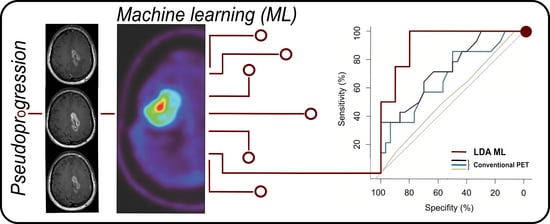
A Preliminary Study on Machine Learning-Based Evaluation of Static and Dynamic FET-PET for the Detection of Pseudoprogression in Patients with IDH-Wildtype Glioblastoma
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
- Patients with a neuropathological diagnosis of an IDH-wildtype glioblastoma;
- Completed radiotherapy plus concomitant chemotherapy with either temozolomide (TMZ) or temozolomide/lomustine (TMZ/CCNU);
- Dynamic FET-PET performed shortly after CE-MRI;
- Confirmation of PSP or TP by histopathology or confirmatory MRI.
2.2. PET Imaging with FET
2.3. FET-PET Data Analysis
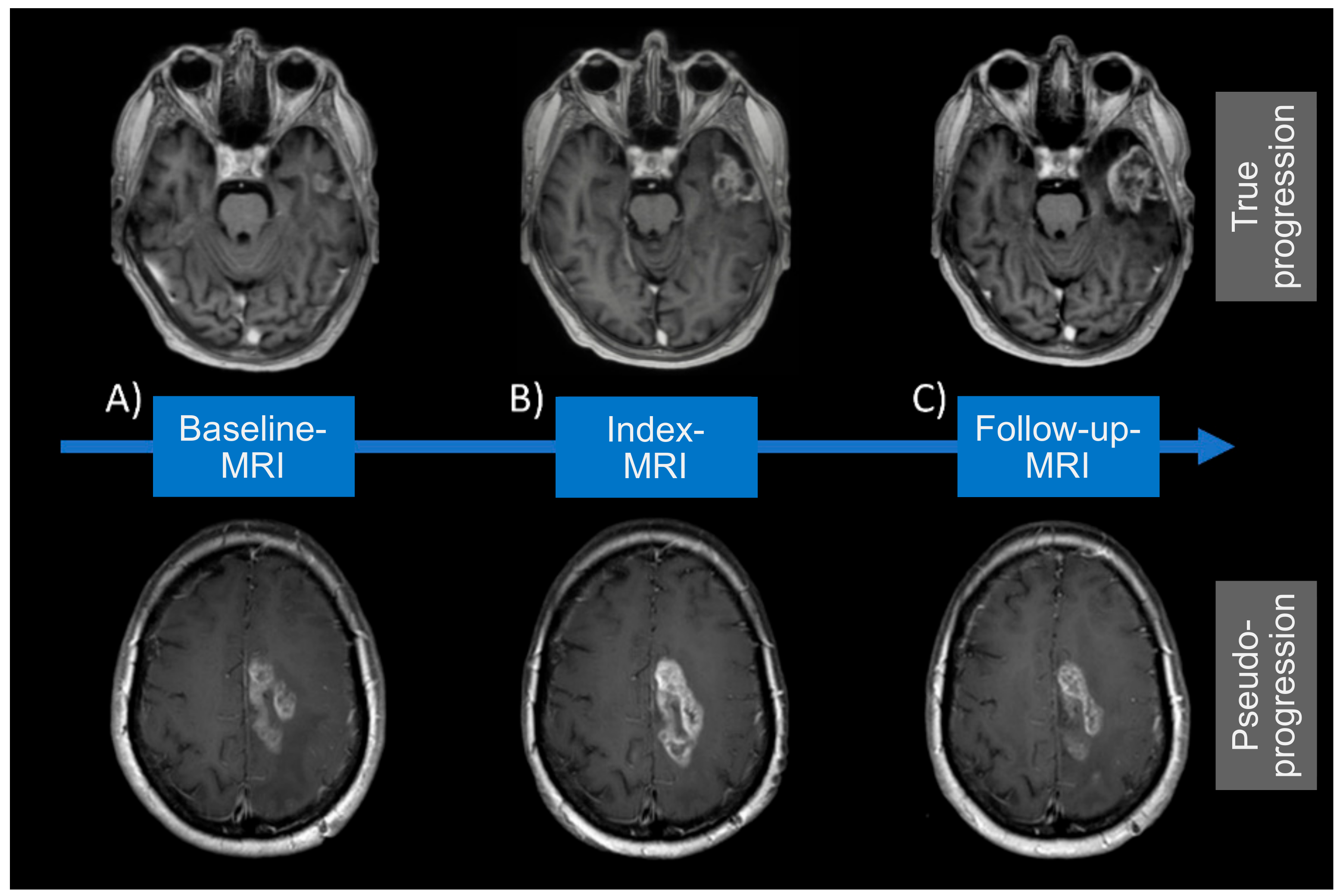
2.4. Definition of MRI Acquisition Time Points
2.5. Diagnosis of True Progression/Pseudoprogression
2.6. Machine Learning Algorithm
2.7. Statistical Analysis
2.8. Data Availability Statement
3. Results
3.1. Patients’ Characteristics
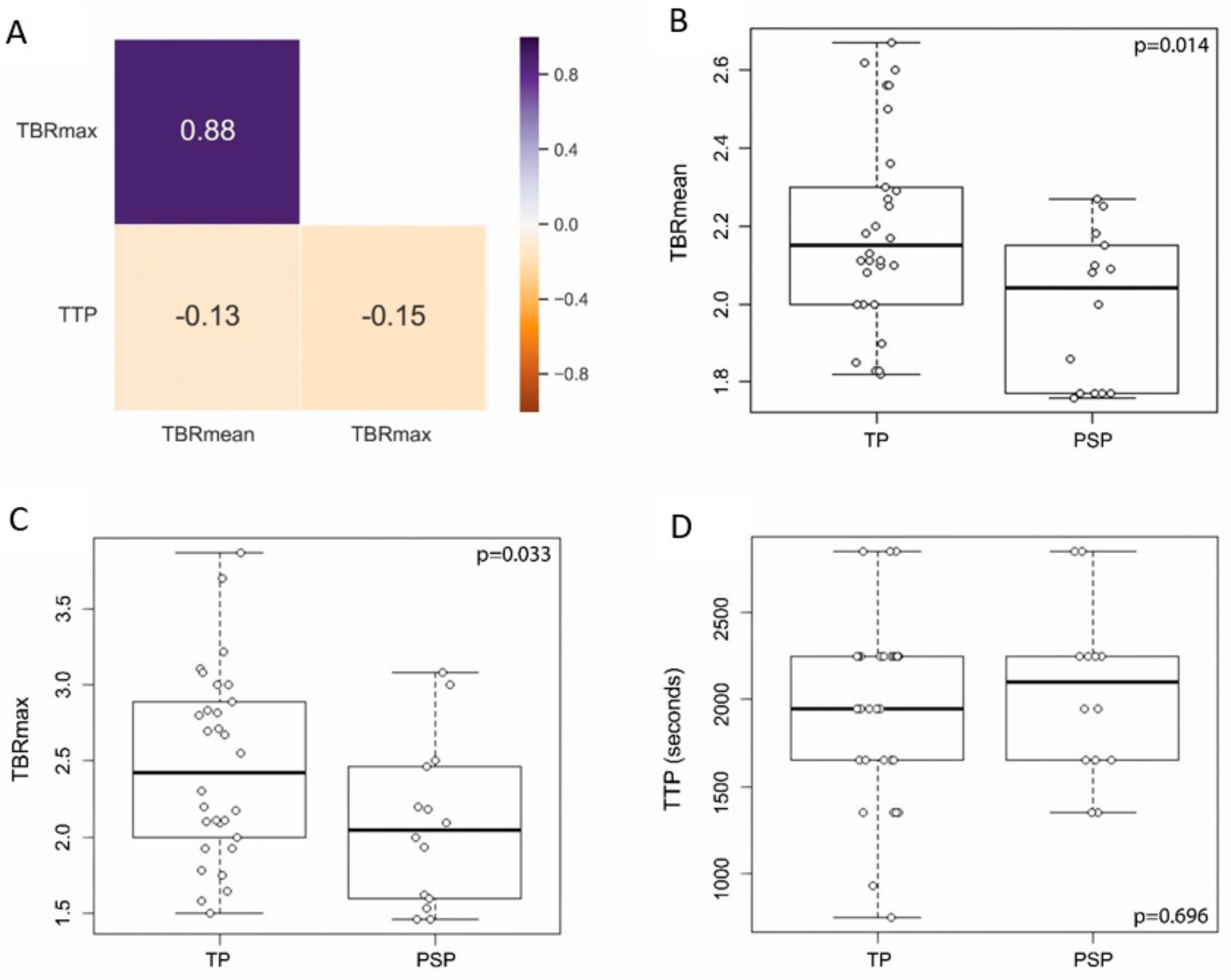
3.2. Distribution of Conventional PET Features between PSP and TP
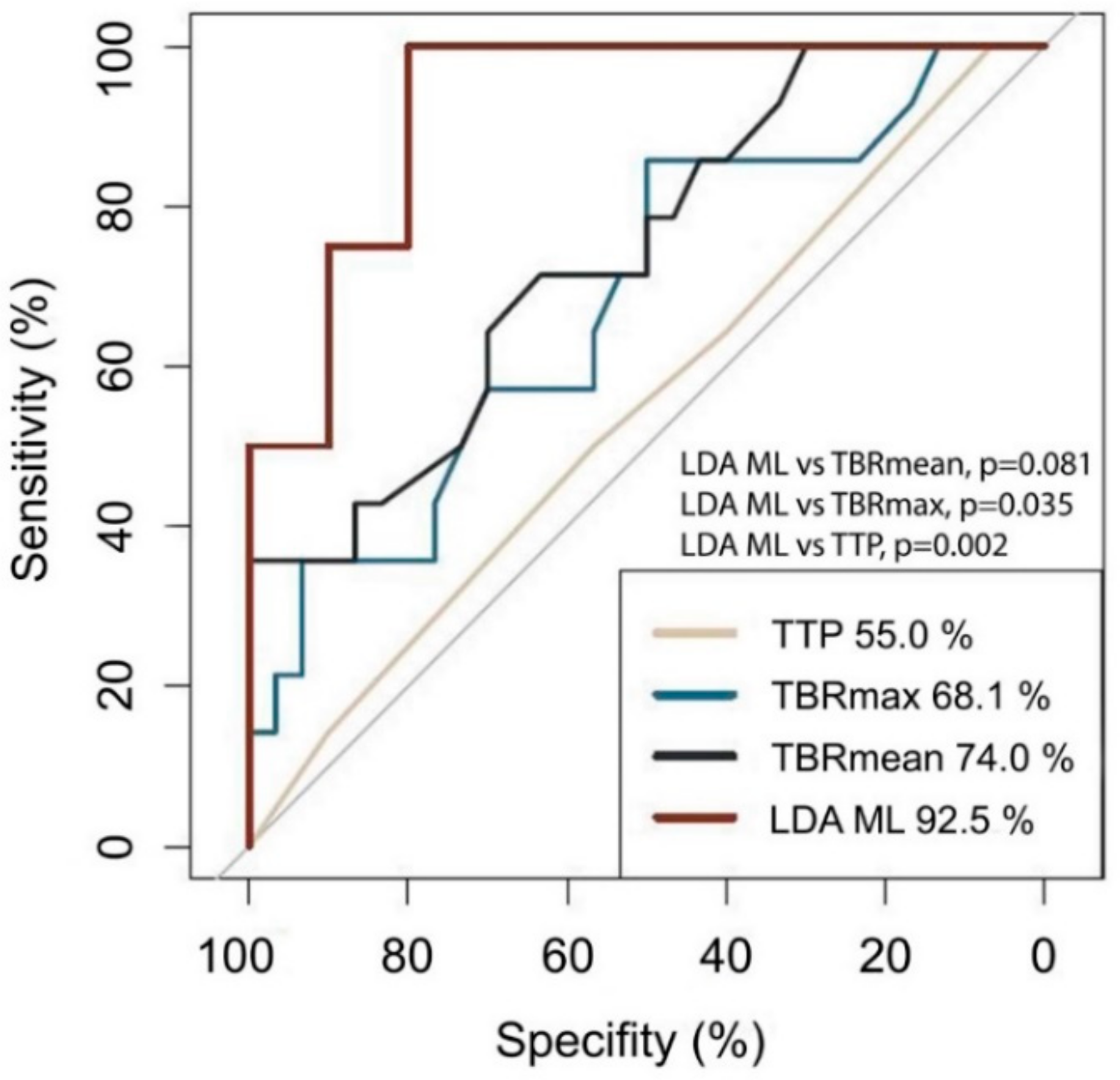
3.3. PSP Detection Applying Conventional PET Analysis
3.4. Implementation of The Machine Learning Algorithm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Herrlinger, U.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Sabel, M.; Hau, P.; Kortmann, R.-D.; Krex, D.; Grauer, O.; et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA–09): A randomised, open-label, phase 3 trial. Lancet 2019, 393, 678–688. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Malmström, A.; Grønberg, B.H.; Marosi, C.; Stupp, R.; Frappaz, D.; Schultz, H.; Abacioglu, U.; Tavelin, B.; Lhermitte, B.; E Hegi, M.; et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 2012, 13, 916–926. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Seystahl, K.; Stoecklein, V.; Schüller, U.; Rushing, E.; Nicolas, G.; Schäfer, N.; Ilhan, H.; Pangalu, A.; Weller, M.; Tonn, J.