Subtraction Maps Derived from Longitudinal Magnetic Resonance Imaging in Patients with Glioma Facilitate Early Detection of Tumor Progression
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Inclusion
2.2. Magnetic Resonance Imaging
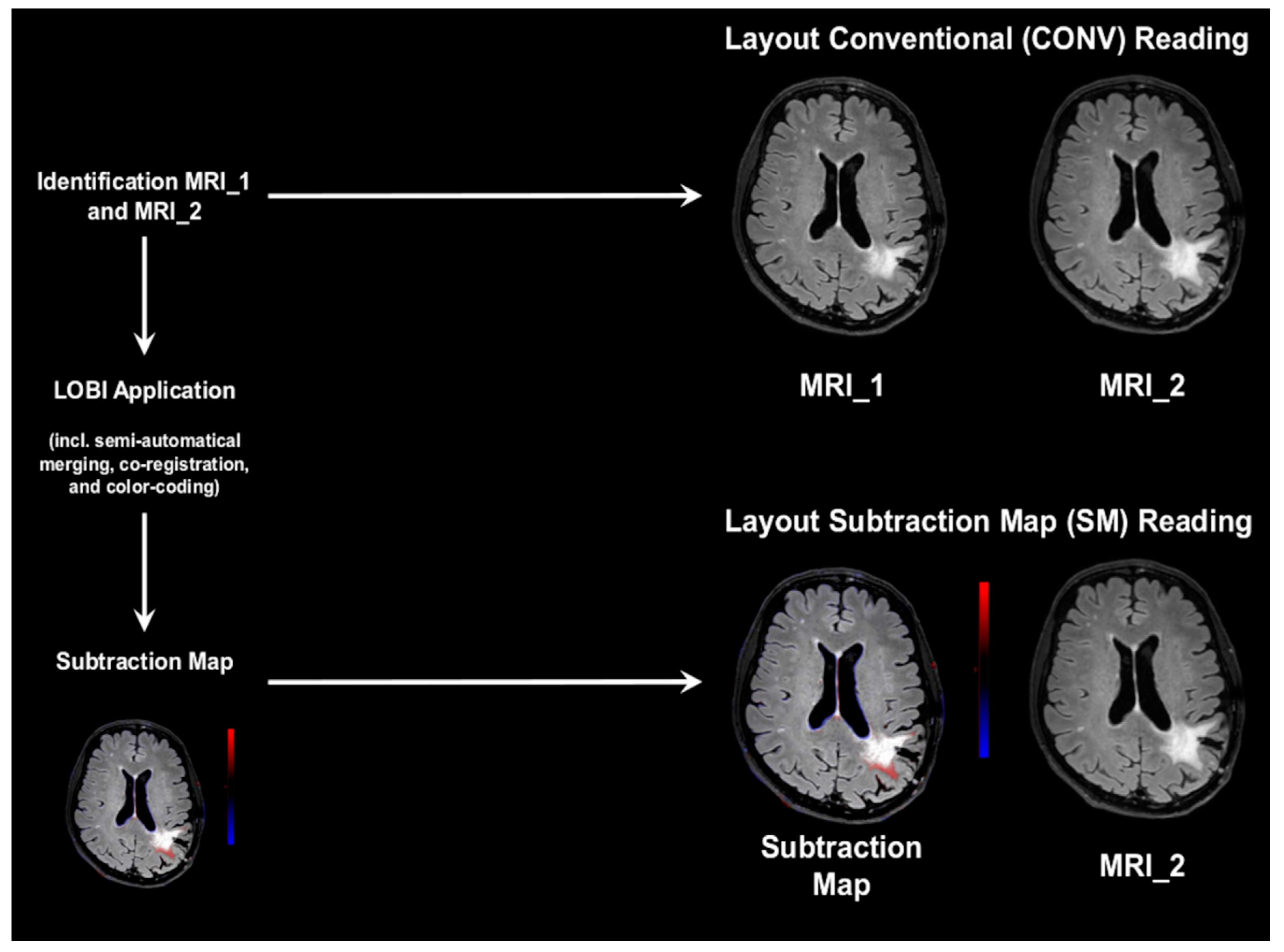
2.3. Conventional and Subtraction Map Reading
2.3.1. Setup and Scoring
2.3.2. Conventional Reading
2.3.3. Subtraction Map Reading
2.4. Gold-Standard Reading
2.5. Statistical Analysis
3. Results
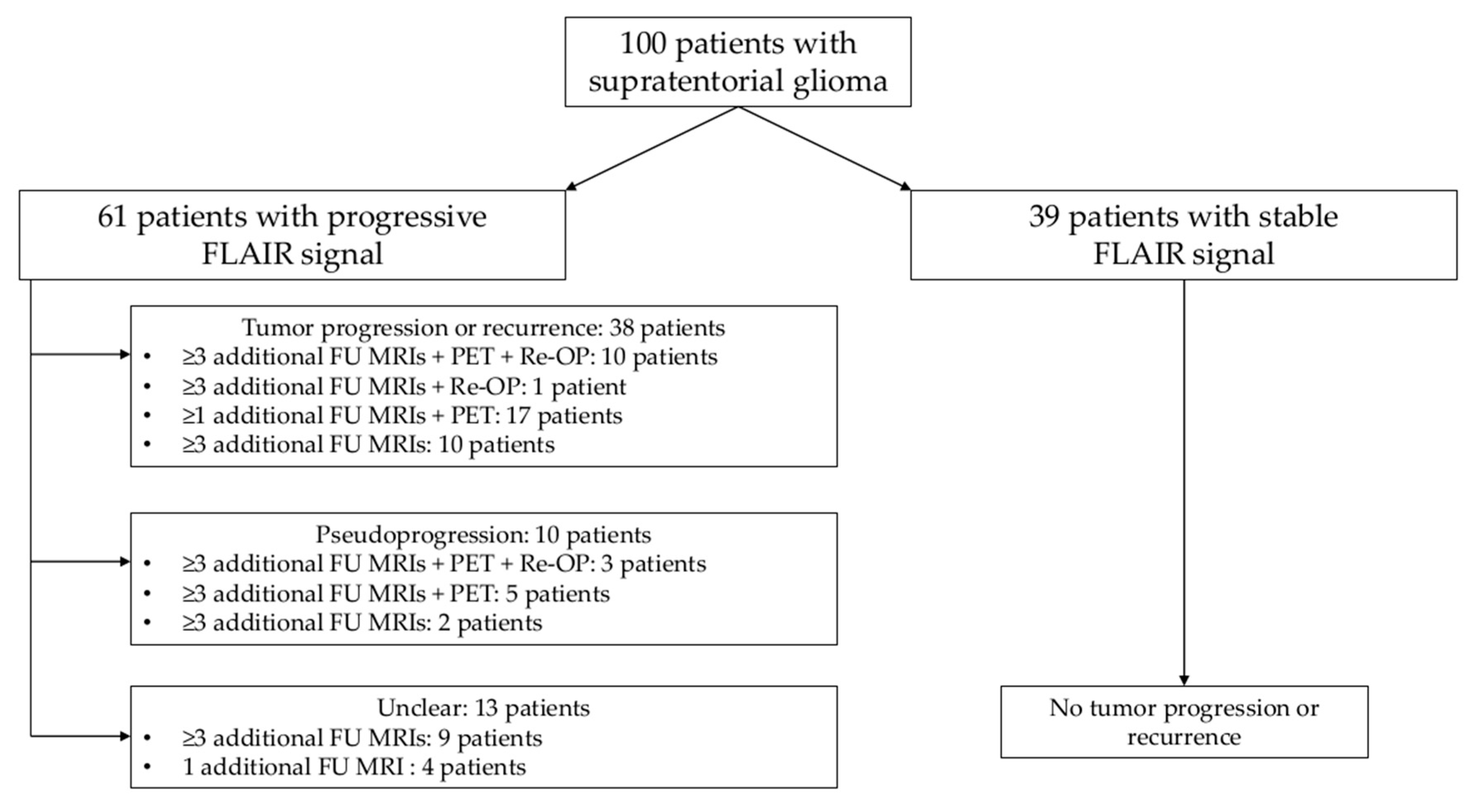
3.1. Patient Cohort
3.2. Image Quality and Artifacts
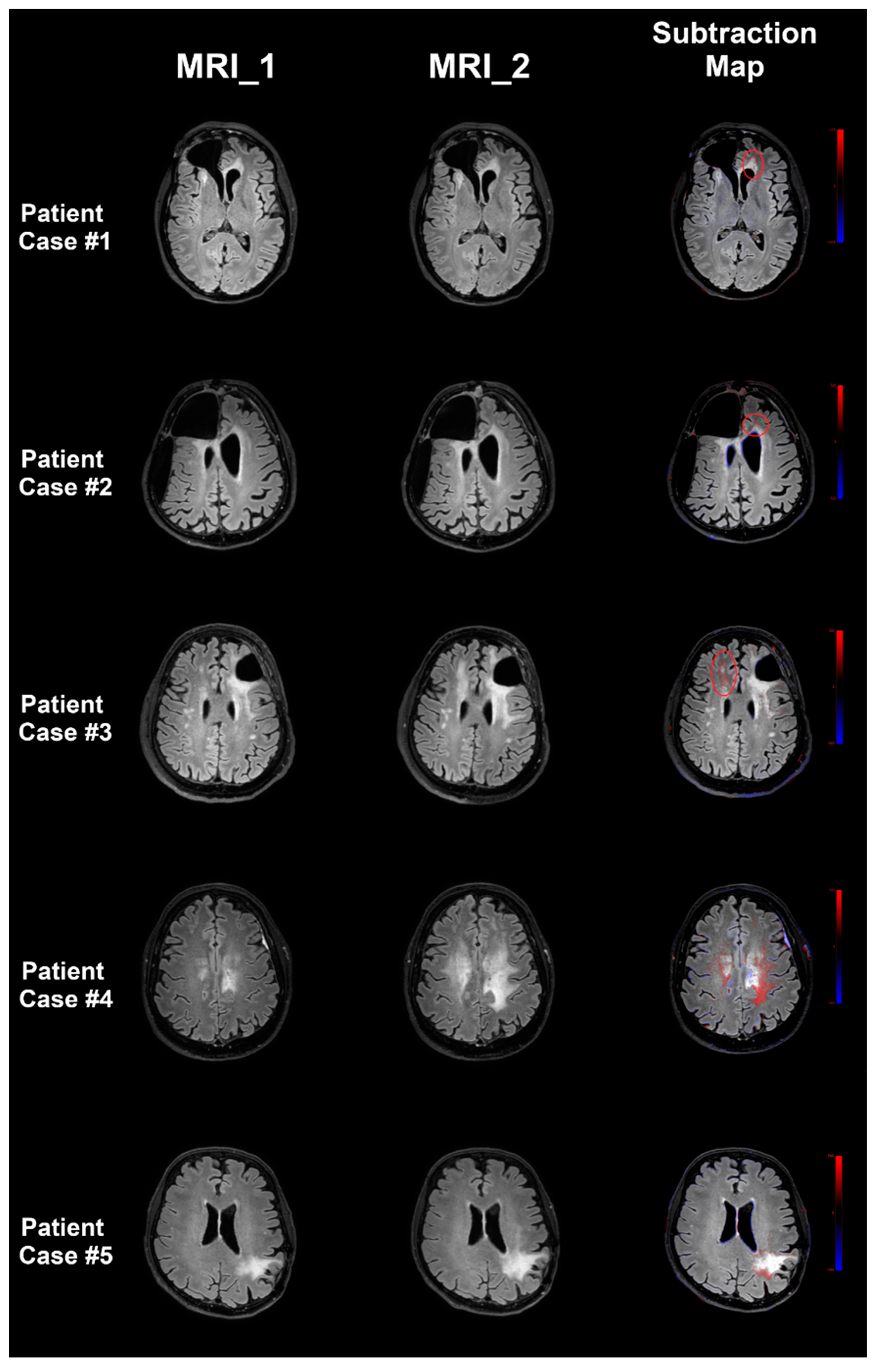
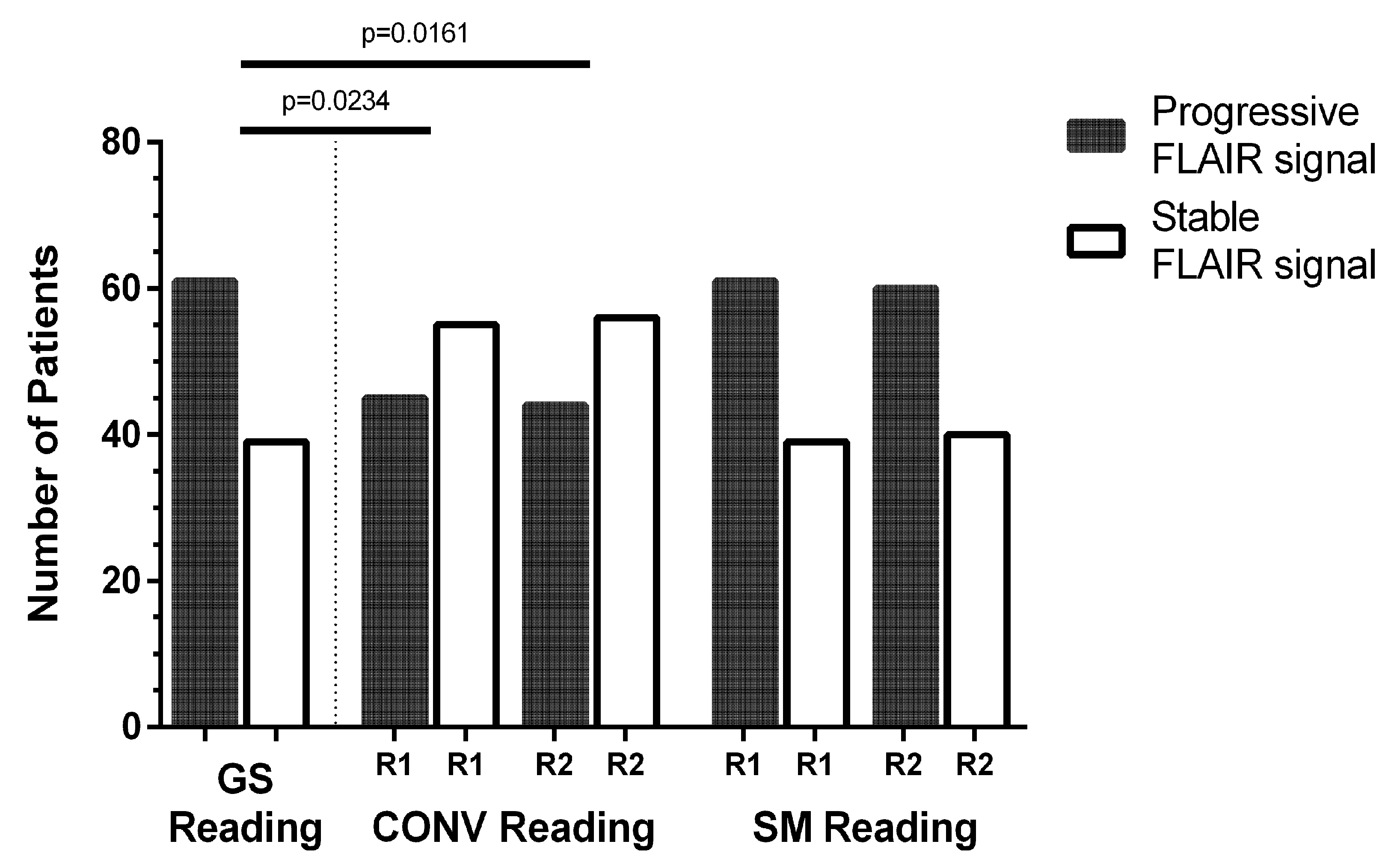
3.3. Evaluation of FLAIR Signal Changes
3.3.1. Progressive Versus Stable FLAIR Signal
3.3.2. Tumor Progression Versus Pseudoprogression
3.3.3. ROC Analysis
3.4. Reading Time and Diagnostic Confidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| CE | Conformité Européenne |
| CONV | Conventional |
| CTX | Chemotherapy |
| FDA | Food and Drug Administration |
| FLAIR | Fluid attenuated inversion recovery |
| FU | Follow-up |
| GS | Gold standard |
| κ | Cohen’s kappa |
| MRI | Magnetic resonance imaging |
| LOBI | Longitudinal Brain Imaging |
| PACS | Picture Archiving and Communication System |
| PET | Positron emission tomography |
| R1 | Reader 1 |
| R2 | Reader 2 |
| ROC | Receiver operating characteristics |
| RTX | Radiotherapy |
| SD | Standard deviation |
| SM | Subtraction map |
| WHO | World Health Organization |
References
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro Oncol. 2014, 16, 896–913. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cote, D.J.; Ascha, M.; Kruchko, C.; Barnholtz-Sloan, J.S. Adult glioma incidence and survival by race or ethnicity in the USA from 2000 to 2014. JAMA Oncol. 