Hypothyroidism Is a Predictive Factor for Better Clinical Outcomes in Patients with Advanced Hepatocellular Carcinoma Undergoing Lenvatinib Therapy
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Baseline Patient Characteristics
2.2. Treatment Efficacy
2.3. Adverse Events
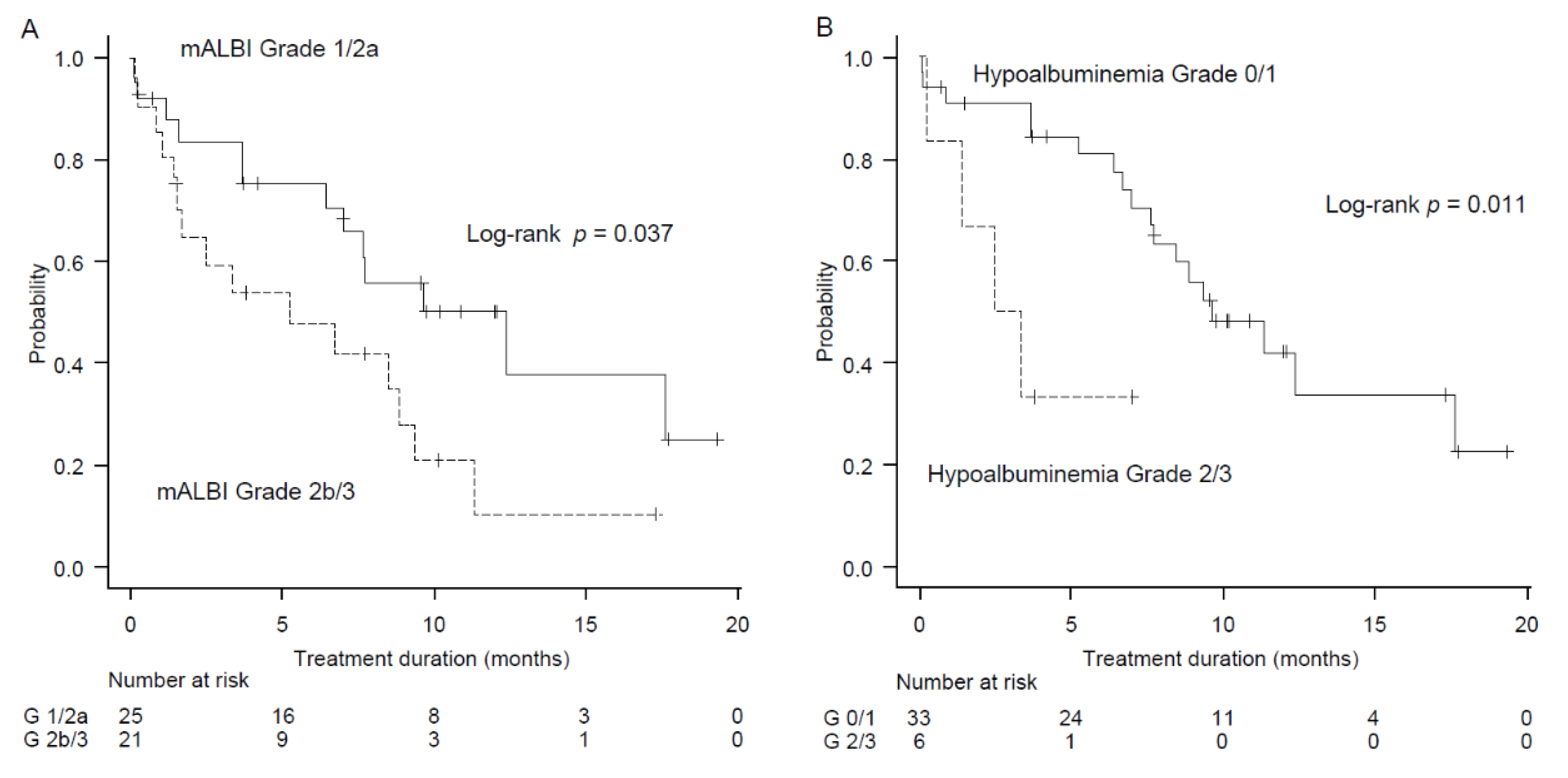
2.4. Factors Associated with Treatment Duration
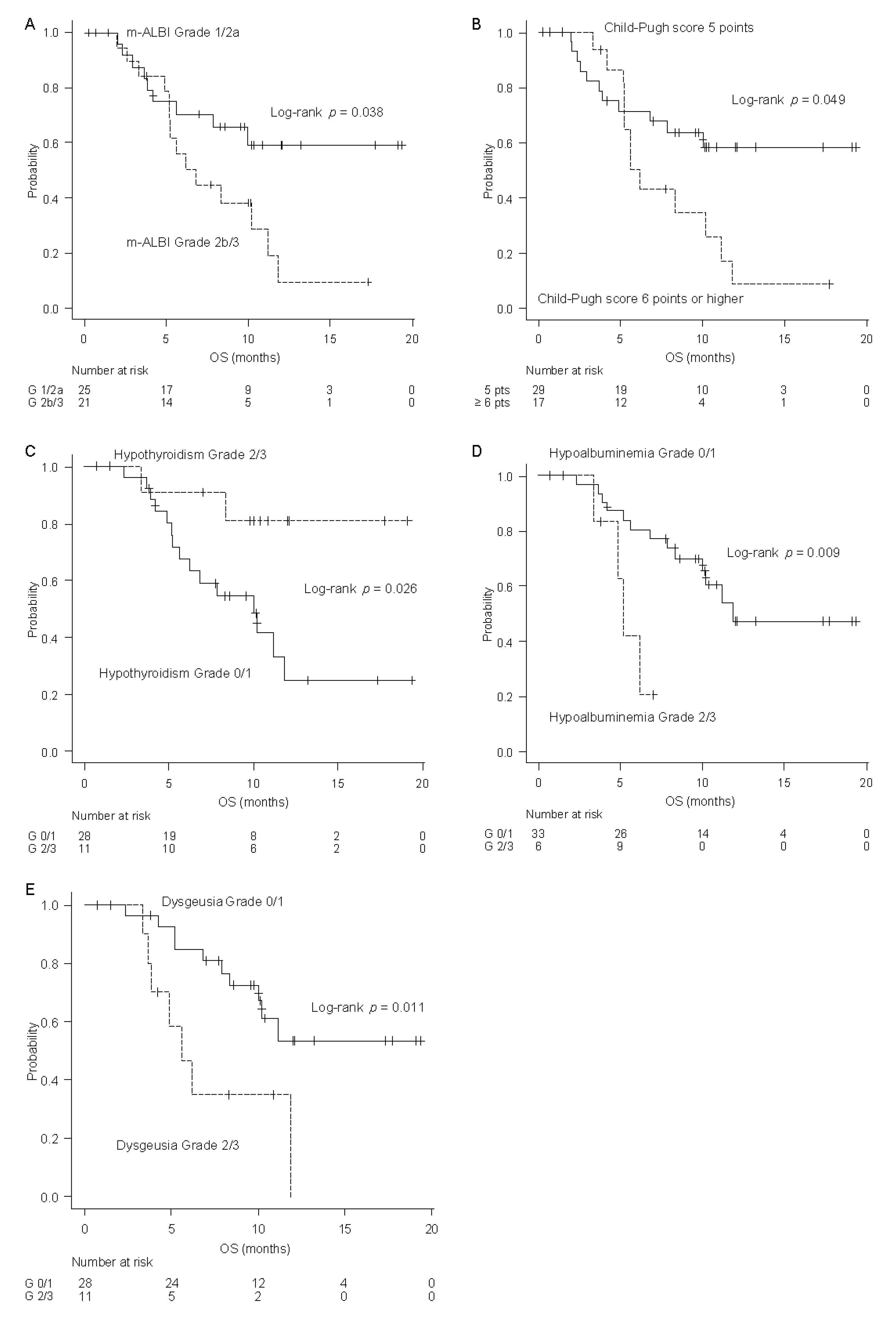
2.5. Factors Associated with OS
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Patients
4.3. Treatment Procedures
4.4. Clinical Evaluation
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, J.Y. Hepatocellular carcinoma: Current situation and challenge. Hepatobiliary Pancreat. Dis. Int. 2019, 18, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, A.A.; Singal, A.G.; Marrero, J.A.; Zhu, H.; Yopp, A.C. Vascular invasion and metastasis is predictive of outcome in Barcelona Clinic Liver Cancer stage c hepatocellular carcinoma. J. Natl. Compr. Cancer Netw. 2017, 15, 197–204. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Ikeda, K.; Kudo, M.; Kawazoe, S.; Osaki, Y.; Ikeda, M.; Okusaka, T.; Tamai, T.; Suzuki, T.; Hisai, T.; Hayato, S.; et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J. Gastroenterol. 2017, 52, 512–519. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Shomura, M.; Kagawa, T.; Shiraishi, K.; Hirose, S.; Arase, Y.; Koizumi, J.; Mine, T. Skin toxicity predicts efficacy to sorafenib in patients with advanced hepatocellular carcinoma. World J. Hepatol. 2014, 6, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Estfan, B.; Byrne, M.; Kim, R. Sorafenib in advanced hepatocellular carcinoma: Hypertension as a potential surrogate marker for efficacy. Am. J. Clin. Oncol. 