High PD-L1/CD274 Expression of Monocytes and Blood Dendritic Cells Is a Risk Factor in Lung Cancer Patients Undergoing Treatment with PD1 Inhibitor Therapy
Simple Summary
Abstract
1. Introduction
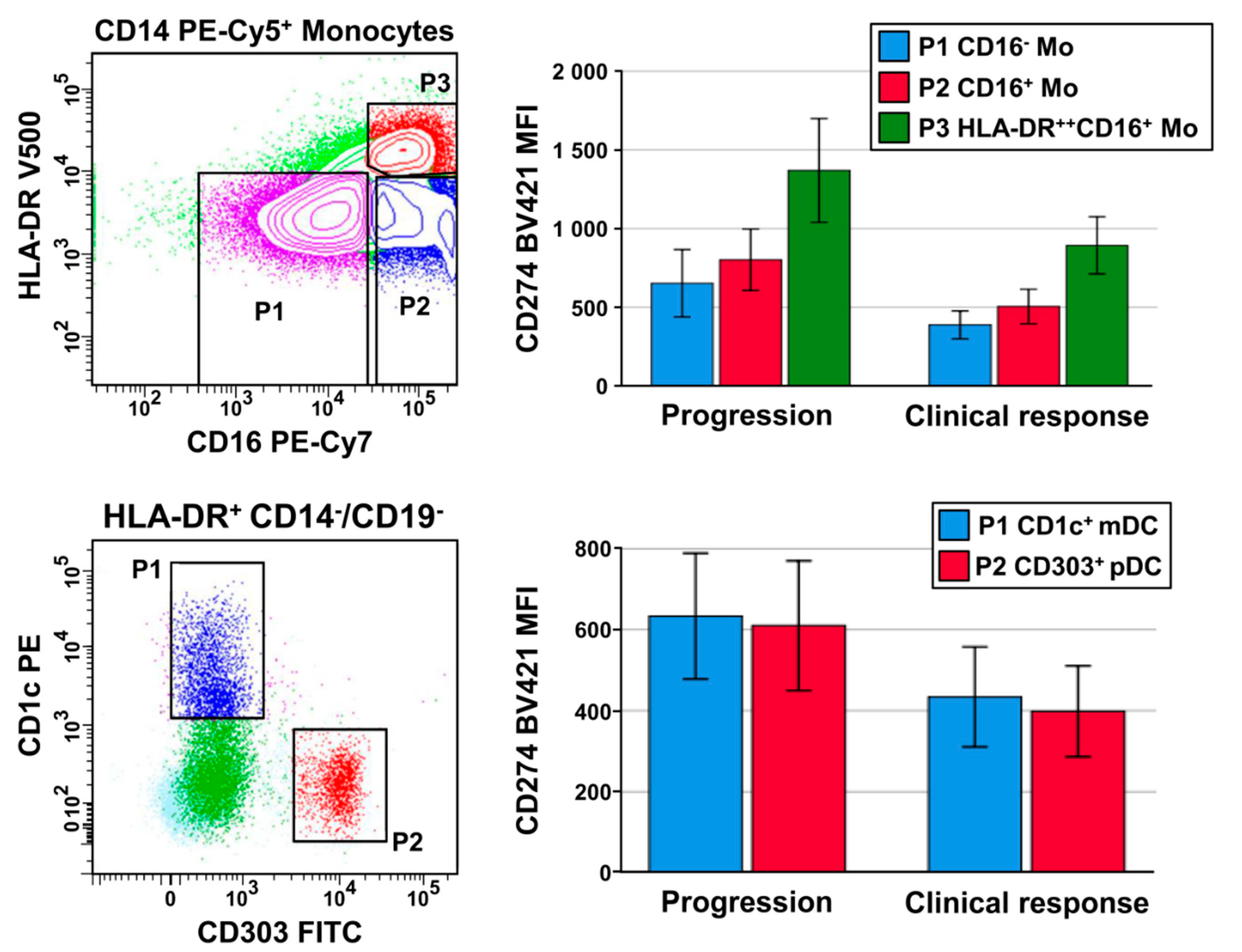
2. Results
3. Discussion
4. Materials and Methods
4.1. Patient Cohort
4.2. Antibody Staining and Flow Cytometry
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the Pd-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Curiel, T.J.; Wei, S.; Dong, H.; Alvarez, X.; Cheng, P.; Mottram, P.; Krzysiek, R.; Knutson, K.L.; Daniel, B.; Zimmermann, M.C.; et al. Blockade of B7-H1 improves myeloid dendritic cell–mediated antitumor immunity. Nat. Med. 2003, 9, 562–567. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Govindan, R.; Anders, R.A.; Antonia, S.J.; Bonerigo, S.; Davies, M.; Dubinett, S.M.; Ferris, A.; Gandhi, L.; Garon, E.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J. Immunother. Cancer 2018, 6, 75. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Möller, M.; Turzer, S.; Schütte, W.; Seliger, B.; Riemann, D. Blood Immune Cell Biomarkers in Patient With Lung Cancer Undergoing Treatment With Checkpoint Blockade. J. Immunother. 2020, 43, 57–66. [Google Scholar] [CrossRef]
- Hart, D.N. Dendritic Cells: Unique Leukocyte Populations Which Control the Primary Immune Response. Blood 1997, 90, 3245–3287. [Google Scholar] [CrossRef]
- Macdonald, K.P.A.; Munster, D.J.; Clark, G.J.; Dzionek, A.; Schmitz, J.; Hart, D.N.J. Characterization of human blood dendritic cell subsets. Blood 2002, 100, 4512–4520. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): A multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017, 18, 1600–1609. [Google Scholar] [CrossRef]
- Velcheti, V.; A Schalper, K.; E Carvajal, D.; Anagnostou, V.K.; Syrigos, K.N.; Sznol, M.; Herbst, R.S.; Gettinger, S.N.; Chen, L.; Rimm, D.L. Programmed death ligand-1 expression in non-small cell lung cancer. Lab. Investig. 2013, 94, 107–116. [Google Scholar] [CrossRef]
- Ali, H.R.; Glont, S.-E.; Blows, F.M.; Provenzano, E.; Dawson, S.-J.; Liu, B.; Hiller, L.; Dunn, J.; Poole, C.J.; Bowden, S.; et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann. Oncol. 2015, 26, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Obeid, J.M.; Erdag, G.; Smolkin, M.E.; Deacon, D.H.; Patterson, J.W.; Chen, L.; Bullock, T.N.; SlingluffJr, C.L. PD-L1, PD-L2 and PD-1 expression in metastatic melanoma: Correlation with tumor-infiltrating immune cells and clinical outcome. OncoImmunology 2016, 5, e1235107. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Kowanetz, M.; Zou, W.; Gettinger, S.N.; Koeppen, H.; Kockx, M.; Schmid, P.; Kadel, E.E.; Wistuba, I.; Chaft, J.; Rizvi, N.A.; et al. Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti–PD-L1). Proc. Natl. Acad. Sci. USA 2018, 115, E10119–E10126. [Google Scholar] [CrossRef]
