Progression-Free Survival and Overall Survival in Patients with Advanced HER2-Positive Breast Cancer Treated with Trastuzumab Emtansine (T-DM1) after Previous Treatment with Pertuzumab
Simple Summary
Abstract
1. Introduction
2. Results
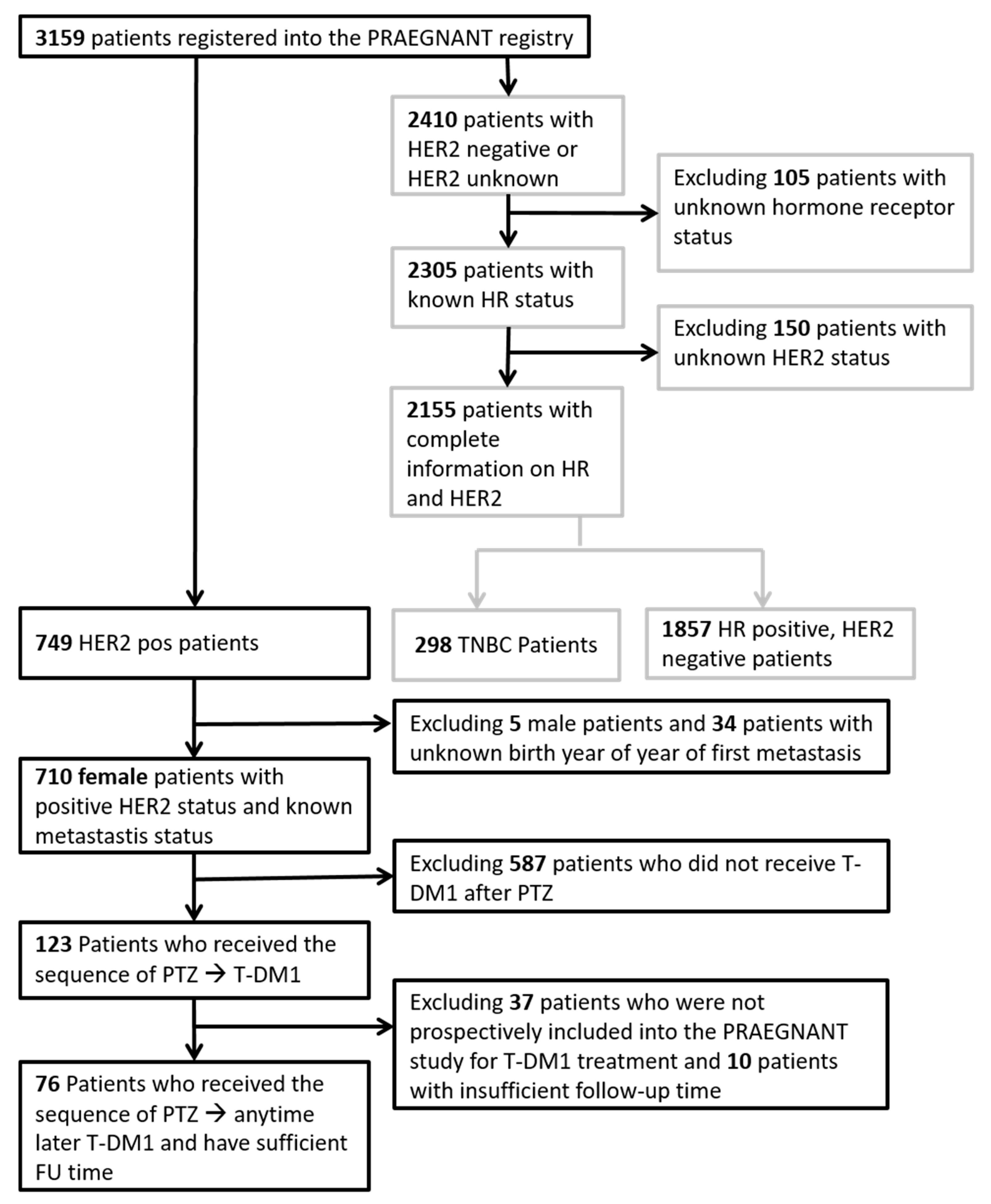
2.1. Patient Characteristics
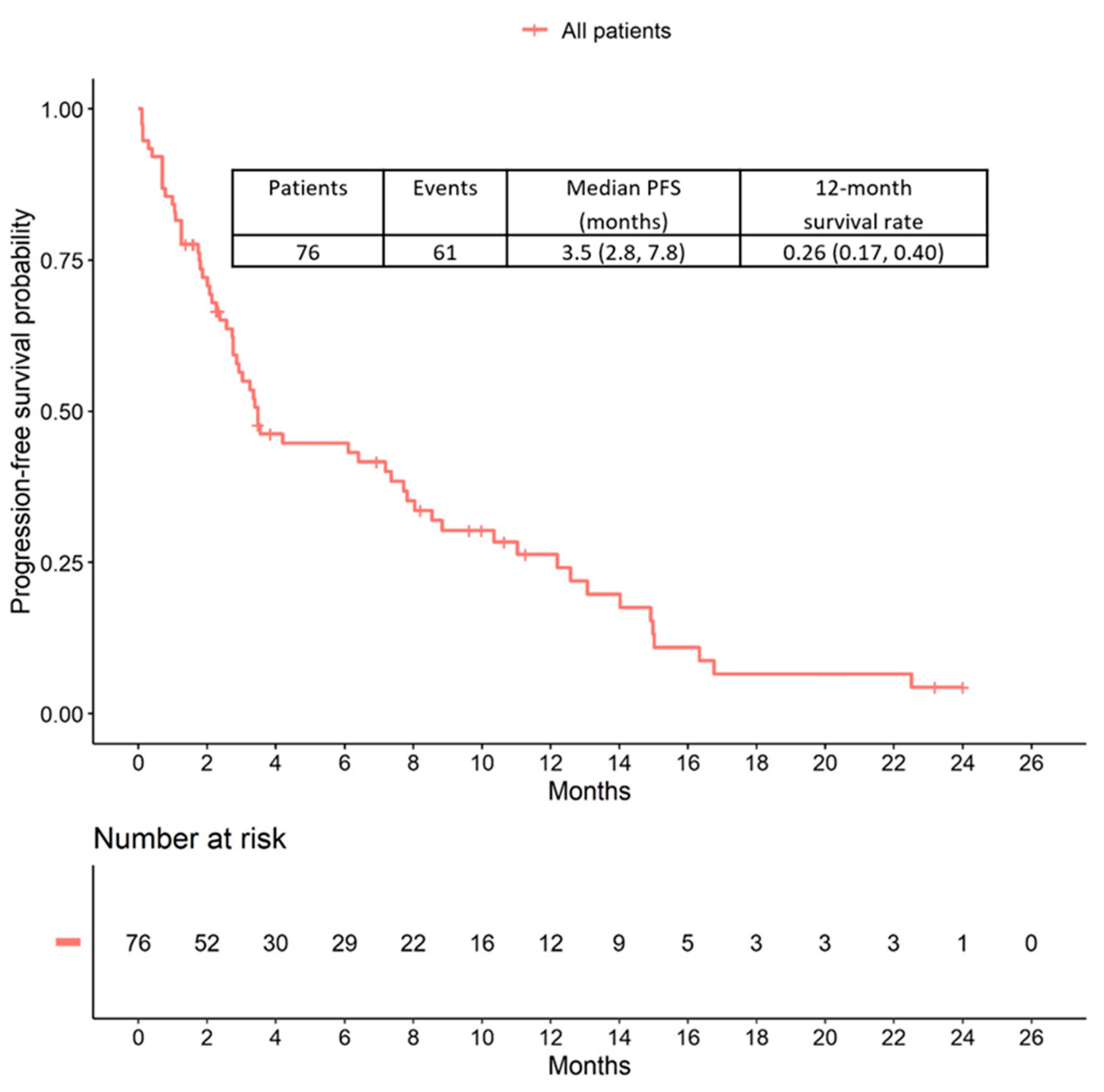
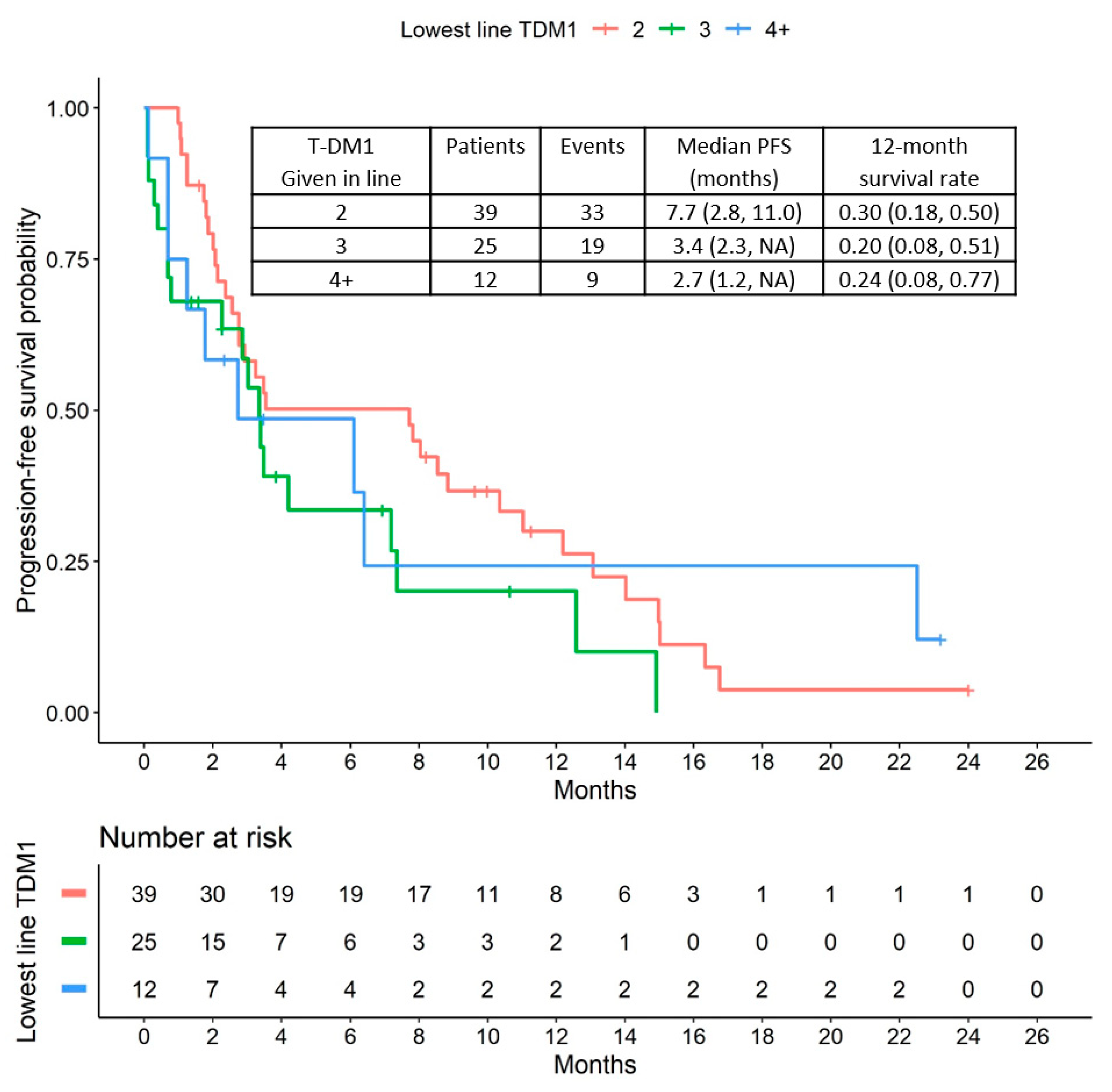
2.2. Progression-Free Survival
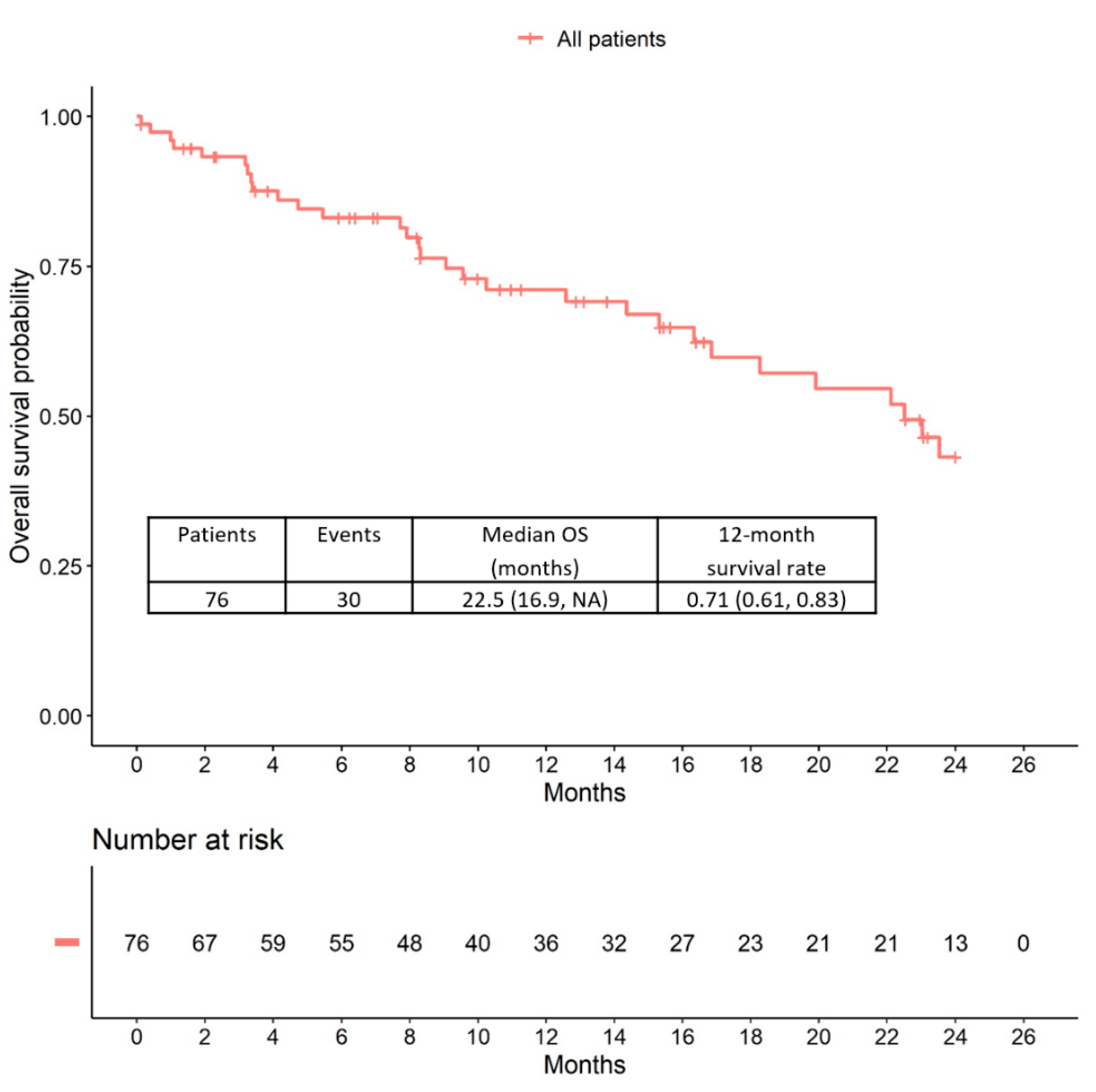
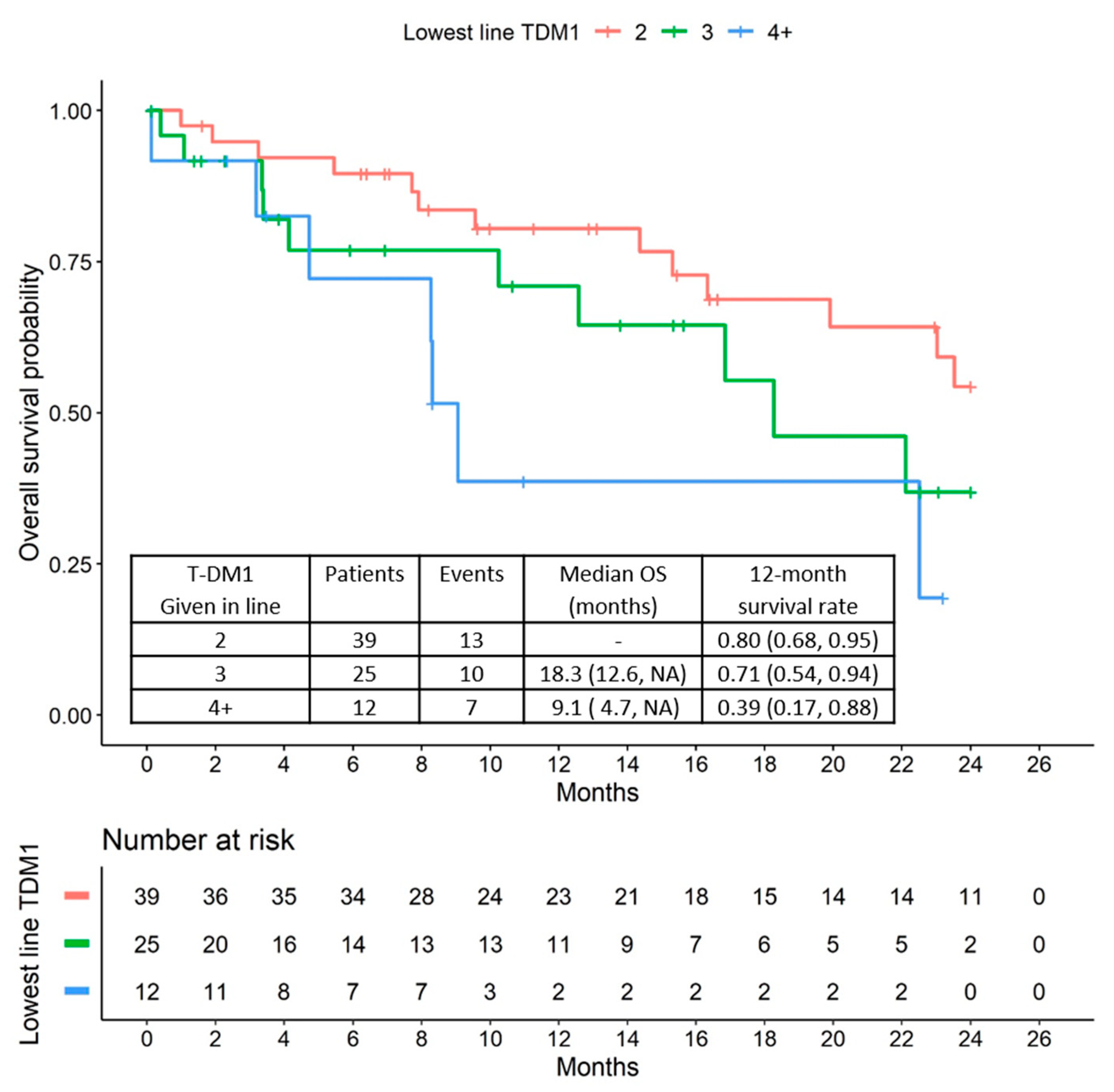
2.3. Overall Survival
3. Discussion
4. Materials and Methods
4.1. The PRAEGNANT Research Network
4.2. Patients
4.3. Data Collection
4.4. Definition of Hormone Receptors, HER2 Status, Grading, and Metastatic Patterns
4.5. Statistical Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADCC | Antibody-dependent, cell-mediated cytotoxicity |
| BC | Breast cancer |
| CI | Confidence interval |
| CISH | Competitive in Situ Hybridization |
| DFS | Disease-free survival |
| ECOG | Eastern Cooperative Oncology Group |
| EGFR | Epidermal growth factor receptor |
| FDA | U.S. Food and Drug Administration |
| FISH | Fluorescence in Situ Hybridization |
| mBC | Metastatic breast cancer |
| OS | Overall survival |
| PFS | Progression-free survival |
| RCT | Randomized controlled trial |
References
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Gilles Romieu, C.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef]