2013, 36, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Koschny, R.; Gotthardt, D.; Koehler, C.; Jaeger, D.; Stremmel, W.; Ganten, T.M. Diarrhea is a positive outcome predictor for sorafenib treatment of advanced hepatocellular carcinoma. Oncology 2013, 84, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.R.J.; Kudo, M.; Finn, R.S.; Han, K.H.; Cheng, A.L.; Ikeda, M.; Kraljevic, S.; Ren, M.; Dutcus, C.E.; Piscaglia, F.; et al. Correction: Urine protein:creatinine ratio vs 24-hour urine protein for proteinuria management: Analysis from the phase 3 REFLECT study of lenvatinib vs sorafenib in hepatocellular carcinoma. Br. J. Cancer 2019, 121, 625. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; Itobayashi, E.; Tajiri, K.; et al. Prognostic factor of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions-multicenter analysis. Cancer Med. 2019, 8, 3719–3728. [Google Scholar] [CrossRef]
- Takahashi, A.; Moriguchi, M.; Seko, Y.; Shima, T.; Mitsumoto, Y.; Takashima, H.; Kimura, H.; Fujii, H.; Ishikawa, H.; Takaharu, Y.; et al. Early tumor shrinkage as a predictive factor for outcomes in hepatocellular carcinoma patients treated with lenvatinib: A multicenter analysis. Cancers 2020, 12, 754. [Google Scholar] [CrossRef]
- Wirth, L.J.; Tahara, M.; Robinson, B.; Francis, S.; Brose, M.S.; Habra, M.A.; Newbold, K.; Kiyota, N.; Dutcus, C.E.; Mathias, E.; et al. Treatment-emergent hypertension and efficacy in the phase 3 Study of (E7080) lenvatinib in differentiated cancer of the thyroid (SELECT). Cancer 2018, 124, 2365–2372. [Google Scholar] [CrossRef]
- Haddad, R.I.; Schlumberger, M.; Wirth, L.J.; Sherman, E.J.; Shah, M.H.; Robinson, B.; Dutcus, C.E.; Teng, A.; Gianoukakis, A.G.; Sherman, S.I.; et al. Incidence and timing of common adverse events in Lenvatinib-treated patients from the SELECT trial and their association with survival outcomes. Endocrine 2017, 56, 121–128. [Google Scholar] [CrossRef]