- Reck, M.; Kerr, K.M.; Grohé, C.; Manegold, C.; Pavlakis, N.; Paz-Ares, L.; Huber, R.M.; Popat, S.; Thatcher, N.; Park, K.; et al. Defining aggressive or early progressing nononcogene-addicted non-small-cell lung cancer: A separate disease entity? Future Oncol. 2019, 15, 1363–1383. [Google Scholar] [CrossRef] [PubMed]
- Rollins, M.R.; Johnson, R.M.G. CD80 Expressed by CD8+ T Cells Contributes to PD-L1-Induced Apoptosis of Activated CD8+ T Cells. J. Immunol. Res. 2017, 2017, 7659462. [Google Scholar] [CrossRef]
- Park, J.J.; Omiya, R.; Matsumura, Y.; Sakoda, Y.; Kuramasu, A.; Augustine, M.M.; Yao, S.; Tsushima, F.; Narazaki, H.; Anand, S.; et al. B7-h1/cd80 interaction is required for the induction and maintenance of peripheral t-cell tolerance. Blood 2010, 116, 1291–1298. [Google Scholar] [CrossRef]
- Sugiura, D.; Maruhashi, T.; Okazaki, I.M.; Shimizu, K.; Maeda, T.K.; Takemoto, T.; Okazaki, T. Restriction of pd-1 function by cis-pd-l1/cd80 interactions is required for optimal t cell responses. Science 2019, 364, 558–566. [Google Scholar] [CrossRef]
- Chen, S.; Crabill, G.A.; Pritchard, T.S.; McMiller, T.L.; Wei, P.; Pardoll, D.M.; Pan, F.; Topalian, S.L. Mechanisms regulating PD-L1 expression on tumor and immune cells. J. Immunother. Cancer 2019, 7, 1–12. [Google Scholar] [CrossRef]
- Casey, S.C.; Tong, L.; Li, Y.; Do, R.; Walz, S.; Fitzgerald, K.N.; Gouw, A.M.; Baylot, V.; Gütgemann, I.; Eilers, M.; et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 2016, 352, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Beckebaum, S. Increased Levels of Interleukin-10 in Serum from Patients with Hepatocellular Carcinoma Correlate with Profound Numerical Deficiencies and Immature Phenotype of Circulating Dendritic Cell Subsets. Clin. Cancer Res. 2004, 10, 7260–7269. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Galluzzi, L.; Kepp, O.; Smyth, M.J.; Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 2015, 15, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Villadangos, J.A.; Young, L. Antigen-Presentation Properties of Plasmacytoid Dendritic Cells. Immunity 2008, 29, 352–361. [Google Scholar] [CrossRef]
- Riemann, D.; Cwikowski, M.; Turzer, S.; Giese, T.; Grallert, M.; Schütte, W.; Seliger, B. Blood immune cell biomarkers in lung cancer. Clin. Exp. Immunol. 2018, 195, 179–189. [Google Scholar] [CrossRef]
