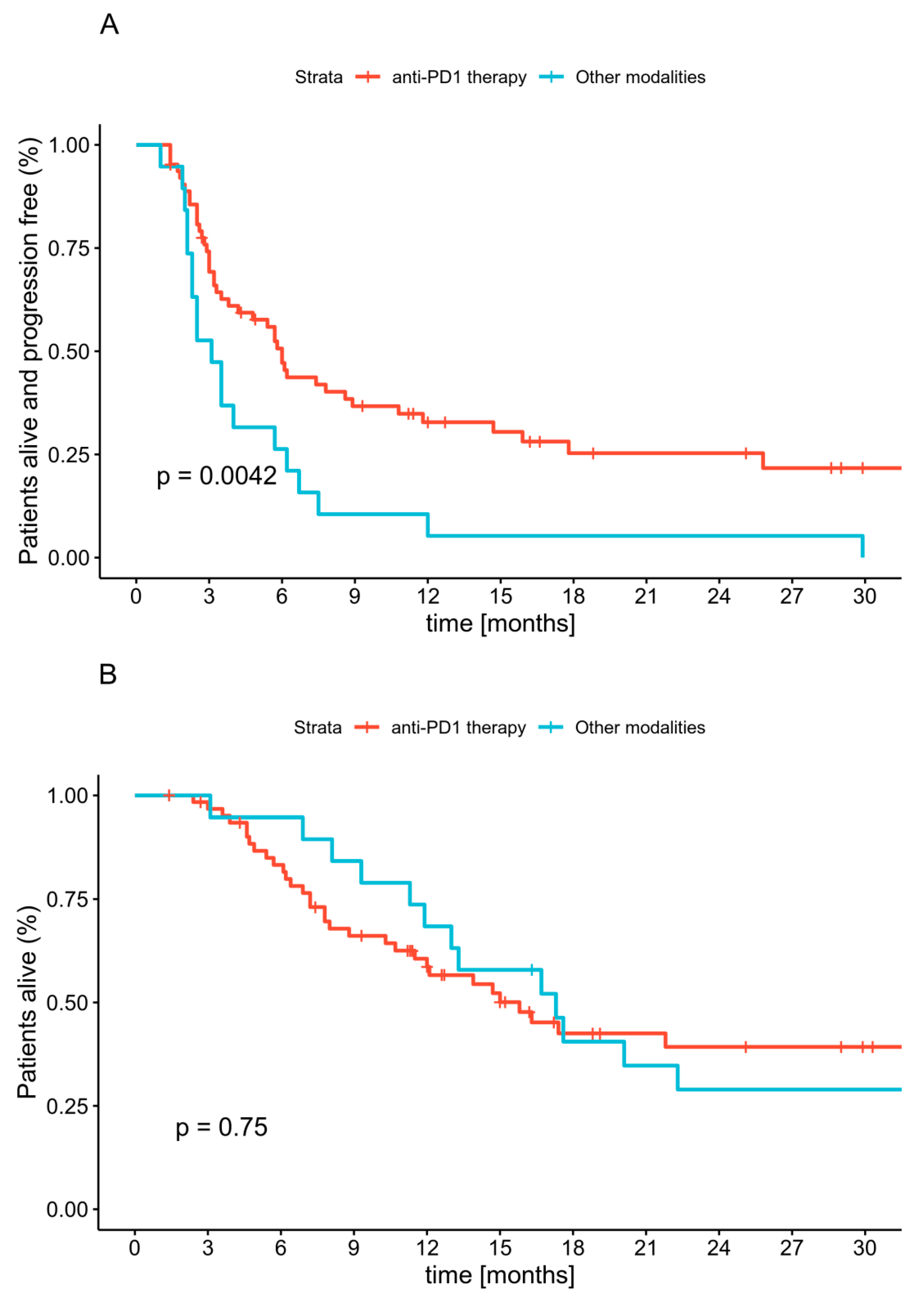
2.4. Progression-Free Survival
2.5. Overall Survival
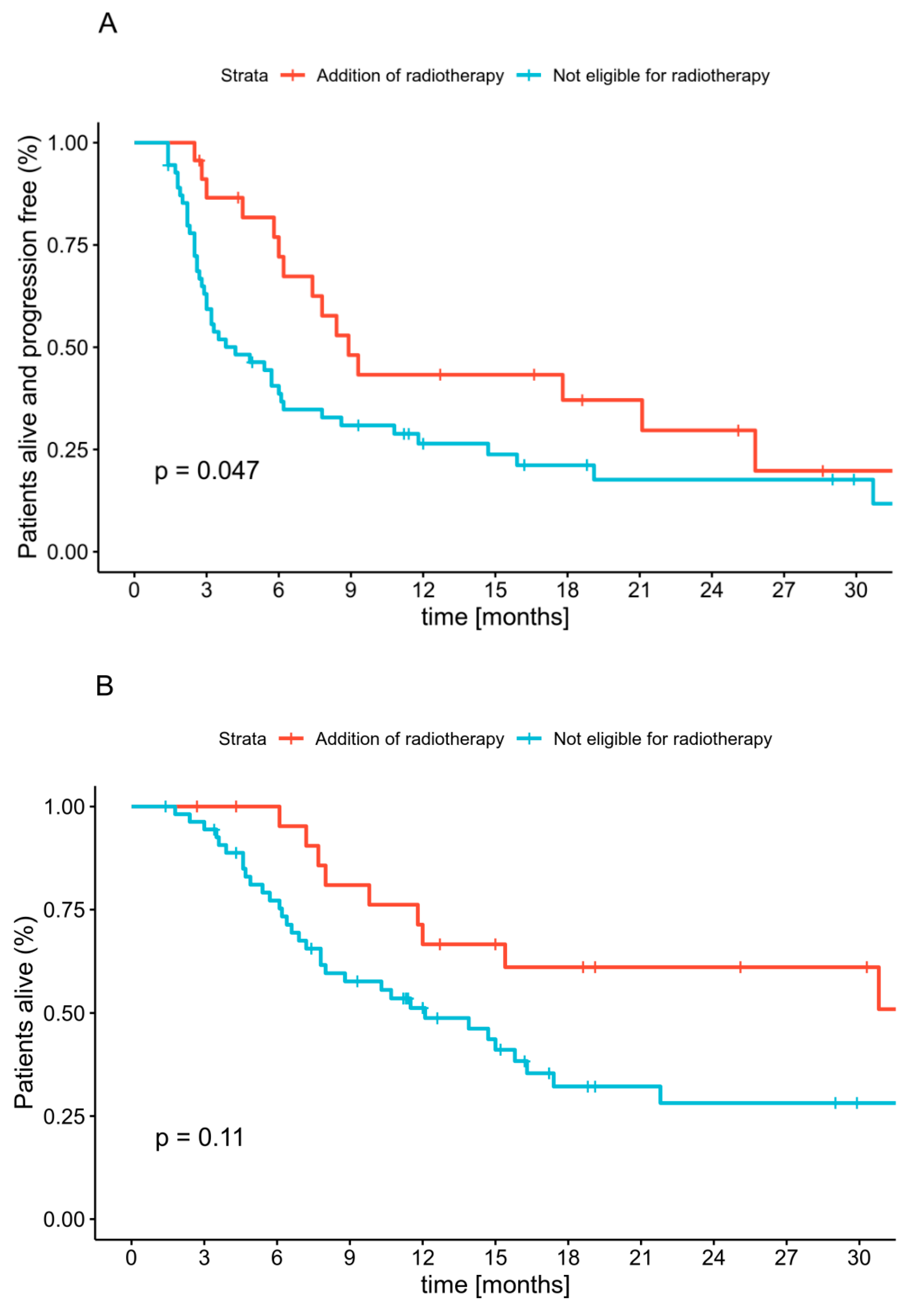
2.6. Radiotherapy
3. Discussion
4. Materials and Methods
4.1. Cohort
4.2. Treatment
4.3. Response and Survival Analysis
4.4. Statistical Analysis
4.5. Ethical Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Topić, B.; Mašić, T.; Radović, S.; Lincender, I.; Muhić, E. Primary Oral Mucosal Melanomas-Two Case Reports and Comprehensive Literature Review. Acta Clin. Croat 2017, 56, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, M.; Vlajkovic, S.; Jovanovic, P.; Stefanovic, V. Primary mucosal melanomas: A comprehensive review. Int. J. Clin. Exp. Pathol. 2012, 5, 739–753. [Google Scholar] [PubMed]
- Lerner, B.A.; Stewart, L.A.; Horowitz, D.P.; Carvajal, R.D. Mucosal Melanoma: New Insights and Therapeutic Options for a Unique and Aggressive Disease. Oncology 2017, 31, e23–e32. [Google Scholar] [PubMed]
- Ss, T. Re: Cancers with increasing incidence trends in the United States: 1999 through 2008. J. Urol. 2012, 188, 1120–1121. [Google Scholar] [CrossRef]
- Simard, E.P.; Ward, E.M.; Siegel, R.; Jemal, A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J. Clin. 2012, 62, 118–128. [Google Scholar] [CrossRef]
- Yde, S.S.; Sjoegren, P.; Heje, M.; Stolle, L.B. Mucosal Melanoma: A Literature Review. Curr. Oncol. Rep. 2018, 20, 28. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, X.; Qi, Y.; Gao, Q. A study of the clinical characteristics and prognosis of advanced mucosal and cutaneous melanoma in a Chinese population. Immunotherapy 2018, 11, 91–99. [Google Scholar] [CrossRef]
- Alicea, G.M.; Rebecca, V.W. Emerging strategies to treat rare and intractable subtypes of melanoma. Pigment Cell Melanoma Res. 2020. [Google Scholar] [CrossRef]
- Werdin, C.; Limas, C.; Knodell, R.G. Primary malignant melanoma of the rectum. Evidence for origination from rectal mucosal melanocytes. Cancer 1988, 61, 1364–1370. [Google Scholar] [CrossRef]
- Lundberg, R.; Brytting, M.; Dahlgren, L.; Kanter-Lewensohn, L.; Schloss, L.; Dalianis, T.; Ragnarsson-Olding, B. Human herpes virus DNA is rarely detected in non-UV light-associated primary malignant melanomas of mucous membranes. Anticancer Res. 2006, 26, 3627–3631. [Google Scholar]