-C.; et al. Somatostatin receptor-targeted radionuclide therapy for progressive meningioma: Benefit linked to 68Ga-DOTATATE/-TOC uptake. Neuro-Oncology 2016, 18, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Taal, W.; Brandsma, D.; De Bruin, H.; Bromberg, J.; Swaak-Kragten, A.T.; Smitt, P.A.E.S.; Van Es, C.; van den Bent, M. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 2008, 113, 405–410. [Google Scholar] [CrossRef]
- Kebir, S.; Fimmers, R.; Galldiks, N.; Schäfer, N.; Mack, F.; Schaub, C.; Stuplich, M.; Niessen, M.; Tzaridis, T.; Simon, M.; et al. Late Pseudoprogression in Glioblastoma: Diagnostic Value of Dynamic O-(2-[18F]fluoroethyl)-L-Tyrosine PET. Clin. Cancer Res. 2016, 22, 2190–2196. [Google Scholar] [CrossRef]
- Young, R.J.; Gupta, A.; Shah, A.D.; Graber, J.J.; Zhang, Z.; Shi, W.; Holodny, A.I.; Omuro, A.M.P. Potential utility of conventional MRI signs in diagnosing pseudoprogression in glioblastoma. Neurology 2011, 76, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, D.; Bent, M.J.V.D. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr. Opin. Neurol. 2009, 22, 633–638. [Google Scholar] [CrossRef]
- Werner, J.-M.; Stoffels, G.; Lichtenstein, T.; Borggrefe, J.; Lohmann, P.; Ceccon, G.; Shah, N.J.; Fink, G.R.; Langen, K.-J.; Kabbasch, C.; et al. Differentiation of treatment-related changes from tumour progression: A direct comparison between dynamic FET PET and ADC values obtained from DWI MRI. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1889–1901. [Google Scholar] [CrossRef]
- Brandes, A.A.; Tosoni, A.; Spagnolli, F.; Frezza, G.; Leonardi, M.; Calbucci, F.; Franceschi, E. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: Pitfalls in neurooncology. Neuro-Oncology 2008, 10, 361–367. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Wen, P.Y.; Cloughesy, T.F. Modified Criteria for Radiographic Response Assessment in Glioblastoma Clinical Trials. Neurotherapeutics 2017, 14, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Chang, S.M.; Bent, M.J.V.D.; Vogelbaum, M.A.; Macdonald, D.R.; Lee, E.Q. Response Assessment in Neuro-Oncology Clinical Trials. J. Clin. Oncol. 