- Cameron, D.; Casey, M.; Press, M.; Lindquist, D.; Pienkowski, T.; Gilles Romieu, C.; Chan, S.; Jagiello-Gruszfeld, A.; Kaufman, B.; Crown, J.; et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res. Treat. 2008, 112, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R.; Tausch, C.; Seo, J.H.; Tsai, Y.-F.; Ratnayake, J.; et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 2013, 24, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Waldron-Lynch, M.; Eng-Wong, J.; Kirk, S.; Cortés, J. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur. J. Cancer 2018, 89, 27–35. [Google Scholar] [PubMed]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Roman, L.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Staroslawska, E.; De la Haba-Rodriguez, J.; Im, S.A.; et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Starosławska, E.; De la Haba-Rodriguez, J.; Im, S.A.; Luiz Pedrini, J.; et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Procter, M.; De Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Welslau, M.; Hartkopf, A.D.; Muller, V.; Wöckel, A.; Lux, M.P.; Janni, W.; Ettl, J.; Lüftner, D.; Belleville, E.; Schutz, F.; et al. Update Breast Cancer 2019 Part 5—Diagnostic and Therapeutic Challenges of New, Personalised Therapies in Patients with Advanced Breast Cancer. Geburtshilfe Frauenheilkd 2019, 79, 1090–1099. [Google Scholar] [CrossRef]
- Schutz, F.; Fasching, P.A.; Welslau, M.; Hartkopf, A.D.; Wöckel, A.; Lux, M.P.; Janni, W.; Ettl, J.; Lüftner, D.; Belleville, E.; et al. Update Breast Cancer 2019 Part 4—Diagnostic and Therapeutic Challenges of New, Personalised Therapies for Patients with Early Breast Cancer. Geburtshilfe Frauenheilkd 2019, 79, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, A.; Lux, M.P.; Janni, W.; Hartkopf, A.D.; Nabieva, N.; Taran, F.A.; Overkamp, F.; Kolberg, H.C.; Hadji, P.; Tesch, H.; et al. Update Breast Cancer 2018 (Part 2)—Advanced Breast Cancer, Quality of Life and Prevention. Geburtshilfe Frauenheilkd 2018, 78, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Taran, F.A.; Schneeweiss, A.; Lux, M.P.; Janni, W.; Hartkopf, A.D.; Nabieva, N.; Overkamp, F.; Kolberg, H.C.; Hadji, P.; Tesch, H.; et al. Update Breast Cancer 2018 (Part 1)—Primary Breast Cancer and Biomarkers. Geburtshilfe Frauenheilkd 2018, 78, 237–245. [Google Scholar] [CrossRef]
- Lux, M.P.; Janni, W.; Hartkopf, A.D.; Taran, F.A.; Overkamp, F.; Kolberg, H.C.; Hadji, P.; Tesch, H.; Ettl, J.; Huober, J.B.; et al. Update Breast Cancer 2017—Implementation of Novel Therapies. Geburtshilfe Frauenheilkd 2017, 77, 1281–1290. [Google Scholar] [CrossRef]
- Untch, M.; Huober, J.; Jackisch, C.; Schneeweiss, A.; Brucker, S.Y.; Dall, P.; Denkert, C.; Fasching, P.A.; Fehm, T.; Gerber, B.; et al. Initial Treatment of Patients with Primary Breast Cancer: Evidence, Controversies, Consensus: Spectrum of Opinion of German Specialists at the 15th International St. Gallen Breast Cancer Conference (Vienna 2017). Geburtshilfe Frauenheilkd 2017, 77, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Kim, S.B.; Cortes, J.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Knott, A.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013, 14, 461–471. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Dieras, V.; Miles, D.; Verma, S.; Pegram, M.; Welslau, M.; Baselga, J.; Krop, I.E.; Blackwell, K.; Hoersch, S.; Xu, J.; et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): A descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 732–742. [Google Scholar] [CrossRef]