- Giraud, G.; Ramqvist, T.; Ragnarsson-Olding, B.; Dalianis, T. DNA from BK virus and JC virus and from KI, WU, and MC polyomaviruses as well as from simian virus 40 is not detected in non-UV-light-associated primary malignant melanomas of mucous membranes. J. Clin. Microbiol. 2008, 46, 3595–3598. [Google Scholar] [CrossRef]
- Lourenço, S.V.; Fernandes, J.D.; Hsieh, R.; Coutinho-Camillo, C.M.; Bologna, S.; Sangueza, M.; Nico, M.M. Head and Neck Mucosal Melanoma: A Review. Am. J. Dermatopathol. 2014, 36, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Axéll, T.; Hedin, C.A. Epidemiologic study of excessive oral melanin pigmentation with special reference to the influence of tobacco habits. Scand. J. Dent. Res. 1982, 90, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Purdue, M.P. Re: Determinants of BRAF mutations in primary melanomas. J. Natl. Cancer Inst. 2005, 97, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Kirchoff, D.D.; Deutsch, G.B.; Foshag, L.J.; Lee, J.H.; Sim, M.-S.; Faries, M.B. Evolving Therapeutic Strategies in Mucosal Melanoma Have Not Improved Survival Over Five Decades. Am. Surg. 2016, 82, 1–5. [Google Scholar] [CrossRef] [PubMed]
- DeMatos, P.; Tyler, D.S.; Seigler, H.F. Malignant melanoma of the mucous membranes: A review of 119 cases. Ann. Surg. Oncol. 1998, 5, 733–742. [Google Scholar] [CrossRef]
- Pittaka, M.; Kardamakis, D.; Spyropoulou, D. Comparison of International Guidelines on Mucosal Melanoma of the Head and Neck: A Comprehensive Review of the Role of Radiation Therapy. In Vivo 2016, 30, 165–170. [Google Scholar]
- Yii, N.W.; Eisen, T.; Nicolson, M.; A’Hern, R.; Rhys-Evans, P.; Archer, D.; Henk, J.M.; Gore, M.E. Mucosal malignant melanoma of the head and neck: The Marsden experience over half a century. Clin. Oncol. (R. Coll. Radiol.) 2003, 15, 199–204. [Google Scholar] [CrossRef]
- Temam, S.; Mamelle, G.; Marandas, P.; Wibault, P.; Avril, M.F.; Janot, F.; Julieron, M.; Schwaab, G.; Luboinski, B. Postoperative radiotherapy for primary mucosal melanoma of the head and neck. Cancer 2005, 103, 313–319. [Google Scholar] [CrossRef]