- Ma, C.; Su, M.; Shen, K.; Chen, J.; Ning, Y.; Qi, C. Key genes and pathways in tumor-educated dendritic cells by bioinformatical analysis. Microbiol. Immunol. 2019, 64, 63–71. [Google Scholar] [CrossRef]
- Hobo, W.; Novobrantseva, T.I.; Fredrix, H.; Wong, J.; Milstein, S.; Epstein-Barash, H.; Liu, J.; Schaap, N.; Van Der Voort, R.; Dolstra, H. Improving dendritic cell vaccine immunogenicity by silencing PD-1 ligands using siRNA-lipid nanoparticles combined with antigen mRNA electroporation. Cancer Immunol. Immunother. 2012, 62, 285–297. [Google Scholar] [CrossRef]
- Hassannia, H.; Chaleshtari, M.G.; Atyabi, F.; Nosouhian, M.; Masjedi, A.; Hojjat-Farsangi, M.; Namdar, A.; Azizi, G.; Mohammadi, H.; Ghalamfarsa, G.; et al. Blockage of immune checkpoint molecules increases T-cell priming potential of dendritic cell vaccine. Immunology 2019, 159, 75–87. [Google Scholar] [CrossRef]
- Carenza, C.; Calcaterra, F.; Oriolo, F.; Di Vito, C.; Ubezio, M.; Della Porta, M.G.; Mavilio, D.; Della Bella, S. Costimulatory Molecules and Immune Checkpoints Are Differentially Expressed on Different Subsets of Dendritic Cells. Front. Immunol. 2019, 10, 10. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Tian, K.; Chen, X.; Zhang, R.; Mu, X.; Wu, Y.; Wang, D.; Wang, S.; Liu, F.; et al. Apigenin suppresses PD-L1 expression in melanoma and host dendritic cells to elicit synergistic therapeutic effects. J. Exp. Clin. Cancer Res. 2018, 37, 261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, W.; Zhang, X.; Qu, Q.; Zhang, L. Expression and clinical significance of programmed death-1 on lymphocytes and programmed death ligand-1 on monocytes in the peripheral blood of patients with cervical cancer. Oncol. Lett. 2017, 14, 7225–7231. [Google Scholar] [CrossRef]
- Seliger, B. Basis of pd1/pd-l1 therapies. J. Clin. Med. 2019, 8, 2168. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, B.; Mitsdoerffer, M.; Kieseier, B.C.; Chen, L.; Hartung, H.-P.; Weller, M.; Wiendl, H. Interferon-β enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: Relevance for the immune modulatory effect in multiple sclerosis. J. Neuroimmunol. 2004, 155, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Flies, D.B.; Chen, L. The New B7s: Playing a Pivotal Role in Tumor Immunity. J. Immunother. 2007, 30, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Xiao, X.; Wu, Y.; Wei, Y.; Zhu, L.-Y.; Zhou, J.; Kuang, D.-M. Interleukin-17-educated monocytes suppress cytotoxic T-cell function through B7-H1 in hepatocellular carcinoma patients. Eur. J. Immunol. 2011, 41, 2314–2322. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Nie, H.; Liu, A.; Feng, G.; He, N.; Xu, R.; Zhang, Q.; Dong, C.; Zhang, J.Z. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J. Immunol. 2006, 177, 8844–8850. [Google Scholar] [CrossRef]
