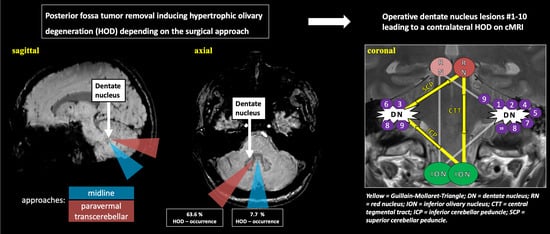
A Paravermal Trans-Cerebellar Approach to the Posterior Fossa Tumor Causes Hypertrophic Olivary Degeneration by Dentate Nucleus Injury
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
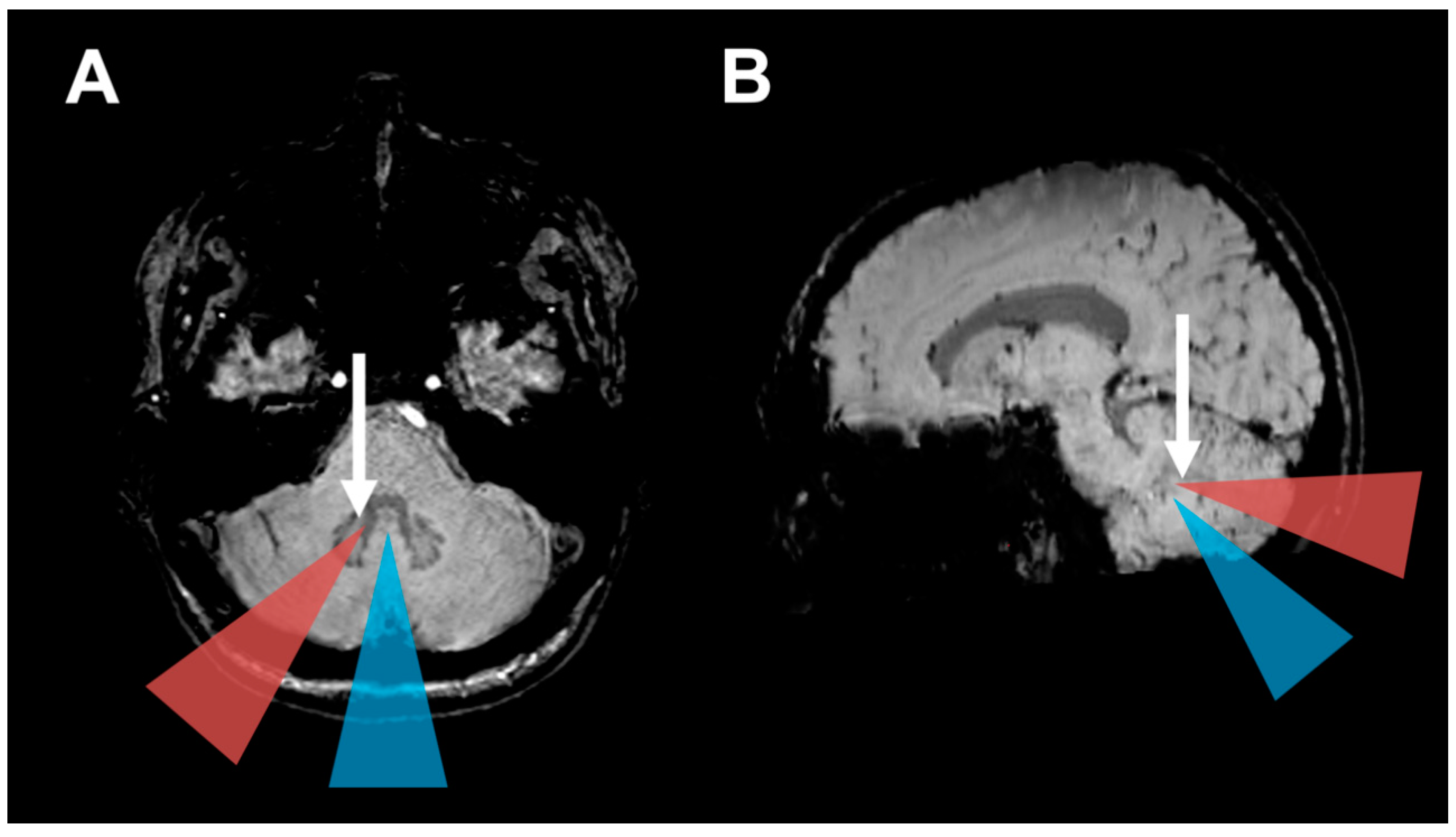
2.2. Surgical Approach and HOD Development
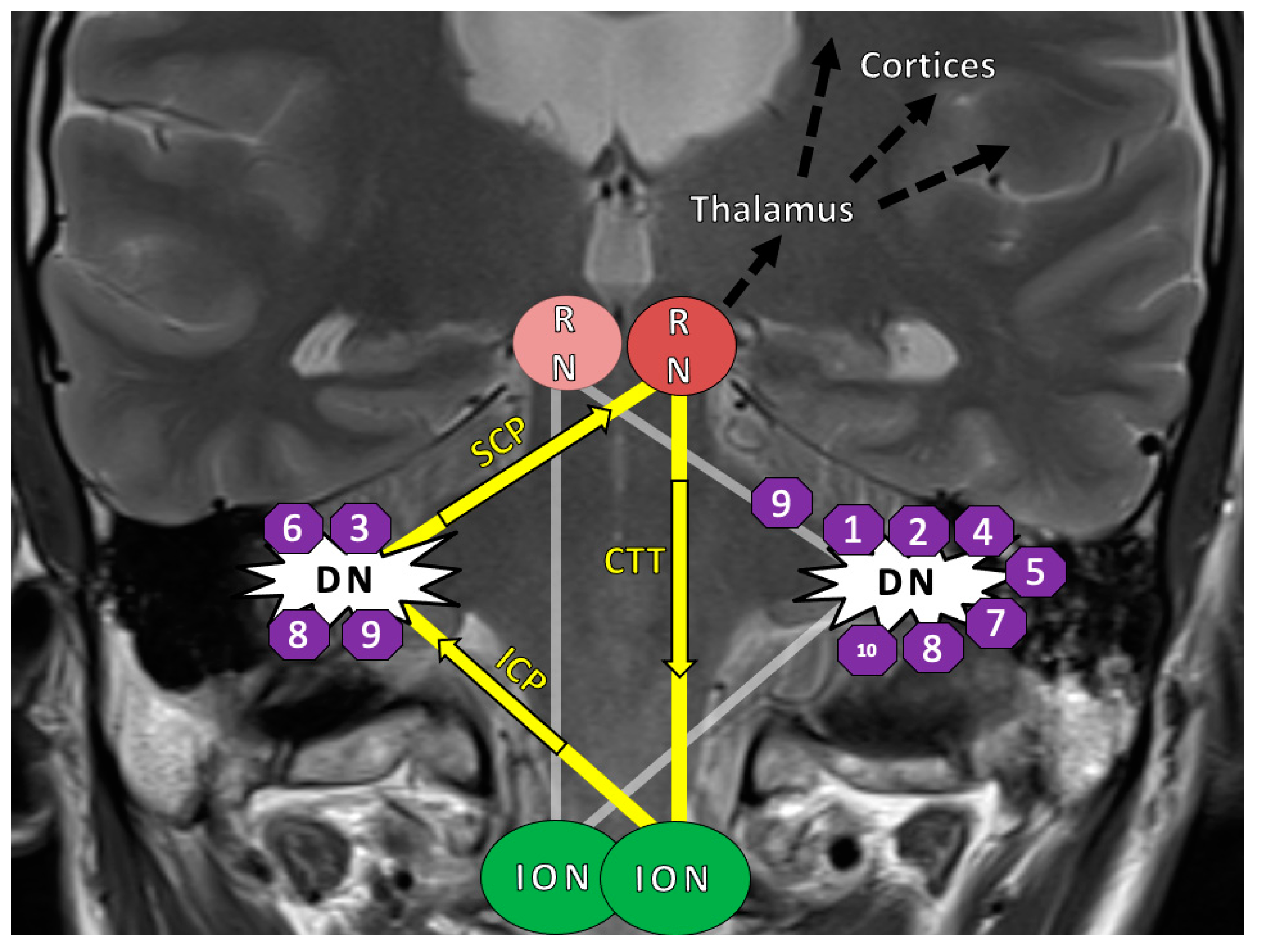
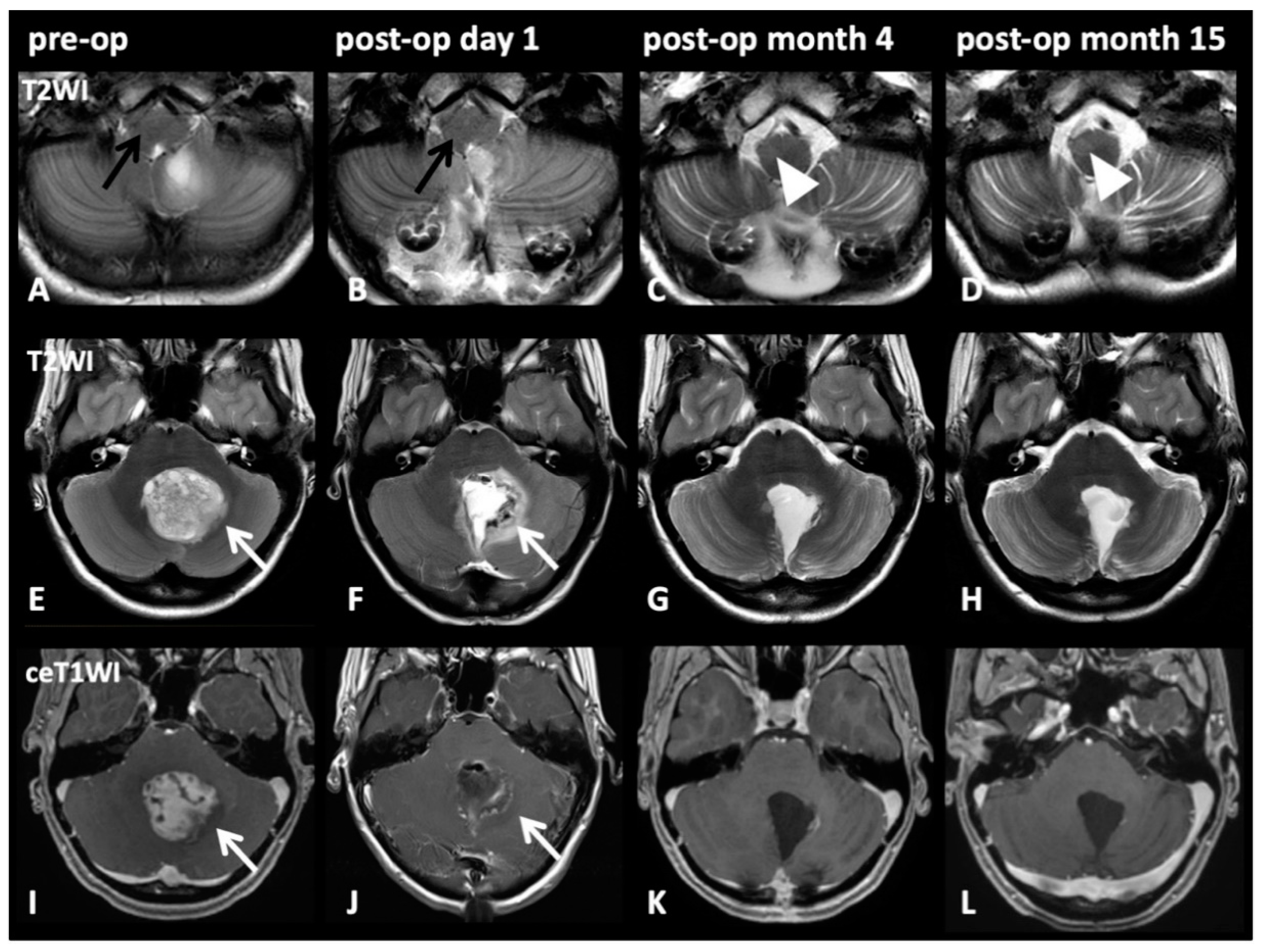
2.3. HOD-Patient Characteristics and Disease Pattern
2.4. Surgical Approach and CMS
3. Discussion
Limitations
4. Materials and Methods
4.1. Study Population
4.2. Magnetic Resonance Imaging
4.3. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variable | Odds Ratio | p-Value |
|---|---|---|
| Paravermal trans-cerebellar approach | 39.26 | 0.0041 |
| Age | 1.06 | 0.1059 |
| Sex | 1.90 | 0.5646 |
| Volume | 1.00 | 0.4982 |
| Chemotherapy | 0.42 | 0.5743 |
| Radiotherapy | 4.83 | 0.3133 |
| Variable | Odds Ratio | p-Value |
|---|---|---|
| Paravermal trans-cerebellar approach | 54.72 | 0.008 |
| Age | 1.07 | 0.110 |
| Sex | 1.89 | 0.588 |
| Volume | 1.00 | 0.285 |
| Chemotherapy | 0.84 | 0.919 |
| Radiotherapy | 5.79 | 0.298 |
| Location 4th Ventricle | 5.09 | 0.294 |
| Age, Sex | Diagnosis WHO° | Later HOD | Tumor Localization | Associated Cyst | Original Operative Approach | New Recommended Approach |
|---|---|---|---|---|---|---|
| 12, m | pilocytic astrocytoma I° | no | 4th ventricle to cerebellum | yes | paravermal trans-cerebellar | telovelar |
| 20, f | pilocytic astrocytoma I° | yes | 4th ventricle | no | paravermal trans-cerebellar | telovelar |
| 12, f | pilocytic astrocytoma I° | no | 4th ventricle | yes | paravermal trans-cerebellar | supracerebellar, then transcerebellar |
| 2, m | ependymoma II° | no | 4th ventricle | no | paravermal trans-cerebellar | combined telovelar and retrosigmoid |
| 60, f | pilocytic astrocytoma I° | yes | 4th ventricle to vermis | yes | paravermal trans-cerebellar | original approach, but without cyst membrane removal |
| 32, m | pilocytic astrocytoma I° | yes | 4th ventricle to vermis | yes | paravermal trans-cerebellar | telovelar |
| 14, f | pilocytic astrocytoma I° | yes | vermis | yes | paravermal trans-cerebellar | supracerebellar, then transcerebellar |
| 17, f | pilocytic astrocytoma I° | yes | 4th ventricle | no | paravermal trans-cerebellar | telovelar |