- Moreno, M.A.; Roberts, D.B.; Kupferman, M.E.; DeMonte, F.; El-Naggar, A.K.; Williams, M.; Rosenthal, D.S.; Hanna, E.Y. Mucosal melanoma of the nose and paranasal sinuses, a contemporary experience from the M. D. Anderson Cancer Center. Cancer 2010, 116, 2215–2223. [Google Scholar] [CrossRef]
- Krengli, M.; Masini, L.; Kaanders, J.H.; Maingon, P.; Oei, S.B.; Zouhair, A.; Ozyar, E.; Roelandts, M.; Amichetti, M.; Bosset, M.; et al. Radiotherapy in the treatment of mucosal melanoma of the upper aerodigestive tract: Analysis of 74 cases. A Rare Cancer Network study. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 751–759. [Google Scholar] [CrossRef]
- Koto, M.; Demizu, Y.; Saitoh, J.I.; Suefuji, H.; Tsuji, H.; Okimoto, T.; Ohno, T.; Shioyama, Y.; Ikawa, H.; Nemoto, K.; et al. Definitive Carbon-Ion Radiation Therapy for Locally Advanced Sinonasal Malignant Tumors: Subgroup Analysis of a Multi-center Study by the Japan Carbon-Ion Radiation Oncology Study Group (J-CROS). Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Fuji, H.; Yoshikawa, S.; Kasami, M.; Murayama, S.; Onitsuka, T.; Kashiwagi, H.; Kiyohara, Y. High-dose proton beam therapy for sinonasal mucosal malignant melanoma. Radiat. Oncol. 2014, 9, 162. [Google Scholar] [CrossRef]
- Kim, K.B.; Alrwas, A. Treatment of KIT-mutated metastatic mucosal melanoma. Chin. Clin. Oncol. 2014, 3, 12. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Larkin, J.; Sosman, J.A.; Lebbé, C.; Brady, B.; Neyns, B.; Schmidt, H.; Hassel, J.C.; Hodi, F.S.; Savage, K.J.; et al. Efficacy and Safety of Nivolumab Alone or in Combination with Ipilimumab in Patients With Mucosal Melanoma: A Pooled Analysis. JCO 2016, 35, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Studentova, H.; Kalabova, H.; Koranda, P.; Chytilova, K.; Kucerova, L.; Melichar, B.; Vrana, D. Immunotherapy in mucosal melanoma: A case report and review of the literature. Oncotarget 2018, 9, 17971–17977. [Google Scholar] [CrossRef] [PubMed]
- Napierała, M.J.; Czarnecka, A.M. Mucosal melanoma—clinical presentation and treatment based on a case series. Oncol. Clin. Pract. 2019, 15, 223–230. [Google Scholar] [CrossRef]
- Mignard, C.; Deschamps-Huvier, A.; Duval-Modeste, A.B.; Dutriaux, C.; Khammari, A.; Avril, M.F.; Kramkimel, N.; Machet, L.; Marcant, P.; Lesimple, T.; et al. Efficacy of Immunotherapy in Patients with Metastatic Mucosal or Uveal Melanoma. J. Oncol. 2018, 2018, 1908065. [Google Scholar] [CrossRef]