- Ni, X.Y.; Sui, H.X.; Liu, Y.; Ke, S.Z.; Wang, Y.N.; Gao, F.G. Tgf-beta of lung cancer microenvironment upregulates b7h1 and gitrl expression in dendritic cells and is associated with regulatory t cell generation. Oncol. Rep. 2012, 28, 615–621. [Google Scholar] [CrossRef]
- Daassi, D.; Mahoney, K.M.; Freeman, G.J. The importance of exosomal PDL1 in tumour immune evasion. Nat. Rev. Immunol. 2020, 20, 209–215. [Google Scholar] [CrossRef]
- Costantini, A.; Julie, C.; Dumenil, C.; Hélias-Rodzewicz, Z.; Tisserand, J.; Dumoulin, J.; Giraud, V.; Labrune, S.; Chinet, T.; Emile, J.-F.; et al. Predictive role of plasmatic biomarkers in advanced non-small cell lung cancer treated by nivolumab. OncoImmunology 2018, 7, e1452581. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, H.; Choi, Y.J.; Kim, S.Y.; Lee, J.-E.; Sung, K.J.; Sung, Y.H.; Pack, C.G.; Jung, M.-K.; Han, B.; et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Patel, A.A.; Zhang, Y.; Fullerton, J.N.; Boelen, L.; Rongvaux, A.; Maini, A.A.; Bigley, V.; Flavell, R.A.; Gilroy, D.W.; Asquith, B.; et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 2017, 214, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Skrzeczyńska-Moncznik, J.; Bzowska, M.; Loseke, S.; Grage-Griebenow, E.; Zembala, M.; Pryjma, J. Peripheral Blood CD14high CD16+Monocytes are Main Producers of IL-10. Scand. J. Immunol. 2008, 67, 152–159. [Google Scholar] [CrossRef]
- Koppelman, B.; Neefjes, J.J.; de Vries, J.E.; de Waal Malefyt, R. Interleukin-10 down-regulates mhc class ii alphabeta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity 1997, 7, 861–871. [Google Scholar] [CrossRef]
- Moldawer, L.L.; Hotchkiss, R. Immunotherapy: It is not just for cancer anymore. J. Leukoc. Biol. 2018, 103, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Zhou, T.; Li, L.; Liu, Z.; Chen, Y.; Mao, E.; Li, M.; Qu, H.; Liu, J. Monocyte programmed death ligand-1 expression is an early marker for predicting infectious complications in acute pancreatitis. Crit. Care 2017, 21, 186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Lou, J.; Zhou, Y.; Bo, L.; Zhu, J.; Zhu, K.; Wan, X.; Cai, Z.; Deng, X. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit. Care 2011, 15, R70. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Fang, Y.; Yu, H.; Zhao, L.; Jiang, Z.; Li, C.S. Monocyte programmed death ligand-1 expression after 3–4 days of sepsis is associated with risk stratification and mortality in septic patients: A prospective cohort study. Crit. Care 2016, 20, 124. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-Y.; Lee, Y.-J.; Seo, S.-K.; Lee, S.-W.; Park, S.-J.; Lee, J.-N.; Sohn, H.-S.; Yao, S.; Chen, L.; Choi, I. Blocking of monocyte-associated B7-H1 (CD274) enhances HCV-specific T cell immunity in chronic hepatitis C infection. J. Leukoc. Biol. 2007, 83, 755–764. [Google Scholar] [CrossRef]