2017, 35, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Bsc, B.R.V.D.; Van Laar, P.J.; Smits, M.; Dankbaar, J.W.; Enting, R.H.; Van Der Hoorn, A. Perfusion MRI in treatment evaluation of glioblastomas: Clinical relevance of current and future techniques. J. Magn. Reson. Imaging 2019, 49, 11–22. [Google Scholar] [CrossRef]
- Galldiks, N.; Dunkl, V.; Stoffels, G.; Hutterer, M.; Rapp, M.; Sabel, M.; Reifenberger, G.; Kebir, S.; Dorn, F.; Blau, T.; et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-l-tyrosine PET. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 685–695. [Google Scholar] [CrossRef]
- Maurer, G.; Brucker, D.P.; Stoffels, G.; Filipski, K.; Filss, C.P.; Mottaghy, F.M.; Galldiks, N.; Steinbach, J.P.; Hattingen, E.; Langen, K.-J. 18F-FET PET Imaging in Differentiating Glioma Progression from Treatment-Related Changes: A Single-Center Experience. J. Nucl. Med. 2020, 61, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Lynch, C.J.; Liston, C. New machine-learning technologies for computer-aided diagnosis. Nat. Med. 2018, 24, 1304–1305. [Google Scholar] [CrossRef]
- Ardila, D.; Kiraly, A.P.; Bharadwaj, S.; Choi, B.; Reicher, J.J.; Peng, L.; Tse, D.; Etemadi, M.; Ye, W.; Corrado, G.; et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat. Med. 2019, 25, 954–961. [Google Scholar] [CrossRef]
- Thrall, J.H.; Li, X.; Li, Q.; Cruz, C.; Do, S.; Dreyer, K.; Brink, J. Artificial Intelligence and Machine Learning in Radiology: Opportunities, Challenges, Pitfalls, and Criteria for Success. J. Am. Coll. Radiol. 2018, 15, 504–508. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; DeGroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sharma, M.C.; Jha, P.; Pathak, P.; Suri, V.; Sarkar, C.; Chosdol, K.; Suri, A.; Kale, S.S.; Mahapatra, A.K. Comparative study of IDH1 mutations in gliomas by immunohistochemistry and DNA