2018, 4, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W.; et al. The 2016 world health organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Reifenberger, G.; Wirsching, H.G.; Knobbe-Thomsen, C.B.; Weller, M. Advances in the molecular genetics of gliomas—Implications for classification and therapy. Nat. Rev. Clin. Oncol. 2017, 14, 434–452. [Google Scholar] [CrossRef]
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef]
- Claes, A.; Idema, A.J.; Wesseling, P. Diffuse glioma growth: A guerilla war. Acta Neuropathol. 2007, 114, 443–458. [Google Scholar] [CrossRef]
- Shaw, E.G.; Berkey, B.; Coons, S.W.; Bullard, D.; Brachman, D.; Buckner, J.C.; Stelzer, K.J.; Barger, G.R.; Brown, P.D.; Gilbert, M.R.; et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: Results of a prospective clinical trial. J. Neurosurg. 2008, 109, 835–841. [Google Scholar] [CrossRef]
- De Bonis, P.; Anile, C.; Pompucci, A.; Fiorentino, A.; Balducci, M.; Chiesa, S.; Lauriola, L.; Maira, G.; Mangiola, A. The influence of surgery on recurrence pattern of glioblastoma. Clin. Neurol. Neurosurg. 2013, 115, 37–43. [Google Scholar] [CrossRef]
- Chaichana, K.L.; McGirt, M.J.; Laterra, J.; Olivi, A.; Quinones-Hinojosa, A. Recurrence and malignant degeneration after resection of adult hemispheric low-grade gliomas. J. Neurosurg. 2010, 112, 10–17. [Google Scholar] [CrossRef]
- Sughrue, M.E.; Sheean, T.; Bonney, P.A.; Maurer, A.J.; Teo, C. Aggressive repeat surgery for focally recurrent primary glioblastoma: Outcomes and theoretical framework. Neurosurg. Focus 2015, 38, E11. [Google Scholar] [CrossRef]
- Ringel, F.; Pape, H.; Sabel, M.; Krex, D.; Bock, H.C.; Misch, M.; Weyerbrock, A.; Westermaier, T.; Senft, C.; Schucht, P.; et al. Clinical benefit from resection of recurrent glioblastomas: Results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol. 2016, 18, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Dictus, C.; Hartmann, C.; Zurn, O.; Edler, L.; Hartmann, M.; Combs, S.; Herold-Mende, C.; Wirtz, C.R.; Unterberg, A.; et al. Long-term outcome and survival of surgically treated supratentorial low-grade glioma in adult patients. Acta Neurochir. 