| 3, m | medulloblastoma IV° | no | cerebellum | yes | paravermal trans-cerebellar | original approach, but without cyst membrane removal |
| 9, m | medulloblastoma IV° | yes | vermis and cerebellum | no | paravermal trans-cerebellar | original approach, respect DN as feasible |
| 40, m | medulloblastoma IV° | yes | 4th ventricle | no | paravermal trans-cerebellar | telovelar |
References
- Goto, N.; Kaneko, M. Olivary enlargement: Chronological and morphometric analyses. Acta Neuropathol. 1981, 54, 275–282. [Google Scholar] [CrossRef]
- Lapresle, J. Rhythmic palatal myoclonus and the dentato-olivary pathway. J. Neurol. 1979, 220, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.F.C. Sur la dégénération pseudo-hypertrophique de l’olive bulbaire. Rev. Neurol. 1913, 26, 48–52. [Google Scholar]
- Murdoch, S.; Shah, P.; Jampana, R. The Guillain-Mollaret triangle in action. Pract. Neurol. 2016, 16, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Schaller-Paule, M.A.; Foerch, C.; Kluge, S.; Baumgarten, P.; Konczalla, J.; Steinbach, J.P.; Wagner, M.; Luger, A.-L. Delayed occurrence of hypertrophic olivary degeneration after therapy of posterior fossa tumors: A single institution retrospective analysis. J. Clin. Med. 2019, 8, 2222. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Hatzoglou, V.; Karimi, S.; Young, R.J. Hypertrophic olivary degeneration resulting from posterior fossa masses and their treatments. Clin. Imaging 2015, 39, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Versnick, E.; Tuite, P.; Cyr, J.S.; Kucharczyk, W.; Montanera, W.; Willinsky, R.; Mikulis, D. Hypertrophic olivary degeneration: Metaanalysis of the temporal evolution of MR findings. AJNR Am. J. Neuroradiol. 2000, 21, 1073–1077. [Google Scholar]
- Gautier, J.C.; Blackwood, W. Enlargement of the inferior olivary nucleus in association with lesions of the central tegmental tract or dentate nucleus. Brain 1961, 84, 341–361. [Google Scholar] [CrossRef]
- Onen, M.R.; Moore, K.; Cikla, U.; Ucer, M.; Schmidt, B.; Field, A.S.; Baskaya, M.K. Hypertrophic olivary degeneration: Neurosurgical perspective and literature review. World Neurosurg. 2018, 112, e763–e771. [Google Scholar] [CrossRef]