- Perez, E.A.; Barrios, C.; Eiermann, W.; Toi, M.; Im, Y.-H.; Conte, P.; Martin, M.; Pienkowski, T.; Pivot, X.; Burris, H. 3rd.; et al. Trastuzumab Emtansine With or Without Pertuzumab Versus Trastuzumab Plus Taxane for Human Epidermal Growth Factor Receptor 2-Positive, Advanced Breast Cancer: Primary Results From the Phase III MARIANNE Study. J. Clin. Oncol. 2017, 35, 141–148. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Deeks, E.D. Neratinib: First Global Approval. Drugs 2017, 77, 1695–1704. [Google Scholar] [CrossRef]
- Saura, C.; Oliveira, M.; Feng, Y.H.; Dai, M.S.; Chen, S.W.; Hurvitz, S.A.; Kim, S.B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With >/= 2 HER2-Directed Regimens: Phase III NALA Trial. J. Clin. Oncol. 2020, 38, 3138–3149. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Giaccone, G.; Im, S.A.; Oh, D.Y.; Bauer, T.M.; Nordstrom, J.L.; Li, H.; Chichili, G.R.; Moore, P.A.; Hong, S.; et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann. Oncol. 2017, 28, 855–861. [Google Scholar] [CrossRef]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 13, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 13, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Murthy, R.K.; Anders, C.K.; Murthy, R.K.; Paplomata, E.; Hamilton, E.; Hurvitz, S.; Loi, S.; Okines, A.; Abramson, V.; et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with previously treated HER2+ metastatic breast cancer with brain metastases (HER2CLIMB). J. Clin. Oncol. 2020, 38, 1005. [Google Scholar] [CrossRef]
- Lux, M.P.; Nabieva, N.; Hartkopf, A.D.; Huober, J.; Volz, B.; Taran, F.A.; Overkamp, F.; Kolberg, H.C.; Hadji, P.; Tesch, H.; et al. Therapy Landscape in Patients with Metastatic HER2-Positive Breast Cancer: Data from the PRAEGNANT Real-World Breast Cancer Registry. Cancers 2018, 11, 10. [Google Scholar] [CrossRef]