2009, 151, 1359–1365. [Google Scholar] [CrossRef]
- Capelle, L.; Fontaine, D.; Mandonnet, E.; Taillandier, L.; Golmard, J.L.; Bauchet, L.; Pallud, J.; Peruzzi, P.; Baron, M.H.; Kujas, M.; et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization grade II gliomas: A series of 1097 cases: Clinical article. J. Neurosurg. 2013, 118, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.H.; Berger, M.S.; Lamborn, K.R.; Aldape, K.; McDermott, M.W.; Prados, M.D.; Chang, S.M. Repeated operations for infiltrative low-grade gliomas without intervening therapy. J. Neurosurg. 2003, 98, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.G.; Garside, R.; Rogers, G.; Stein, K.; Grant, R. Temozolomide for high grade glioma. Cochrane Database Syst. Rev. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.T.; Brem, S. Taming glioblastoma by targeting angiogenesis: 3 years later. J. Clin. Oncol. 2011, 29, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; Van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H.; Taillandier, L. New concepts in the management of diffuse low-grade glioma: Proposal of a multistage and individualized therapeutic approach. Neuro Oncol. 2015, 17, 332–342. [Google Scholar] [CrossRef]
- Villanueva-Meyer, J.E.; Mabray, M.C.; Cha, S. Current clinical brain tumor imaging. Neurosurgery 2017, 81, 397–415. [Google Scholar] [CrossRef]