sequencing. Neuro-Oncology 2013, 15, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Felsberg, J.; Wolter, M.; Seul, H.; Friedensdorf, B.; Göppert, M.; Sabel, M.C.; Reifenberger, G. Rapid and sensitive assessment of the IDH1 and IDH2 mutation status in cerebral gliomas based on DNA pyrosequencing. Acta Neuropathol. 2010, 119, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Felsberg, J.; Rapp, M.; Loeser, S.; Fimmers, R.; Stummer, W.; Goeppert, M.; Steiger, H.-J.; Friedensdorf, B.; Reifenberger, G.; Sabel, M.C. Prognostic Significance of Molecular Markers and Extent of Resection in Primary Glioblastoma Patients. Clin. Cancer Res. 2009, 15, 6683–6693. [Google Scholar] [CrossRef]
- Hamacher, K.; Coenen, H. Efficient routine production of the 18F-labelled amino acid O-(2-[18F]fluoroethyl)-l-tyrosine. Appl. Radiat. Isot. 2002, 57, 853–856. [Google Scholar] [CrossRef]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; La Fougère, C.; Langen, K.-J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 540–557. [Google Scholar] [CrossRef]
- Langen, K.-J.; Weirich, C.; Kops, E.R.; Kaffanke, J.; Tellmann, L.; Scheins, J.; Neuner, I.; Stoffels, G.; Fischer, K.; Caldeira, L.; et al. High resolution BrainPET combined with simultaneous MRI. Nuklearmedizin 2011, 50, 74–82. [Google Scholar] [CrossRef]
- Lohmann, P.; Herzog, H.; Kops, E.R.; Stoffels, G.; Judov, N.; Filss, C.; Galldiks, N.; Tellmann, L.; Weiss, C.; Sabel, M.; et al. Dual-time-point O-(2-[18F]fluoroethyl)-L-tyrosine PET for grading of cerebral gliomas. Eur. Radiol. 2015, 25, 3017–3024. [Google Scholar] [CrossRef]
- Pauleit, D.; Floeth, F.; Hamacher, K.; Riemenschneider, M.J.; Reifenberger, G.; Müller, H.-W.; Zilles, K.; Coenen, H.H.; Langen, K.-J. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 2005, 128 Pt 3, 678–687. [Google Scholar] [CrossRef]