- Sung, M.W.; Finn, R.S.; Qin, S.; Han, K.; Ikeda, K.; Cheng, A.; Kudo, M.; Tateishi, R.; Ikeda, M.; Breder, V.; et al. Association between overall survival and adverse events with Lenvatinib treatment in patients with hepatocellular carcinoma (REFRECT). J. Clin. Oncol. 2019, 37, 317. [Google Scholar] [CrossRef]
- Ohki, T.; Sato, K.; Kondo, M.; Goto, E.; Sato, T.; Kondo, Y.; Akamatsu, M.; Sato, S.; Yoshida, H.; Koike, Y.; et al. Impact of adverse events on the progression-free survival of patients with advanced hepatocellular carcinoma treated with lenvatinib: A multicenter retrospective study. Drugs Real World Outcomes 2020, 7, 141–149. [Google Scholar] [CrossRef]
- Koizumi, Y.; Hirooka, M.; Hiraoka, A.; Ochi, H.; Tanaka, T.; Yukimoto, A.; Imai, Y.; Watanabe, T.; Yoshida, O.; Miyake, T.; et al. Lenvatinib-induced thyroid abnormalities in unresectable hepatocellular carcinoma. Endocr. J. 2019, 66, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Arizumi, T.; Ueshima, K.; Iwanishi, M.; Minami, T.; Chishina, H.; Kono, M.; Takita, M.; Kitai, S.; Inoue, T.; Yada, N.; et al. Validation of Kinki Criteria, a modified substaging system, in patients with intermediate stage hepatocellular carcinoma. Dig. Dis. 2016, 34, 671–678. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, K.; Nishida, N.; Hagiwara, S.; Aoki, T.; Minami, T.; Chishina, H.; Takita, M.; Minami, Y.; Ida, H.; Takenaka, M.; et al. Impact of baseline ALBI grade on the outcomes of hepatocellular carcinoma patients treated with lenvatinib: A multicenter study. Cancers 2019, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; Itobayashi, E.; Tajiri, K.; et al. Early relative change in hepatic function with lenvatinib for unresectable hepatocellular carcinoma. Oncology 2019, 97, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; Itobayashi, E.; Tajiri, K.; et al. Important clinical factors in sequential therapy including lenvatinib against unresectable hepatocellular