- Rees, J. Advances in magnetic resonance imaging of brain tumors. Curr. Opin. Neurol. 2003, 16, 643–650. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Van den Bent, M.J.; Wefel, J.S.; Schiff, D.; Taphoorn, M.J.; Jaeckle, K.; Junck, L.; Armstrong, T.; Choucair, A.; Waldman, A.D.; Gorlia, T.; et al. Response assessment in neuro-oncology (a report of the RANO group): Assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011, 12, 583–593. [Google Scholar] [CrossRef]
- Okada, H.; Weller, M.; Huang, R.; Finocchiaro, G.; Gilbert, M.R.; Wick, W.; Ellingson, B.M.; Hashimoto, N.; Pollack, I.F.; Brandes, A.A.; et al. Immunotherapy response assessment in neuro-oncology: A report of the RANO working group. Lancet Oncol. 2015, 16, e534–e542. [Google Scholar] [CrossRef]
- Zopfs, D.; Laukamp, K.R.; Paquet, S.; Lennartz, S.; Pinto Dos Santos, D.; Kabbasch, C.; Bunck, A.; Schlamann, M.; Borggrefe, J. Follow-up MRI in multiple sclerosis patients: Automated co-registration and lesion color-coding improves diagnostic accuracy and reduces reading time. Eur. Radiol. 2019, 29, 7047–7054. [Google Scholar] [CrossRef]
- Eichinger, P.; Wiestler, H.; Zhang, H.; Biberacher, V.; Kirschke, J.S.; Zimmer, C.; Muhlau, M.; Wiestler, B. A novel imaging technique for better detecting new lesions in multiple sclerosis. J. Neurol. 2017, 264, 1909–1918. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Linker, R.A.; Lang, S.; Lucking, H.; Engelhorn, T.; Kloska, S.; Uder, M.; Cavallaro, A.; Dorfler, A.; Dankerl, P.; et al. FLAIR fusion processing with contrast inversion: Improving detection and reading time of new cerebral MS lesions. Clin. Neuroradiol. 2018, 28, 367–376. [Google Scholar] [CrossRef]
- Van Heerden, J.; Rawlinson, D.; Zhang, A.M.; Chakravorty, R.; Tacey, M.A.; Desmond, P.M.; Gaillard, F. Improving Multiple Sclerosis Plaque Detection Using a Semiautomated Assistive Approach. Am. J. Neuroradiol. 2015, 36, 1465–1471. [Google Scholar] [CrossRef]