- Zheng, J.; Liang, H.; Xu, C.; Xu, Q.; Zhang, T.; Shen, T.; Lu, F. An unbalanced pd-l1/cd86 ratio in cd14(++)cd16(+) monocytes is correlated with hcv viremia during chronic hcv infection. Cell Mol. Immunol. 2014, 11, 294–304. [Google Scholar] [CrossRef]
- Fares, C.M.; Van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 147–164. [Google Scholar] [CrossRef]
- Dzionek, A.; Fuchs, A.; Schmidt, P.; Cremer, S.; Zysk, M.; Miltenyi, S.; Buck, D.W.; Schmitz, J. BDCA-2, BDCA-3, and BDCA-4: Three Markers for Distinct Subsets of Dendritic Cells in Human Peripheral Blood. J. Immunol. 2000, 165, 6037–6046. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Characteristics | N (%) |
|---|---|---|
| Age at start of immunotherapy, years n (%) | Median | 65 |
| Range | 24–85 | |
| >75 years | 6 (17) | |
| Sex, n (%) | Male Female | 19 (54) 16 (46) |
| Histology, n (%) | Adenocarcinoma Squamous cell carcinoma mixed | 23 (66) 7 (20) 5 (14) |
| Smoking status | Current or former smokers Never smokers | 30 (86) 5 (14) |
| PD-L1/CD274 tumor expression, n (%) | <1% ≥1–49% >49 Missing | 11 (31) 9 (26) 14 (40) 1 |
| Response, n (%) | Stop after 2 treatment cycles Progressive disease after ≥3 cycles disease stabilization Partial remission | 9 (25.7) 12 (34.3) 7 (20.0) 7 (20.0) |
| Immune Cell Subtypes | Clinical Response | Progression | p-Value | AUC | Cut-off Value |
|---|---|---|---|---|---|
| Leukocytes (cells/μL) | 8597 ± 2262 | 9600 ± 3175 | |||
| Neutrophils (cells/μL) | 6214 ± 1948 | 7326 ± 3140 | |||
| Monocyte counts (cells/μL) | 626 ± 160 | 672 ± 261 | |||
| CD14+CD16+ monocytes (% of monocytes) | 23.6 ± 19.3 | 16.4 ± 11.6 | |||
| CD14+HLA-DR++CD16+ monocytes (% of monocytes) | 8.3 ± 3.8 | 7.7 ± 4.3 | |||
| Lymphocytes (cells/μL) | 1459 ± 520 | 1413 ± 628 | |||
| CD303+ pDC counts (cells/μL) | 10.6 ± 6.2 | 5.9 ± 3.9 | 0.009 | 0.745 | 7.01 |
| CD303+ pDC (% of leukocytes) | 0.119 ± 0.054 | 0.070 ± 0.050 | 0.011 | 0.769 | 0.061 |
| CD1c+ mDC (cells/μL) | 13.2 ± 8.5 | 9.3 ± 8.6 | |||
| CD1c+ mDC (% of leukocytes) | 0.146 ± 0.068 | 0.089 ± 0.064 | 0.018 | 0.755 | 0.104 |
| CD141+ mDC (% of leukocytes) | 0.0122 ± 0.009 | 0.006 ± 0.001 | 0.019 | ||
| Monocytic CD274 intensity (MFI) | 450 ± 180 | 757 ± 468 | 0.027 | 0.750 | 480 |
| CD274 intensity of pDC (MFI) | 398 ± 194 | 609 ± 331 | 0.042 | 0.722 | 440 |
| CD274+ pDC (% of pDC) | 12.5 ± 11.0 | 24.5 ± 15.4 | 0.022 | 0.730 | 16.0 |