- Maeda, T.; Yoshino, K.; Nagai, K.; Oaku, S.; Kato, M.; Hiura, A.; Hata, H. Efficacy of nivolumab monotherapy against acral lentiginous melanoma and mucosal melanoma in Asian patients. Br. J. Dermatol. 2019, 180, 1230–1231. [Google Scholar] [CrossRef]
- Nomura, M.; Oze, I.; Masuishi, T.; Yokota, T.; Satake, H.; Iwasawa, S.; Kato, K.; Andoh, M. Multi-center prospective phase II trial of nivolumab in patients with unresectable or metastatic mucosal melanoma. Int. J. Clin. Oncol. 2020, 25, 972–977. [Google Scholar] [CrossRef]
- Moya-Plana, A.; Gómez, R.G.H.; Rossoni, C.; Dercle, L.; Ammari, S.; Girault, I.; Roy, S.; Scoazec, J.-Y.; Vagner, S.; Janot, F.; et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol. Immunother. 2019, 68, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, A.M.; Teterycz, P.; Mariuk-Jarema, A.; Lugowska, I.; Rogala, P.; Dudzisz-Sledz, M.; Switaj, T.; Rutkowski, P. Treatment Sequencing and Clinical Outcomes in BRAF-Positive and BRAF-Negative Unresectable and Metastatic Melanoma Patients Treated with New Systemic Therapies in Routine Practice. Target Oncol. 2019, 14, 729–742. [Google Scholar] [CrossRef]
- Shoushtari, A.N.; Munhoz, R.R.; Kuk, D.; Ott, P.A.; Johnson, D.B.; Tsai, K.K.; Rapisuwon, S.; Eroglu, Z.; Sullivan, R.J.; Luke, J.J.; et al. Efficacy of Anti-PD-1 Agents in Acral and Mucosal Melanoma. Cancer 2016, 122, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Ribas, A.; Hodi, F.S.; Walpole, E.; Daud, A.; Arance, A.S.; Brown, E.; Hoeller, C.; Mortier, L.; et al. Antitumour activity of pembrolizumab in advanced mucosal melanoma: A post-hoc analysis of KEYNOTE-001, 002, 006. British J. Cancer 2018, 119, 670–674. [Google Scholar] [CrossRef]
- Urasaki, T.; Ono-Fuchiwaki, M.; Tomomatsu, J.; Nakano, K.; Taira, S.; Takahashi, S. Eight patients with mucosal malignant melanoma treated by nivolumab: A retrospective analysis in a single institution. Ann. Oncol. 2017, 28, ix102–ix103. [Google Scholar] [CrossRef]