- Huober, J.; Weder, P.; Veyret, C.; Thürlimann, B.; Xyrafas, A.; Vanlemmens, L.; Guiu, S.; Brain, E.; Grenier, J.; Dalenc, F.; et al. PERNETTA – A non comparative randomized open label phase II trial of pertuzumab (P) + trastuzumab (T) with or without chemotherapy both followed by T-DM1 in case of progression, in patients with HER2 positive metastatic breast cancer (MBC): (SAKK 22/10 / UNICANCER UC-0140/1207). Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Vici, P.; Pizzuti, L.; Michelotti, A.; Sperduti, I.; Natoli, C.; Mentuccia, L.; Di Lauro, L.; Sergi, D.; Marchetti, P.; Santini, D.; et al. A retrospective multicentric observational study of trastuzumab emtansine in HER2 positive metastatic breast cancer: A real-world experience. Oncotarget 2017, 8, 56921–56931. [Google Scholar] [CrossRef]
- Dzimitrowicz, H.; Berger, M.; Vargo, C.; Hood, A.; Abdelghany, O.; Raghavendra, A.S.; Tripathy, D.; Valero, V.; Hatzis, C.; Pusztai, L.; et al. T-DM1 Activity in Metastatic Human Epidermal Growth Factor Receptor 2-Positive Breast Cancers That Received Prior Therapy With Trastuzumab and Pertuzumab. J. Clin. Oncol. 2016, 34, 3511–3517. [Google Scholar] [CrossRef]
- Urruticoechea, A.; Im, S.A.; Munoz, M.; Baselga, J.; Yardley, D.A.; Heeson, S.; Jones, S.; Knott, A.; Douthwaite, H.; Badovinac Crnjevic, T.; et al. Efficacy of trastuzumab emtansine (T-DM1) in patients (pts) with HER2+metastatic breast cancer (MBC) previously treated with pertuzumab (P). J. Clin. Oncol. 2017, 35, 1023. [Google Scholar] [CrossRef]