- Avula, S.; Spiteri, M.; Kumar, R.; Lewis, E.; Harave, S.; Windridge, D.; Ong, C.; Pizer, B. Post-operative pediatric cerebellar mutism syndrome and its association with hypertrophic olivary degeneration. Quant. Imaging Med. Surg. 2016, 6, 535–544. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Wang, R.; Li, Y.; Wang, P.; Li, J.; Du, J. Hypertrophic olivary degeneration: A comprehensive review focusing on etiology. Brain Res. 2019, 1718, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Foerch, C.; Schaller, M.A.; Lapa, S.; Filipski, K.; Steinmetz, H.; Kang, J.-S.; Zöllner, J.P.; Wagner, M. Hypertrophe degeneration der olive: Ursache neuerlicher neurologischer symptome nach schlaganfall. Nervenarzt 2019, 90, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Tilikete, C.; Desestret, V. Hypertrophic olivary degeneration and palatal or oculopalatal tremor. Front. Neurol. 2017, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- van de Loo, S.; Somasundaram, S.; Wagner, M.; Singer, O.C.; Steinmetz, H.; Hilker, R. Dysphagia in symptomatic palatal tremor. Mov. Disord. 2010, 25, 1304–1305. [Google Scholar] [CrossRef]
- Deuschl, G.; Mischke, G.; Schenck, E.; Schulte-Mönting, J.; Lücking, C.H. Symptomatic and essential rhythmic palatal myoclonus. Brain 1990, 113 Pt 6, 1645–1672. [Google Scholar] [CrossRef]
- Beez, T.; Munoz-Bendix, C.; Steiger, H.-J.; Hänggi, D. Functional tracts of the cerebellum-essentials for the neurosurgeon. Neurosurg. Rev. 2020. [Google Scholar] [CrossRef]
- Albazron, F.M.; Bruss, J.; Jones, R.M.; Yock, T.I.; Pulsifer, M.B.; Cohen, A.L.; Nopoulos, P.C.; Abrams, A.N.; Sato, M.; Boes, A.D. Pediatric postoperative cerebellar cognitive affective syndrome follows outflow pathway lesions. Neurology 2019, 93, e1561–e1571. [Google Scholar] [CrossRef]
- Schmahmann, J.D. Neuroanatomy of pediatric postoperative cerebellar cognitive affective syndrome and mutism. Neurology 2019, 93, 693–694. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Schmahmann, J.D. Functional topography of the human cerebellum. Handb. Clin. Neurol. 2018, 154, 59–70. [Google Scholar] [CrossRef]
- Law, N.; Greenberg, M.; Bouffet, E.; Taylor, M.D.; Laughlin, S.; Strother, D.; Fryer, C.; McConnell, D.; Hukin, J.; Kaise, C.; et al. Clinical and neuroanatomical predictors of cerebellar mutism syndrome. Neuro-Oncology 2012, 14, 1294–1303. [Google Scholar] [CrossRef]