- Kato, J.; Hida, T.; Someya, M.; Sato, S.; Sawada, M.; Horimoto, K.; Fujioka, M.; Minowa, T.; Matsui, Y.; Tsuchiya, T.; et al. Efficacy of combined radiotherapy and anti-programmed death 1 therapy in acral and mucosal melanoma. J. Dermatol. 2019, 46, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, Y.; Tanemura, A.; Takafuji, M.; Kiyohara, E.; Arase, N.; Suzuki, O.; Isohashi, F.; Ogawa, K.; Fujimoto, M. Local and disease control for nasal melanoma treated with radiation and concomitant anti-programmed death 1 antibody. J. Dermatol. 2020, 47, 423–425. [Google Scholar] [CrossRef]
- Kim, H.J.; Chang, J.S.; Roh, M.R.; Oh, B.H.; Chung, K.Y.; Shin, S.J.; Koom, W.S. Effect of Radiotherapy Combined With Pembrolizumab on Local Tumor Control in Mucosal Melanoma Patients. Front. Oncol. 2019, 9, 835. [Google Scholar] [CrossRef]
- Otsuka, M.; Sugihara, S.; Mori, S.; Hamada, K.; Sasaki, Y.; Yoshikawa, S.; Kiyohara, Y. Immune-related adverse events correlate with improved survival in patients with advanced mucosal melanoma treated with nivolumab: A single-center retrospective study in Japan. J. Dermatol. 2020, 47, 356–362. [Google Scholar] [CrossRef]
- Tyrrell, H.; Payne, M. Combatting mucosal melanoma: Recent advances and future perspectives. Melanoma Manag. 2018, 5, MMT11. [Google Scholar] [CrossRef]
- Johnson, D.B.; Carlson, J.A.; Elvin, J.A.; Vergilio, J.A.; Suh, J.; Ramkissoon, S.; Daniel, S.; Fabrizio, D.; Frampton, G.; Ali, S.M.; et al. Landscape of genomic alterations (GA) and tumor mutational burden (TMB) in different metastatic melanoma (MM) subtypes. JCO 2017, 35, 9536. [Google Scholar] [CrossRef]
- Furney, S.J.; Turajlic, S.; Stamp, G.; Nohadani, M.; Carlisle, A.; Thomas, J.M.; Hayes, A.; Strauss, D.; Gore, M.; van den Oord, J.; et al. Genome sequencing of mucosal melanomas reveals that they are driven by distinct mechanisms from cutaneous melanoma. J. Pathol. 2013, 230, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Wang, Y.A.; Livingston, J.A.; Zhang, J.; Futreal, P.A. A computational network approach to identify predictive biomarkers and therapeutic combinations for anti-PD-1 immunotherapy in cancer. bioRxiv 2020. [Google Scholar] [CrossRef]
- Cristescu, R.; Mogg, R.; Ayers, M.; Albright, A.; Murphy, E.; Yearley, J.; Sher, X.; Liu, X.Q.; Lu, H.; Nebozhyn, M.; et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade–based immunotherapy. Science 2018, 362. [Google Scholar] [CrossRef]