- Rapp, M.; Heinzel, A.; Galldiks, N.; Stoffels, G.; Felsberg, J.; Ewelt, C.; Sabel, M.; Steiger, H.J.; Reifenberger, G.; Beez, T.; et al. Diagnostic Performance of 18F-FET PET in Newly Diagnosed Cerebral Lesions Suggestive of Glioma. J. Nucl. Med. 2013, 54, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Stoffels, G.; Filss, C.; Rapp, M.; Blau, T.; Tscherpel, C.; Ceccon, G.; Dunkl, V.; Weinzierl, M.; Stoffel, M.; et al. The use of dynamic O-(2-18F-fluoroethyl)-L-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro-Oncology 2015, 17, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Swets, J.A. ROC Analysis Applied to the Evaluation of Medical Imaging Techniques. Investig. Radiol. 1979, 14, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Mihovilovic, M.I.; Kertels, O.; Hänscheid, H.; Löhr, M.; Monoranu, C.-M.; Kleinlein, I.; Samnick, S.; Kessler, A.F.; Linsenmann, T.; Ernestus, R.-I.; et al. O-(2-(18F)fluoroethyl)-L-tyrosine PET for the differentiation of tumour recurrence from late pseudoprogression in glioblastoma. J. Neurol. Neurosurg. Psychiatry 2019, 90, 238–239. [Google Scholar] [CrossRef]
- Zou, Y.; Bai, H.X.; Wang, Z.; Yang, L. Comparison of immunohistochemistry and DNA sequencing for the detection of IDH1 mutations in gliomas. Neuro-Oncology 2015, 17, 477–478. [Google Scholar] [CrossRef] [PubMed]
- Elshafeey, N.; Kotrotsou, A.; Hassan, A.; Elshafei, N.; Hassan, I.; Ahmed, S.; Abrol, S.; Agarwal, A.; El Salek, K.; Bergamaschi, S.; et al. Multicenter study demonstrates radiomic features derived from magnetic resonance perfusion images identify pseudoprogression in glioblastoma. Nat. Commun. 2019, 10, 3170. [Google Scholar] [CrossRef] [PubMed]
- Boxerman, J.L.; Quarles, C.C.; Hu, L.S.; Erickson, B.J.; Gerstner, E.R.; Smits, M.; Kaufmann, T.J.; Barboriak, D.P.; Huang, R.H.; Wick, W.; et al. Consensus recommendations for a dynamic susceptibility contrast MRI protocol for use in high-grade gliomas. Neuro-Oncology 2020, 22, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Thust, S.C.; Bent, M.J.V.D.; Smits, M. Pseudoprogression of brain tumors. J. Magn. Reson. Imaging 2018, 48, 571–589. [Google Scholar] [CrossRef]