- Gudrunardottir, T.; Morgan, A.T.; Lux, A.L.; Walker, D.A.; Walsh, K.S.; Wells, E.M.; Wisoff, J.H.; Juhler, M.; Schmahmann, J.D.; Keating, R.F.; et al. Consensus paper on post-operative pediatric cerebellar mutism syndrome: The Iceland Delphi results. Childs. Nerv. Syst. 2016, 32, 1195–1203. [Google Scholar] [CrossRef]
- Al-Afif, S.; Krauss, J.K.; Helms, F.; Angelov, S.; John, N.; Schwabe, K.; Hermann, E.J. Long-term impairment of social behavior, vocalizations and motor activity induced by bilateral lesions of the fastigial nucleus in juvenile rats. Brain Struct. Funct. 2019, 224, 1739–1751. [Google Scholar] [CrossRef] [PubMed]
- Lanier, J.C.; Abrams, A.N. Posterior fossa syndrome: Review of the behavioral and emotional aspects in pediatric cancer patients. Cancer 2017, 123, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Hoche, F.; Guell, X.; Vangel, M.G.; Sherman, J.C.; Schmahmann, J.D. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain 2018, 141, 248–270. [Google Scholar] [CrossRef] [PubMed]
- Levisohn, L.; Cronin-Golomb, A.; Schmahmann, J.D. Neuropsychological consequences of cerebellar tumour resection in children: Cerebellar cognitive affective syndrome in a paediatric population. Brain 2000, 123 Pt 5, 1041–1050. [Google Scholar] [CrossRef]
- Wisoff, J.H.; Epstein, F.J. Pseudobulbar palsy after posterior fossa operation in children. Neurosurgery 1984, 15, 707–709. [Google Scholar] [CrossRef]
- Renne, B.; Radic, J.; Agrawal, D.; Albrecht, B.; Bonfield, C.M.; Cohrs, G.; Davis, T.; Gupta, A.; Hebb, A.L.O.; Lamberti-Pasculli, M.; et al. Cerebellar mutism after posterior fossa tumor resection in children: A multicenter international retrospective study to determine possible modifiable factors. Childs. Nerv. Syst. 2019. [Google Scholar] [CrossRef]
- de Smet, H.J.; Baillieux, H.; Wackenier, P.; de Praeter, M.; Engelborghs, S.; Paquier, P.F.; de Deyn, P.P.; Mariën, P. Long-term cognitive deficits following posterior fossa tumor resection: A neuropsychological and functional neuroimaging follow-up study. Neuropsychology 2009, 23, 694–704. [Google Scholar] [CrossRef]