carcinoma. Oncology 2019, 97, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, P.; Ferrari, S.M.; Vita, R.; Di Domenicantonio, A.; Corrado, A.; Benvenga, S.; Antonelli, A. Thyroid dysfunctions induced by tyrosine kinase inhibitors. Expert Opin. Drug Saf. 2014, 13, 723–733. [Google Scholar] [CrossRef]
- Buda-Nowak, A.; Kucharz, J.; Dumnicka, P.; Kuzniewski, M.; Herman, R.M.; Zygulska, A.L.; Kusnierz-Cabala, B. Sunitinib-induced hypothyroidism predicts progression-free survival in metastatic renal cell carcinoma patients. Med. Oncol. 2017, 34, 68. [Google Scholar] [CrossRef]
- Vasileiadis, T.; Chrisofos, M.; Safioleas, M.; Kontzoglou, K.; Papazisis, K.; Sdrolia, A. Impact of sunitinib-induced hypothyroidism on survival of patients with metastatic renal cancer. BMC Cancer 2019, 19, 407. [Google Scholar] [CrossRef]
- Lechner, M.G.; Vyas, C.M.; Hamnvik, O.R.; Alexander, E.K.; Larsen, P.R.; Choueiri, T.K.; Angell, T.E. Hypothyroidism during tyrosine kinase inhibitor therapy is associated with longer survival in patients with advanced nonthyroidal cancers. Thyroid 2018, 28, 445–453. [Google Scholar] [CrossRef]
- Torino, F.; Corsello, S.M.; Longo, R.; Barnabei, A.; Gasparini, G. Hypothyroidism related to tyrosine kinase inhibitors: An emerging toxic effect of targeted therapy. Nat. Rev. Clin. Oncol. 2009, 6, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, E.; Antoniades, K. Molecularly targeted drugs for the treatment of cancer: Oral complications and pathophysiology. Hippokratia 2012, 16, 196–199. [Google Scholar] [PubMed]
- The Japanese Ministry of Health, Labor and Welfare. Manual for Handling Disorders Due to Adverse Drug Reactions-Hypothyroidism; The Japanese Ministry of Health, Labor and Welfare: Tokyo, Japan, 2009; pp. 8–36. Available online: https://www.mhlw.go.jp/topics/2006/11/dl/tp1122-1d09.pdf (accessed on 14 July 2020).