- Galletto Pregliasco, A.; Collin, A.; Gueguen, A.; Metten, M.A.; Aboab, J.; Deschamps, R.; Gout, O.; Duron, L.; Sadik, J.C.; Savatovsky, J.; et al. Improved detection of new MS lesions during follow-up using an automated MR coregistration-fusion method. Am. J. Neuroradiol. 2018, 39, 1226–1232. [Google Scholar] [CrossRef]
- Monch, S.; Sollmann, N.; Hock, A.; Zimmer, C.; Kirschke, J.S.; Hedderich, D.M. Magnetic resonance imaging of the brain using compressed sensing—Quality assessment in daily clinical routine. Clin. Neuroradiol. 2019, 30, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Regnery, S.; Knowles, B.R.; Paech, D.; Behl, N.; Meissner, J.E.; Windisch, P.; Ben Harrabi, S.; Bernhardt, D.; Schlemmer, H.P.; Ladd, M.E.; et al. High-resolution FLAIR MRI at 7 Tesla for treatment planning in glioblastoma patients. Radiother. Oncol. 2019, 130, 180–184. [Google Scholar] [CrossRef]
- Poser, B.A.; Setsompop, K. Pulse sequences and parallel imaging for high spatiotemporal resolution MRI at ultra-high field. NeuroImage 2018, 168, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Lennartz, S.; Zopfs, D.; Nobis, A.; Paquet, S.; Hoyer, U.C.I.; Zaske, C.; Goertz, L.; Kabbasch, C.; Laukamp, K.R.; Grosse Hokamp, N.; et al. MRI Follow-up of astrocytoma: Automated coregistration and color-coding of FLAIR sequences improves diagnostic accuracy with comparable reading time. J. Magn. Reson. Imaging 2020, 52, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Horsfield, M.A.; Banahan, C.; Thomas, A.G.; Nath, M.; Nath, J.; Ambrosi, P.B.; Chung, E.M.L. Detection of focal longitudinal changes in the brain by subtraction of MR images. Am. J. Neuroradiol. 2017, 38, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Chang, S.M.; Van den Bent, M.J.; Vogelbaum, M.A.; Macdonald, D.R.; Lee, E.Q. Response assessment in neuro-oncology clinical trials. J. Clin. Oncol. 2017, 35, 2439–2449. [Google Scholar] [CrossRef]