- Roh, W.; Chen, P.-L.; Reuben, A.; Spencer, C.N.; Prieto, P.A.; Miller, J.P.; Gopalakrishnan, V.; Wang, F.; Cooper, Z.A.; Reddy, S.M.; et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zbytek, B.; Slominski, R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int. J. Cancer 2009, 124, 1470–1477. [Google Scholar] [CrossRef]
- Feller, L.; Khammissa, R.A.G.; Lemmer, J. A Review of the Aetiopathogenesis and Clinical and Histopathological Features of Oral Mucosal Melanoma. Sci. World J. 2017, 2017, 9189812. [Google Scholar] [CrossRef] [PubMed]
- Brozyna, A.A.; Jozwicki, W.; Roszkowski, K.; Filipiak, J.; Slominski, A.T. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget 2016, 7, 17844–17853. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.M.; Lee, K.G.; Choi, W.; Cheong, S.H.; Myung, K.B.; Hahn, H.J. An updated review of mucosal melanoma: Survival meta-analysis. Mol. Clin. Oncol. 2019, 11, 116–126. [Google Scholar] [CrossRef]
- Demaria, S.; Formenti, S.C. Radiation as an immunological adjuvant: Current evidence on dose and fractionation. Front. Oncol. 2012, 2, 153. [Google Scholar] [CrossRef]
- Yentz, S.; Lao, C.D. Immunotherapy for mucosal melanoma. Ann. Transl. Med. 2019, 7, S118. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Wickham, H. tidyverse: Easily Install and Load “Tidyverse” Packages. R Package Version 2017, 1, 51. [Google Scholar]
- Kassambara, A.; Kosinski, M. survminer: Drawing Survival Curves Using “ggplot2”; R Package Version 0.4; R Foundation for Statistical Computing: Vienna, Austria, 2018; Volume 3. [Google Scholar]
| Variable | Value | Number (Percentage) |
|---|---|---|
| n | 82 (100) | |
| Sex (%) | female | 53 (64.6) |
| male | 29 (35.4) | |
| Age (median [IQR]) | 67.50 [57.25, 75.75] | |
| BRAF V600 mutation(%) | negative | 77 (93.9) |
| positive | 5 (6.1) | |
| Disease stage at the start of the treatment (%) | Localized, nonresectable disease | 14 (17.1) |
| M1a | 6 (7.3) | |
| M1b | 14 (17.1) | |
| M1c | 43 (52.4) | |
| M1d | 5 (6.1) | |
| LDH at the start of the first-line (%) | Normal | 43 (52.4) |
| Over ULN | 31 (37.8) | |
| Unknown | 8 (9.8) | |
| ECOG score at the start of first-line(%) | 0 | 40 (48.8) |
| 1+ | 42 (51.2) | |
| Localization (%) | Gastrointestinal system * | 27 (33.0) |
| Genitourinary tract | 22 (26.8) | |