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Japan Clinical Oncology Group. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Available online: http://www.jcog.jp/doctor/tool/CTCAEv4J_20170912_v20_1.pdf (accessed on 10 July 2020).
- Anderson, J.R.; Cain, K.C.; Gelber, R.D. Analysis of survival by tumor response. J. Clin. Oncol. 1983, 1, 710–719. [Google Scholar] [CrossRef]
- Kabata, D.; Shintani, A. Statistical analysis of observational study. JJSPC 2019, 26, 1–6. [Google Scholar]
- Guo, X.; Xu, Y.; Wang, X.; Lin, F.; Wu, H.; Duan, J.; Xiong, Y.; Han, X.; Baklaushev, V.P.; Xiong, S.; et al. Advanced hepatocellular carcinoma with bone metastases: Prevalence, associated factors, and survival estimation. Med. Sci. Monit. 2019, 25, 1105–1112. [Google Scholar] [CrossRef]
- Fuchigami, A.; Imai, Y.; Uchida, Y.; Uchiya, H.; Fujii, Y.; Nakazawa, M.; Ando, S.; Sugawara, K.; Nakayama, N.; Tomiya, T.; et al. Therapeutic efficacy of lenvatinib for patients with unresectable hepatocellular carcinoma based on the middle-term outcome. PLoS ONE 2020, 15, e0231427. [Google Scholar] [CrossRef]
| Variable | Number of Cases (%) | |
|---|---|---|
| Sex | Male | 37 (80) |
| Female | 9 (20) | |
| Age, years | <75 | 24 (52) |
| ≥75 | 22 (48) | |
| Body mass index | ≥21 | 37 (80) |
| <21 | 9 (20) | |
| Etiology | HCV | 14 (30) |
| HBV | 7 (15) | |
| Alcoholic | 7 (15) | |
| NASH | 4 (9) | |
| unknown | 14 (30) | |
| Child-Pugh score | 5 | 29 (63) |
| 6 | 10 (22) | |
| ≥7 | 7 (15) | |
| mALBI grade | 1 | 14 (30) |
| 2a | 11 (24) | |
| 2b | 18 (39) | |
| 3 | 3 (7) | |
| TNM stage | III | 18 (39) |
| IV | 28 (61) | |
| BCLC (Kindai criteria) | B1 | 2 (4) |
| B2 | 19 (41) | |
| C | 25 (54) | |
| Tumor size, mm | <50 | 27 (59) |
| ≥50 | 19 (41) | |
| Extrahepatic invasion | Yes | 20 (43) |
| Vascular invasion | Yes | 9 (20) |
| Previous curative treatment | Yes | 26 (56) |
| AFP, ng/mL | ≥100 | 24 (52) |
| <100 | 21 (48) | |
| DCP, mAU/mL | ≥1000 | 19 (41) |
| <1000 | 27 (59) | |
| Initial dose of lenvatinib | 4 mg | 18 (40) |
| 8 mg | 15 (33) | |
| 12 mg | 13 (28) | |
| Adverse Events | Any Grade | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| Any adverse events | 46 (100) | 6 (13) | 19 (41) | 21 (46) |
| Fatigue | 39 (85) | 23 (50) | 9 (20) | 7 (15) |
| Anorexia | 31 (67) | 8 (17) | 16 (35) | 7 (15) |
| Proteinuria | 23 (50) | 13 (28) | 3 (7) | 7 (15) |