- Kazda, T.; Hardie, J.G.; Pafundi, D.H.; Kaufmann, T.J.; Brinkmann, D.H.; Laack, N.N. Evaluation of RANO response criteria compared to clinician evaluation in WHO grade III anaplastic astrocytoma: Implications for clinical trial reporting and patterns of failure. J. Neuro Oncol. 2015, 122, 197–203. [Google Scholar] [CrossRef]
- Huber, T.; Alber, G.; Bette, S.; Kaesmacher, J.; Boeckh-Behrens, T.; Gempt, J.; Ringel, F.; Specht, H.M.; Meyer, B.; Zimmer, C.; et al. Progressive disease in glioblastoma: Benefits and limitations of semi-automated volumetry. PLoS ONE 2017, 12, e0173112. [Google Scholar] [CrossRef]
- Kumar, A.J.; Leeds, N.E.; Fuller, G.N.; Van Tassel, P.; Maor, M.H.; Sawaya, R.E.; Levin, V.A. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 2000, 217, 377–384. [Google Scholar] [CrossRef]
- Taal, W.; Brandsma, D.; De Bruin, H.G.; Bromberg, J.E.; Swaak-Kragten, A.T.; Smitt, P.A.; Van Es, C.A.; Van den Bent, M.J. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 2008, 113, 405–410. [Google Scholar] [CrossRef]
- Gao, X.Y.; Wang, Y.D.; Wu, S.M.; Rui, W.T.; Ma, D.N.; Duan, Y.; Zhang, A.N.; Yao, Z.W.; Yang, G.; Yu, Y.P.; et al. Differentiation of treatment-related effects from glioma recurrence using machine learning classifiers based upon pre-and post-contrast T1WI and T2 FLAIR subtraction features: A two-center study. Cancer Manag. Res. 2020, 12, 3191–3201. [Google Scholar] [CrossRef]
- Elshafeey, N.; Kotrotsou, A.; Hassan, A.; Elshafei, N.; Hassan, I.; Ahmed, S.; Abrol, S.; Agarwal, A.; El Salek, K.; Bergamaschi, S.; et al. Multicenter study demonstrates radiomic features derived from magnetic resonance perfusion images identify pseudoprogression in glioblastoma. Nat. Commun. 2019, 10, 3170. [Google Scholar] [CrossRef]