- Huber, J.F.; Bradley, K.; Spiegler, B.J.; Dennis, M. Long-term effects of transient cerebellar mutism after cerebellar astrocytoma or medulloblastoma tumor resection in childhood. Childs. Nerv. Syst. 2006, 22, 132–138. [Google Scholar] [CrossRef]
- Cámara, S.; Fournier, M.C.; Cordero, P.; Melero, J.; Robles, F.; Esteso, B.; Vara, M.T.; Rodríguez, S.; Lassaletta, Á.; Budke, M. Neuropsychological profile in children with posterior fossa tumors with or without postoperative cerebellar mutism syndrome (CMS). Cerebellum 2020, 19, 78–88. [Google Scholar] [CrossRef]
- Steinbok, P.; Cochrane, D.D.; Perrin, R.; Price, A. Mutism after posterior fossa tumour resection in children: Incomplete recovery on long-term follow-up. Pediatr. Neurosurg. 2003, 39, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Grieco, J.A.; Abrams, A.N.; Evans, C.L.; Yock, T.I.; Pulsifer, M.B. A comparison study assessing neuropsychological outcome of patients with post-operative pediatric cerebellar mutism syndrome and matched controls after proton radiation therapy. Childs. Nerv. Syst. 2020, 36, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, E.A.; Melzer, C.; Timmermann, L.; von Cramon, D.Y.; Tittgemeyer, M. Basal ganglia and cerebellar interconnectivity within the human thalamus. Brain Struct. Funct. 2017, 222, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.C.; Graff-Radford, J.; Jones, D.T.; Benarroch, E.E. Mediodorsal nucleus and its multiple cognitive functions. Neurology 2016, 87, 2161–2168. [Google Scholar] [CrossRef]
- Patay, Z.; Enterkin, J.; Harreld, J.H.; Yuan, Y.; Löbel, U.; Rumboldt, Z.; Khan, R.; Boop, F. MR imaging evaluation of inferior olivary nuclei: Comparison of postoperative subjects with and without posterior fossa syndrome. AJNR Am. J. Neuroradiol. 2014, 35, 797–802. [Google Scholar] [CrossRef]
- Avula, S.; Kumar, R.; Pizer, B.; Pettorini, B.; Abernethy, L.; Garlick, D.; Mallucci, C. Diffusion abnormalities on intraoperative magnetic resonance imaging as an early predictor for the risk of posterior fossa syndrome. Neuro-Oncology 2015, 17, 614–622. [Google Scholar] [CrossRef]
- Cobourn, K.; Marayati, F.; Tsering, D.; Ayers, O.; Myseros, J.S.; Magge, S.N.; Oluigbo, C.O.; Keating, R.F. Cerebellar mutism syndrome: Current approaches to minimize risk for CMS. Child’s Nerv. Syst. 2019. [Google Scholar] [CrossRef]