| Weight loss | 23 (50) | 13 (28) | 9 (20) | 1 (2) |
| Hypoalbuminemia | 21 (46) | 15 (33) | 6 (13) | 0 |
| Skin toxicity a | 20 (43) | 10 (22) | 6 (13) | 4 (9) |
| Hypothyroidism | 19 (41) | 6 (13) | 12 (26) | 1 (2) |
| Hoarseness | 18 (39) | 16 (35) | 2 (4) | 0 |
| Nausea & vomiting | 17 (37) | 13 (28) | 2 (4) | 2 (4) |
| Diarrhea | 16 (35) | 14 (30) | 1 (2) | 1 (2) |
| Abdominal pain | 16 (35) | 12 (26) | 4 (9) | 0 |
| Dysgeusia | 13 (28) | 2 (4) | 11 (24) | 0 |
| Hypertension | 11 (24) | 5 (11) | 5 (11) | 1 (2) |
| Alopecia | 11 (24) | 8 (17) | 3 (7) | 0 |
| Variable | Age Adjusted HR (95% CI) | Multivariate a | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Baseline characteristics | ||||||
| Body mass index ≥ 21 (vs. < 21) | 0.81 (0.21–1.23) | |||||
| HCV infection (vs. other etiology) | 1.67 (0.77–3.65) | |||||
| Child-Pugh score = 5 (vs. ≥ 6) | 0.46(0.21–1.00) | 0.45 (0.19–1.08) | ||||
| mALBI = 1/2a (vs. other) | 0.43 (0.21–0.99) | |||||
| TNM stage III (vs. IV) | 1.27 (0.59–2.72) | 1.00 (0.39–2.55) | 1.30 (0.52–3.27) | |||
| BCLC B1/2 (vs. C) | 1.42 (0.66–3.04) | 1.62 (0.63–4.18) | 1.35 (0.55–3.30) | |||
| Maximum tumor size < 50 mm (vs. ≥ 50 mm) | 1.02 (0.48–2.16) | |||||
| Extrahepatic invasion − (vs. +) | 1.79 (0.79–4.05) | |||||
| Vascular invasion − (vs. +) | 0.55 (0.24–1.27) | |||||
| Previous curative therapy: Yes (vs. No) | 1.76 (0.84–3.70) | |||||
| AFP < 100 (vs. ≥ 100) | 0.64 (0.30–1.39) | |||||
| DCP < 1000 (vs. ≥ 1000) | 0.56 (0.27–1.20) | |||||
| Reduced initial dose No (vs. Yes) | 0.88 (0.41–1.93) | |||||
| Sex, Male (vs. Female) | 0.87 (0.35–2.16) | 0.87 (0.27–2.87) | ||||
| Adverse events b | ||||||
| Hypoalbuminemia grade 2/3 (vs. grade 0/1) | 4.38 (1.28–15.00) | 5.01 (1.37–18.35) | 5.65 (1.55–20.53) | |||
| Hypothyroidism grade 2/3 (vs. grade 0/1) | 0.49 (0.18–1.36) | 0.57 (0.18–1.74) | 0.44 (0.16–1.23) | 0.52 (0.17–1.56) | 0.46 (0.16–1.33) | |
| Variable | Age Adjusted HR (95% CI) | Multivariate a | |||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Baseline characteristics | |||||
| Body mass index ≥ 21 (vs. < 21) | 0.48 (0.18–1.27) | ||||
| HCV infection (vs. other etiology) | 1.67 (0.70–3.96) | ||||
| Child-Pugh score = 5 (vs. ≥ 6) | 0.44 (0.19–1.00) | 0.30 (0.11–0.82) | |||
| mALBI = 1&2a (vs. other) | 0.44 (0.19–1.04) | ||||
| TNM stage III (vs. IV) | 1.05 (0.45–2.46) | 0.48(0.16–1.50) | 0.59 (0.20–1.68) | ||