- Ismail, M.; Hill, V.; Statsevych, V.; Huang, R.; Prasanna, P.; Correa, R.; Singh, G.; Bera, K.; Beig, N.; Thawani, R.; et al. Shape features of the lesion habitat to differentiate brain tumor progression from pseudoprogression on routine multiparametric MRI: A multisite study. Am. J. Neuroradiol. 2018, 39, 2187–2193. [Google Scholar] [CrossRef]
| Qualitative Image Evaluation | |||||
|---|---|---|---|---|---|
| Item | Score | ||||
| 1 | 2 | 3 | 4 | 5 | |
| Overall Image Quality (for CONV reading) | Very good to perfect quality | Good to very good quality | Medium quality | Adequate quality | Poor quality |
| Overall Artifacts (for SM reading) | No artifacts | Minimal artifacts | Prominent artifacts | Major artifacts | Severe artifacts |
| Diagnostic Confidence (for CONV and SM reading) | Very high | High | Intermediate | Low | Very low |
| Cohort Characteristics | |||
|---|---|---|---|
| Tumor entity (N, patients) | Glioma WHO grade I | 2 | |
| Glioma WHO grade II | 17 | ||
| Glioma WHO grade III | 42 | ||
| Glioma WHO grade IV | 39 | ||
| Tumor location (N, patients) | Left hemisphere | 59 | |
| Right hemisphere | 37 | ||
| Multifocal/Corpus callosum | 4 | ||
| Time since first diagnosis (months, mean ± SD [range]) | 45.8 ± 58.7 [1.1–334.1] | ||
| Tumor resection/biopsy performed (N, patients) | 96/4 | ||
| Time since (last) tumor resection (months, mean ± SD [range]) | 20.9 ± 22.1 [1.0–114.8] | ||
| Interval between MRI_1 and MRI_2 (months, mean ± SD [range]) | 5.4 ± 1.9 [1.0–9.6] | ||
| Adjuvant CTX | CTX performed (N, patients) | 89 | |
| Substance of (last) CTX (N, patients) | Temozolomide | 64 | |
| PCV | 14 | ||
| CCNU | 10 | ||
| Bevacizumab | 1 | ||
| Time since (last) CTX (months, mean ± SD [range]) | 14.4 ± 16.8 [0.3–72.2] | ||
| Adjuvant RTX | RTX performed (N, patients) | 93 | |
| RTX dose of (last) RTX (Gy, mean ± SD [range]) | 53.5 ± 8.8 [20.0–64.0] | ||
| Time since (last) RTX (months, mean ± SD [range]) | 27.7 ± 44.7 [0.1–334.1] | ||