- Mutasa, S.; Sun, S.; Ha, R. Understanding artificial intelligence based radiology studies: What is overfitting? Clin. Imaging 2020, 65, 96–99. [Google Scholar] [CrossRef]
- Jeni, L.A.; Cohn, J.F.; De La Torre, F. Facing Imbalanced Data—Recommendations for the Use of Performance Metrics. In Proceedings of the 2013 Humaine Association Conference on Affective Computing and Intelligent Interaction, Geneva, Switzerland, 2–5 September 2013; IEEE: New York, NY, USA, 2013; pp. 245–251. [Google Scholar] [CrossRef]
- Stuplich, M.; Hadizadeh, D.R.; Kuchelmeister, K.; Scorzin, J.; Filss, C.; Langen, K.-J.; Schäfer, N.; Mack, F.; Schüller, H.; Simon, M.; et al. Late and Prolonged Pseudoprogression in Glioblastoma After Treatment With Lomustine and Temozolomide. J. Clin. Oncol. 2012, 30, e180–e183. [Google Scholar] [CrossRef]

| Characteristic | True Progression (n = 30) | Pseudo-Progression (n = 14) |
|---|---|---|
| Tumor entity, n (%) | ||
| Primary IDH-wildtype glioblastoma | 30 (100) | 14 (100) |
| Gender, n (%) | ||
| Male | 28 (93) | 6 (43) |
| Female | 2 (7) | 8 (57) |
| Age at diagnosis [y], median (range) | 59 (42–79) | 51 (34–76) |
| KPS at the time of index MRI, median (range) | 85 (60–100) | 90 (70–100) |
| Extent of resection, n (%) | ||
| Complete resection | 14 (47) | 5 (36) |
| Partial resection | 11 (37) | 4 (29) |
| Biopsy | 5 (17) | 5 (36) |
| Confirmation by histopathology, n (%) | ||
| Yes | 7 (23) | 3 (21) |
| No | 23 (77) | 11 (79) |
| MGMT promotor methylation, n (%) | ||
| No | 20 (67) | 5 (36) |
| Yes | 9 (30) | 8 (57) |
| Missing | 1 (3) | 1 (7) |
| Prior treatment, n (%) | ||
| Radiotherapy | 30 (100) | 14 (100) |
| TMZ | 30 (100) | 14 (100) |
| CCNU | 3 (10) | 1 (7) |
| No chemotherapy | 0 (0) | 0 (0) |
| Concomitant dexamethasone treatment, n (%) | ||
| No | 20 (67) | 9 (64) |
| Yes | 9 (30) | 5 (36) |
| Missing | 1 (3) | 0 (0) |
| Change in dexamethasone dose between index and follow-up MRI, n (%) | ||