| BCLC B1/2 (vs. C) | 1.24 (0.54–2.85) | 1.00 (0.34–2.92) | 0.76 (0.26–2.22) | ||
| Tumor size < 50 mm (vs. ≥ 50 mm) | 0.90 (0.40–2.06) | ||||
| Extrahepatic metastasis − (vs. +) | 1.32 (0.56–3.13) | ||||
| Vascular invasion − (vs. +) | 0.58 (0.24–1.40) | ||||
| Previous curative therapy: Yes (vs. No) | 1.39 (0.61–3.16) | ||||
| AFP < 100 (vs. ≥ 100) | 0.69 (0.30–1.61) | ||||
| DCP < 1000 (vs. ≥ 1000) | 0.63 (0.28–1.43) | ||||
| Reduced initial dose No (vs. Yes) | 0.55 (0.24–1.26) | ||||
| Sex, Male (vs. Female) | 1.06 (0.37–2.99) | 1.34 (0.36–4.90) | |||
| Adverse events b | |||||
| Dysgeusia grade 2/3 (vs. grade 0/1) | 3.55 (1.27–9.88) | ||||
| Hypoalbuminemia grade 2/3 (vs. grade 0/1) | 4.96 (1.36–18.12) | 5.83 (1.45–23.48) | 5.83 (1.54–22.10) | ||
| Hypothyroidism grade 2/3 (vs. grade 0/1) | 0.21 (0.05–0.94) | 0.20 (0.04–0.98) | 0.19 (0.04–0.87) | 0.18 (0.04–0.87) | 0.16 (0.04–0.77) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shomura, M.; Okabe, H.; Sato, E.; Fukai, K.; Shiraishi, K.; Hirose, S.; Tsuruya, K.; Arase, Y.; Anzai, K.; Kagawa, T. Hypothyroidism Is a Predictive Factor for Better Clinical Outcomes in Patients with Advanced Hepatocellular Carcinoma Undergoing Lenvatinib Therapy. Cancers 2020, 12, 3078. https://doi.org/10.3390/cancers12113078
Shomura M, Okabe H, Sato E, Fukai K, Shiraishi K, Hirose S, Tsuruya K, Arase Y, Anzai K, Kagawa T. Hypothyroidism Is a Predictive Factor for Better Clinical Outcomes in Patients with Advanced Hepatocellular Carcinoma Undergoing Lenvatinib Therapy. Cancers. 2020; 12(11):3078. https://doi.org/10.3390/cancers12113078
Chicago/Turabian StyleShomura, Masako, Haruka Okabe, Emi Sato, Kota Fukai, Koichi Shiraishi, Shunji Hirose, Kota Tsuruya, Yoshitaka Arase, Kazuya Anzai, and Tatehiro Kagawa. 2020. "Hypothyroidism Is a Predictive Factor for Better Clinical Outcomes in Patients with Advanced Hepatocellular Carcinoma Undergoing Lenvatinib Therapy" Cancers 12, no. 11: 3078. https://doi.org/10.3390/cancers12113078
APA StyleShomura, M., Okabe, H., Sato, E., Fukai, K., Shiraishi, K., Hirose, S., Tsuruya, K., Arase, Y., Anzai, K., & Kagawa, T. (2020). Hypothyroidism Is a Predictive Factor for Better Clinical Outcomes in Patients with Advanced Hepatocellular Carcinoma Undergoing Lenvatinib Therapy. Cancers, 12(11), 3078. https://doi.org/10.3390/cancers12113078