- Akakin, A.; Peris-Celda, M.; Kilic, T.; Seker, A.; Gutierrez-Martin, A.; Rhoton, A. The dentate nucleus and its projection system in the human cerebellum: The dentate nucleus microsurgical anatomical study. Neurosurgery 2014, 74, 401–424. [Google Scholar] [CrossRef]
- Morris, E.B.; Phillips, N.S.; Laningham, F.H.; Patay, Z.; Gajjar, A.; Wallace, D.; Boop, F.; Sanford, R.; Ness, K.K.; Ogg, R.J. Proximal dentatothalamocortical tract involvement in posterior fossa syndrome. Brain 2009, 132, 3087–3095. [Google Scholar] [CrossRef]
- Opalak, C.F.; Parry, M.; Rock, A.K.; Sima, A.P.; Carr, M.T.; Chandra, V.; Workman, K.G.; Somasundaram, A.; Broaddus, W.C. Comparison of ABC/2 estimation and a volumetric computerized method for measurement of meningiomas using magnetic resonance imaging. J. Neurooncol. 2019, 144, 275–282. [Google Scholar] [CrossRef]
- Huber, J.F.; Bradley, K.; Spiegler, B.; Dennis, M. Long-term neuromotor speech deficits in survivors of childhood posterior fossa tumors: Effects of tumor type, radiation, age at diagnosis, and survival years. J. Child Neurol. 2007, 22, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Wells, E.M.; Khademian, Z.P.; Walsh, K.S.; Vezina, G.; Sposto, R.; Keating, R.F.; Packer, R.J. Postoperative cerebellar mutism syndrome following treatment of medulloblastoma: Neuroradiographic features and origin. J. Neurosurg. Pediatr. 2010, 5, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Molinari, E.; Pizer, B.; Catsman-Berrevoets, C.; Avula, S.; Keating, R.; Paquier, P.; Wisoff, J.H.; Walsh, K.S. Posterior fossa society consensus meeting 2018: A synopsis. Child’s Nerv. Syst. 2019. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, K.; Yagmurlu, K.; Kohno, M.; Rhoton, A.L. Anatomy and approaches along the cerebellar-brainstem fissures. J. Neurosurg. 2016, 124, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Korogi, Y.; Shimomura, O.; Sakamoto, Y.; Hirai, T.; Miyayama, H.; Takahashi, M. Hypertrophic olivary degeneration: MR imaging and pathologic findings. Radiology 1994, 192, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Carr, C.M.; Hunt, C.H.; Kaufmann, T.J.; Kotsenas, A.L.; Krecke, K.N.; Wood, C.P. Frequency of bilateral hypertrophic olivary degeneration in a large retrospective cohort. J. Neuroimaging 2015, 25, 289–295. [Google Scholar] [CrossRef]