| No | 8 (27) | 5 (36) |
| Yes | 21 (70) | 9 (64) |
| Missing | 1 (3) | 0 (0) |
| FET PET features | ||
| TBRmean, median (range) | 2.15 (1.82–2.67) | 2.04 (1.76–2.27) |
| TBRmax, median (range) | 2.52 (1.82–3.87) | 2.09 (1.76–3.08) |
| TTP [min], median (range) | 32.5 (12.5–47.5) | 35 (22.5–47.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kebir, S.; Schmidt, T.; Weber, M.; Lazaridis, L.; Galldiks, N.; Langen, K.-J.; Kleinschnitz, C.; Hattingen, E.; Herrlinger, U.; Lohmann, P.; et al. A Preliminary Study on Machine Learning-Based Evaluation of Static and Dynamic FET-PET for the Detection of Pseudoprogression in Patients with IDH-Wildtype Glioblastoma. Cancers 2020, 12, 3080. https://doi.org/10.3390/cancers12113080
Kebir S, Schmidt T, Weber M, Lazaridis L, Galldiks N, Langen K-J, Kleinschnitz C, Hattingen E, Herrlinger U, Lohmann P, et al. A Preliminary Study on Machine Learning-Based Evaluation of Static and Dynamic FET-PET for the Detection of Pseudoprogression in Patients with IDH-Wildtype Glioblastoma. Cancers. 2020; 12(11):3080. https://doi.org/10.3390/cancers12113080
Chicago/Turabian StyleKebir, Sied, Teresa Schmidt, Matthias Weber, Lazaros Lazaridis, Norbert Galldiks, Karl-Josef Langen, Christoph Kleinschnitz, Elke Hattingen, Ulrich Herrlinger, Philipp Lohmann, and et al. 2020. "A Preliminary Study on Machine Learning-Based Evaluation of Static and Dynamic FET-PET for the Detection of Pseudoprogression in Patients with IDH-Wildtype Glioblastoma" Cancers 12, no. 11: 3080. https://doi.org/10.3390/cancers12113080
APA StyleKebir, S., Schmidt, T., Weber, M., Lazaridis, L., Galldiks, N., Langen, K.-J., Kleinschnitz, C., Hattingen, E., Herrlinger, U., Lohmann, P., & Glas, M. (2020). A Preliminary Study on Machine Learning-Based Evaluation of Static and Dynamic FET-PET for the Detection of Pseudoprogression in Patients with IDH-Wildtype Glioblastoma. Cancers, 12(11), 3080. https://doi.org/10.3390/cancers12113080