| Head and neck region | 33 (40.2) | |
| First-line treatment (%) | BRAFi+/-MEKi inhibitor | 1 (1.2) |
| Chemotherapy | 5 (6.1) | |
| Anti-CTLA-4 antibody | 13 (15.9) | |
| Anti-PD1 antibody | 63 (76.8) | |
| Nivolumab | 39 (47.6) | |
| Pembrolizumab | 24 (29.2) | |
| The best response to the first-line (%) | Complete response | 4 (4.9) |
| Partial response | 15 (18.3) | |
| Stable disease | 25 (30.5) | |
| Progressive disease | 37 (45.1) | |
| Not evaluable | 1 (1.2) | |
| LDH at the start of the second-line (%) | Normal | 14 (32.6) |
| Over ULN | 17 (39.5) | |
| Unknown | 12 (27.9) | |
| ECOG score at the start of second-line (%) | 0 | 14 (32.6) |
| 1+ | 23 (53.4) | |
| Unknown | 6 (14.0) | |
| Second-line treatment (%) | Chemotherapy | 6 (7.3) |
| Anti-CTLA-4 antibody | 21 (25.6) | |
| Imatinib | 1 (1.2) | |
| Anti-PD1 antibody | 15 (18.3) | |
| None | 39 (47.6) | |
| The best response to the second-line (%) | Complete response | 2 (4.7) |
| Partial response | 1 (9.3) | |
| Stable disease | 11 (25.6) | |
| Progressive disease | 25 (58.1) | |
| Not evaluable | 1 (1.2) | |
| Brain metastases at the start of treatment (%) | Absent | 77 (93.9) |
| Present | 5 (6.1) | |
| Liver metastases at the start of treatment (%) | Absent | 61 (74.3) |
| Present | 21 (25.6) | |
| Number of organs involved at the start of treatment (%) | 1 | 38 (46.3) |
| 2 | 23 (28.0) | |
| ≥3 | 21 (25.6) | |
| Radiotherapy during anti-PD1 treatment (%) | Not performed | 65 (79.3) |
| Performed | 17 (20.7) |
| Variable | Value | HR for OS (Univariable) | HR for PFS (Univariable) |
|---|---|---|---|
| Age | Per one-year change | 1.01 (0.98–1.03, p = 0.579) | 1.01 (0.99–1.03, p = 0.436) |
| Sex | female | - | - |
| male | 1.83 (1.05–3.21, p = 0.034) | 1.35 (0.81–2.26, p = 0.255) | |
| BRAF V600 mutation | negative | - | - |
| positive | 0.62 (0.15–2.58, p = 0.516) | 0.50 (0.15–1.58, p = 0.236) | |
| Localization | Gastrointestinal system | - | - |
| Genitourinary system | 0.90 (0.43–1.88, p = 0.781) | 1.28 (0.66–2.46, p = 0.462) | |
| Head and Neck Region | 1.07 (0.57–2.02, p = 0.834) | 1.39 (0.76–2.53, p = 0.290) | |
| Disease stage at the start of the treatment | Localized, nonoperable disease | - | - |
| M1a | 3.11 (0.77–12.60, p = 0.112) | 1.60 (0.54–4.68, p = 0.394) | |