| n = 50 | All Patients n = 50 | Paravermal Trans-Cerebellar Approach n = 11 | Other Operative Approaches * n = 39 | p-Value | Odds Ratio |
|---|---|---|---|---|---|
| Age (mean ± SD) | 22.7 ± 16.9 | 20.1 ± 17.5 | 23.5 ± 17.0 | 0.57 | |
| Male sex (n) | 30 | 6 (54.5%) | 24 (61.5%) | 0.74 | 0.75 |
| Tumor size 1 (cm3 ± SD) | 27.6 ± 20.6 | 40.8 ± 26.4 | 23.6 ± 16.9 | 0.05 | |
| Histology: | |||||
| 19 | 7 (63.6%) | 12 (30.7%) | 0.08 | 3.94 |
| 9 | 1 (9.1%) | 8 (20.5%) | 0.66 | 0.39 |
| 21 | 3 (27.2%) | 18 (46.1%) | 0.32 | 0.44 |
| Chemotherapy | 24 | 4 (36.3%) | 20 (51.2%) | 0.50 | 0.54 |
| Radiotherapy 2 | 27 | 4 (36.3%) | 23 (58.9%) | 0.17 | 0.35 |
| Associated cyst | 13 | 6 (54.5%) | 7 (17.9%) | 0.02 | 5.49 |
| Location 4th ventricle | 33 | 8 (72.7%) | 25 (64.1%) | 0.73 | 1.49 |
| Operative positioning: | |||||
| 30 | 6 (54.5%) | 24 (61.5%) | 0.09 | 0.29 |
| 15 | 4 (36.3%) | 11 (28.2%) | 0.71 | 1.45 |
| 4 | 3 (27.2%) | 1 (2.6%) | 0.03 | 14.25 |
| Dentate nucleus injury | 21 | 8 (72.7%) | 13 (33.3%) | 0.04 | 5.33 |
| HOD development 4 | 10 | 7 (63.6%) | 3 (7.7%) | 0.0003 | 21 |
| Cerebellar mutism | |||||
| 12 | 2 (18.2%) | 10 (25.6%) | 1.0 | 0.64 |
| 12 | 2 (18.2%) | 10 (25.6%) | 0.41 | 0.40 |
| Pat. | Age, Sex | Diagnosis WHO° | HOD Side | Tumor Localization | Operative Approach | Lesion within GMT | Adjuvant RCT | Tumor Size (mm) [Volume] | Time Lesion to HOD | CMS |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20, f | pilocytic astrocytoma I° | right | 4th ventricle | paravermal trans-cerebellar | both dentate nuclei (left > right) | none | 63 × 42 × 36 [48 cm3] | 2 mo. | no |
| 2 | 60, f | pilocytic astrocytoma I° | right | 4th ventricle to vermis | paravermal trans-cerebellar | both dentate nuclei (left > right) | none | 42 × 63 × 56 [74 cm3] | 8 mo. | no |
| 3 | 32, m | pilocytic astrocytoma I° | left | 4th ventricle to vermis | paravermal trans-cerebellar | right dentate nucleus | none | 37 × 40 × 30 [22 cm3] | 6 mo. | no |
| 4 | 14, f | pilocytic astrocytoma I° | right | cerebellar vermis | paravermal trans-cerebellar | both dentate nuclei | none | 58 × 57 × 57 [94 cm3] | 4 mo. | no |
| 5 | 17, f | pilocytic astrocytoma I° | right | 4th ventricle | paravermal trans-cerebellar | left dentate nucleus | none | 33 × 34 × 39 [22 cm3] | 4 mo. | no |