- Lakdawalla, D.N.; Shafrin, J.; Hou, N.; Peneva, D.; Vine, S.; Park, J.; Zhang, J.; Brookmeyer, R.; Figlin, R.A. Predicting Real-World Effectiveness of Cancer Therapies Using Overall Survival and Progression-Free Survival from Clinical Trials: Empirical Evidence for the ASCO Value Framework. Value Health 2017, 20, 866–875. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Martin, M.; Symmans, W.F.; Jung, K.H.; Huangm, C.S.; Thompson, A.M.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018, 19, 115–126. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Martin, M.; Jung, K.H.; Huang, C.S.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; Campone, M.; Boileau, J.F.; et al. Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Three-Year Outcomes From the Phase III KRISTINE Study. J. Clin. Oncol. 2019, 37, 2206–2216. [Google Scholar] [CrossRef]
- Fasching, P.A.; Brucker, S.Y.; Fehm, T.N.; Overkamp, F.; Janni, W.; Wallwiener, M.; Hadji, P.; Belleville, E.; Häberle, L.; Taran, F.A.; et al. Biomarkers in Patients with Metastatic Breast Cancer and the PRAEGNANT Study Network. Geburtshilfe Frauenheilkd 2015, 75, 41–50. [Google Scholar] [CrossRef]
- Hartkopf, A.D.; Huober, J.; Volz, B.; Nabieva, N.; Taran, F.A.; Schwitulla, J.; Overkamp, F.; Kolberg, H.C.; Hadji, P.; Tesch, H.; et al. Treatment landscape of advanced breast cancer patients with hormone receptor positive HER2 negative tumors—Data from the German PRAEGNANT breast cancer registry. Breast 2018, 37, 42–51. [Google Scholar] [CrossRef]