| M1b | 2.54 (0.82–7.85, p = 0.105) | 1.61 (0.71–3.62, p = 0.252) | |
| M1c | 1.89 (0.66–5.44, p = 0.238) | 1.05 (0.51–2.15, p = 0.897) | |
| M1d | 1.77 (0.39–8.00, p = 0.459) | 1.57 (0.48–5.08, p = 0.455) | |
| Number of organs involved at the start of treatment | 1 | - | - |
| 2 | 0.95 (0.48–1.88, p = 0.893) | 1.26 (0.69–2.29, p = 0.453) | |
| 3+ | 1.30 (0.65–2.58, p = 0.457) | 1.54 (0.84–2.83, p = 0.162) | |
| LDH at the start of the first-line | Normal | - | - |
| overULN | 2.11 (1.16–3.84, p = 0.014) | 1.40 (0.82–2.37, p = 0.215) | |
| (Missing) | 1.61 (0.67–3.89, p = 0.290) | 1.45 (0.64–3.31, p = 0.375) | |
| ECOG score at the start of first-line | 0 | - | - |
| 1+ | 1.19 (0.68–2.08, p = 0.553) | 1.01 (0.61–1.65, p = 0.978) | |
| The first line of treatment | PD1 | - | - |
| Other | 0.90 (0.49–1.68, p = 0.747) | 2.18 (1.26–3.76, p = 0.005) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teterycz, P.; Czarnecka, A.M.; Indini, A.; Spałek, M.J.; Labianca, A.; Rogala, P.; Cybulska-Stopa, B.; Quaglino, P.; Ricardi, U.; Badellino, S.; et al. Multimodal Treatment of Advanced Mucosal Melanoma in the Era of Modern Immunotherapy. Cancers 2020, 12, 3131. https://doi.org/10.3390/cancers12113131
Teterycz P, Czarnecka AM, Indini A, Spałek MJ, Labianca A, Rogala P, Cybulska-Stopa B, Quaglino P, Ricardi U, Badellino S, et al. Multimodal Treatment of Advanced Mucosal Melanoma in the Era of Modern Immunotherapy. Cancers. 2020; 12(11):3131. https://doi.org/10.3390/cancers12113131
Chicago/Turabian StyleTeterycz, Pawel, Anna M. Czarnecka, Alice Indini, Mateusz J. Spałek, Alice Labianca, Pawel Rogala, Bożena Cybulska-Stopa, Pietro Quaglino, Umberto Ricardi, Serena Badellino, and et al. 2020. "Multimodal Treatment of Advanced Mucosal Melanoma in the Era of Modern Immunotherapy" Cancers 12, no. 11: 3131. https://doi.org/10.3390/cancers12113131
APA StyleTeterycz, P., Czarnecka, A. M., Indini, A., Spałek, M. J., Labianca, A., Rogala, P., Cybulska-Stopa, B., Quaglino, P., Ricardi, U., Badellino, S., Szumera-Ciećkiewicz, A., Falkowski, S., Mandala, M., & Rutkowski, P. (2020). Multimodal Treatment of Advanced Mucosal Melanoma in the Era of Modern Immunotherapy. Cancers, 12(11), 3131. https://doi.org/10.3390/cancers12113131