| ROC Analysis | CONV Reading | SM Reading | ||
|---|---|---|---|---|
| R1 | R2 | R1 | R2 | |
| True positive | 45 | 43 | 60 | 60 |
| True negative | 40 | 39 | 39 | 40 |
| False positive | 0 | 1 | 1 | 0 |
| False negative | 15 | 17 | 0 | 0 |
| Sensitivity | 73.3% | 99.9% | ||
| Specificity | 98.8% | 98.1% | ||
| Positive predictive value | 98.9% | 98.8% | ||
| Negative predictive value | 71.2% | 99.9% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sollmann, N.; Gutbrod-Fernandez, M.; Burian, E.; Riederer, I.; Meyer, B.; Hock, A.; Gempt, J.; Zimmer, C.; Kirschke, J.S. Subtraction Maps Derived from Longitudinal Magnetic Resonance Imaging in Patients with Glioma Facilitate Early Detection of Tumor Progression. Cancers 2020, 12, 3111. https://doi.org/10.3390/cancers12113111
Sollmann N, Gutbrod-Fernandez M, Burian E, Riederer I, Meyer B, Hock A, Gempt J, Zimmer C, Kirschke JS. Subtraction Maps Derived from Longitudinal Magnetic Resonance Imaging in Patients with Glioma Facilitate Early Detection of Tumor Progression. Cancers. 2020; 12(11):3111. https://doi.org/10.3390/cancers12113111
Chicago/Turabian StyleSollmann, Nico, Magaly Gutbrod-Fernandez, Egon Burian, Isabelle Riederer, Bernhard Meyer, Andreas Hock, Jens Gempt, Claus Zimmer, and Jan S. Kirschke. 2020. "Subtraction Maps Derived from Longitudinal Magnetic Resonance Imaging in Patients with Glioma Facilitate Early Detection of Tumor Progression" Cancers 12, no. 11: 3111. https://doi.org/10.3390/cancers12113111
APA StyleSollmann, N., Gutbrod-Fernandez, M., Burian, E., Riederer, I., Meyer, B., Hock, A., Gempt, J., Zimmer, C., & Kirschke, J. S. (2020). Subtraction Maps Derived from Longitudinal Magnetic Resonance Imaging in Patients with Glioma Facilitate Early Detection of Tumor Progression. Cancers, 12(11), 3111. https://doi.org/10.3390/cancers12113111