- Muller, V.; Nabieva, N.; Haberle, L.; Taran, F.A.; Hartkopf, A.D.; Volz, B.; Overkamp, F.; Brandl, A.L.; Kolberg, H.C.; Hadji, P.; et al. Impact of disease progression on health-related quality of life in patients with metastatic breast cancer in the PRAEGNANT breast cancer registry. Breast 2018, 37, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Hein, A.; Gass, P.; Walter, C.B.; Taran, F.A.; Hartkopf, A.; Overkamp, F.; Kolberg, H.C.; Hadji, P.; Tesch, H.; Ettl, J.; et al. Computerized patient identification for the EMBRACA clinical trial using real-time data from the PRAEGNANT network for metastatic breast cancer patients. Breast Cancer Res. Treat. 2016, 158, 59–65. [Google Scholar] [CrossRef]
- Brookmeyer, R.; Crowley, J. A Confidence-Interval for the Median Survival-Time. Biometrics 1982, 38, 29–41. [Google Scholar] [CrossRef]
| Characteristic | n | % |
|---|---|---|
| Age (years) | 54.6 ± 10.8 | - |
| BMI (kg/m2) | 26.1 ± 5.7 | - |
| HER2-positive | 76 | 100.0 |
| Hormone receptor status | - | - |
| Negative | 23 | 31.5 |
| Positive | 50 | 68.5 |
| Missing | 3 | - |
| M-stage at diagnosis | - | - |
| cM0 | 44 | 57.9 |
| cM1 | 32 | 42.1 |
| Metastasis pattern | - | - |
| Brain | 17 | 23.0 |
| Visceral | 48 | 64.9 |
| Bone | 3 | 4.1 |
| Other | 6 | 8.1 |
| Missing | 2 | - |
| ECOG status | - | - |
| 0 | 37 | 55.2 |
| 1 | 25 | 37.3 |
| 2 | 3 | 4.5 |
| 3 | 2 | 3.0 |
| Missing | 9 | - |
| Lowest line of pertuzumab | - | - |
| 1 | 61 | 80.3 |
| 2 | 9 | 11.8 |
| 3 | 4 | 5.3 |
| 4+ | 2 | 2.6 |
| Lowest line of T-DM1 | - | - |
| 2 | 39 | 51.3 |
| 3 | 25 | 32.9 |