| 6 | 35, m | ependymoma II° | left | 4th ventricle | midline telovelar | both dentate nuclei (right > left) | none | 42 × 15 × 20 [6 cm3] | 3 mo. | no |
| 7 | 14, m | medulloblastoma IV° | right | 4th ventricle | midline transvermian | left dentate nucleus | yes | 34 × 47 × 37 [30 cm3] | 3 mo. | no |
| 8 | 34, m | medulloblastoma IV° | bilateral | 4th ventricle | midline telovelar | both dentate nuclei | yes | 46 × 35 × 35 [28 cm3] | 10 mo. | no |
| 9 | 9, m | medulloblastoma IV° | bilateral | 4th ventricle and cerebellum | paravermal trans-cerebellar | both dentate nuclei, left SCP | yes | 53 × 37 × 48 [47 cm3] | 4 mo. | yes |
| 10 | 40, m | medulloblastoma IV° | right | 4th ventricle | paravermal trans-cerebellar and telovelar | left dentate nucleus | yes | 31 × 21 × 27 [9 cm3] | 3 mo. | no |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaller-Paule, M.A.; Baumgarten, P.; Seifert, V.; Wagner, M.; Steidl, E.; Hattingen, E.; Wicke, F.; Steinbach, J.P.; Foerch, C.; Konczalla, J. A Paravermal Trans-Cerebellar Approach to the Posterior Fossa Tumor Causes Hypertrophic Olivary Degeneration by Dentate Nucleus Injury. Cancers 2021, 13, 258. https://doi.org/10.3390/cancers13020258
Schaller-Paule MA, Baumgarten P, Seifert V, Wagner M, Steidl E, Hattingen E, Wicke F, Steinbach JP, Foerch C, Konczalla J. A Paravermal Trans-Cerebellar Approach to the Posterior Fossa Tumor Causes Hypertrophic Olivary Degeneration by Dentate Nucleus Injury. Cancers. 2021; 13(2):258. https://doi.org/10.3390/cancers13020258
Chicago/Turabian StyleSchaller-Paule, Martin A., Peter Baumgarten, Volker Seifert, Marlies Wagner, Eike Steidl, Elke Hattingen, Felix Wicke, Joachim P. Steinbach, Christian Foerch, and Juergen Konczalla. 2021. "A Paravermal Trans-Cerebellar Approach to the Posterior Fossa Tumor Causes Hypertrophic Olivary Degeneration by Dentate Nucleus Injury" Cancers 13, no. 2: 258. https://doi.org/10.3390/cancers13020258
APA StyleSchaller-Paule, M. A., Baumgarten, P., Seifert, V., Wagner, M., Steidl, E., Hattingen, E., Wicke, F., Steinbach, J. P., Foerch, C., & Konczalla, J. (2021). A Paravermal Trans-Cerebellar Approach to the Posterior Fossa Tumor Causes Hypertrophic Olivary Degeneration by Dentate Nucleus Injury. Cancers, 13(2), 258. https://doi.org/10.3390/cancers13020258