| 4+ | 12 | 15.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michel, L.L.; Hartkopf, A.D.; Fasching, P.A.; Kolberg, H.-C.; Hadji, P.; Tesch, H.; Häberle, L.; Ettl, J.; Lüftner, D.; Wallwiener, M.; et al. Progression-Free Survival and Overall Survival in Patients with Advanced HER2-Positive Breast Cancer Treated with Trastuzumab Emtansine (T-DM1) after Previous Treatment with Pertuzumab. Cancers 2020, 12, 3021. https://doi.org/10.3390/cancers12103021
Michel LL, Hartkopf AD, Fasching PA, Kolberg H-C, Hadji P, Tesch H, Häberle L, Ettl J, Lüftner D, Wallwiener M, et al. Progression-Free Survival and Overall Survival in Patients with Advanced HER2-Positive Breast Cancer Treated with Trastuzumab Emtansine (T-DM1) after Previous Treatment with Pertuzumab. Cancers. 2020; 12(10):3021. https://doi.org/10.3390/cancers12103021
Chicago/Turabian StyleMichel, Laura L., Andreas D. Hartkopf, Peter A. Fasching, Hans-Christian Kolberg, Peyman Hadji, Hans Tesch, Lothar Häberle, Johannes Ettl, Diana Lüftner, Markus Wallwiener, and et al. 2020. "Progression-Free Survival and Overall Survival in Patients with Advanced HER2-Positive Breast Cancer Treated with Trastuzumab Emtansine (T-DM1) after Previous Treatment with Pertuzumab" Cancers 12, no. 10: 3021. https://doi.org/10.3390/cancers12103021
APA StyleMichel, L. L., Hartkopf, A. D., Fasching, P. A., Kolberg, H.-C., Hadji, P., Tesch, H., Häberle, L., Ettl, J., Lüftner, D., Wallwiener, M., Müller, V., Beckmann, M. W., Belleville, E., Volz, B., Huebner, H., Wimberger, P., Hielscher, C., Mundhenke, C., Kurbacher, C., ... Fehm, T. N. (2020). Progression-Free Survival and Overall Survival in Patients with Advanced HER2-Positive Breast Cancer Treated with Trastuzumab Emtansine (T-DM1) after Previous Treatment with Pertuzumab. Cancers, 12(10), 3021. https://doi.org/10.3390/cancers12103021