| CD274 intensity of CD1c+ mDC (MFI) | 433 ± 214 | 633 ± 322 | 0.041 | 0.705 | 450 |
| CD274+ mDC (% of CD1c+ mDC) | 15.09 ± 13.77 | 26.0 ± 18.11 | 0.062 |
| A | Cut-Off Value | n | Kaplan–Meier | Cox Regression | ||||
|---|---|---|---|---|---|---|---|---|
| % Censored | PFS Time (Months) | Log-Rank Test | HR | 95% CI | p-Value | |||
| Blood pDC counts (cells/μL) | ≤7.0 | 18 | 11.1 | 3.65 ± 1.236 | 0.002 | 3.455 | 1.427–8.365 | 0.006 |
| >7.0 | 17 | 52.9 | 15.46 ± 2.76 | |||||
| Monocytic CD274 expression (MFI) | <480 | 16 | 56.3 | 16.00 ± 2.87 | 0.007 | 3.116 | 1.242–7.814 | 0.015 |
| ≥480 | 18 | 11.1 | 4.62 ± 1.64 | |||||
| CD274 MFI of pDC | ≤440 | 19 | 47.4 | 13.95 ± 2.65 | 0.026 | 2.414 | 1.029–5.660 | 0.043 |
| >440 | 14 | 14.3 | 5.23 ± 2.08 | |||||
| CD274+ pDC (% of pDC) | ≤16 | 17 | 58.8 | 16.66 ± 2.73 | 0.001 | 4.14 | 1589–10,784 | 0.004 |
| >16 | 14 | 7.1 | 3.32 ± 0.96 | |||||
| CD274 MFI of CD1c+ mDC | <450 | 15 | 53.3 | 15.01 ± 3.04 | 0.031 | 2.464 | 0.997–6.086 | 0.051 |
| ≥450 | 18 | 16.7 | 6.57 ± 2.12 | |||||
| B | Cut-Point | n | Kaplan–Meier | Cox Regression | ||||
| % Censored | OS Time (Months) | Log-RankTest | HR | 95% CI | p-Value | |||
| Blood pDC counts (cells/μL) | ≤7.0 | 18 | 11.1 | 5.94 ± 1.27 | 0.002 | 3.548 | 1.477–8.523 | 0.005 |
| >7.0 | 17 | 52.9 | 16.8 ± 2.44 | |||||
| Monocytic CD274 expression (MFI) | <480 | 16 | 56.3 | 17.06 ± 2.61 | 0.004 | 3.343 | 1.334–8.376 | 0.010 |
| ≥480 | 18 | 11.1 | 6.83 ± 1.63 | |||||
| CD274 MFI of pDC | ≤440 | 19 | 47.4 | 15.21 ± 2.41 | 0.028 | 2.397 | 1.024–5.607 | 0.044 |
| >440 | 14 | 14.3 | 7.57 ± 2.04 | |||||
| CD274+ pDC (% of pDC) | ≤16 | 17 | 58.8 | 17.78 ± 2.44 | 0.001 | 4.011 | 1.532–10.501 | 0.005 |
| >16 | 14 | 7.1 | 6.5 ± 1.54 | |||||
| CD274 MFI of CD1c+ mDC | <450 | 15 | 53.3 | 16.33 ± 2.72 | 0.035 | 2.441 | 0.989–6.023 | 0.053 |
| ≥448 | 18 | 16.7 | 8.61 ± 1.99 | |||||
| Correlation of | CC | p-Value |
|---|---|---|
| Monocytic CD274 Expression with | ||
| CD274 expression of pDC | 0.954 | <0.001 |
| CD274 expression of CD1c+ mDC | 0.861 | <0.001 |
| CD274+ pDC (% of pDC) with | ||
| Proportion of pDC (% of leukocytes) | –0.523 | 0.003 |
| Proportion of CD1c+ mDC (% of leukocytes) | –0.416 | 0.022 |
| Lymphocytes (cells/μL) | –0.632 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riemann, D.; Schütte, W.; Turzer, S.; Seliger, B.; Möller, M. High PD-L1/CD274 Expression of Monocytes and Blood Dendritic Cells Is a Risk Factor in Lung Cancer Patients Undergoing Treatment with PD1 Inhibitor Therapy. Cancers 2020, 12, 2966. https://doi.org/10.3390/cancers12102966
Riemann D, Schütte W, Turzer S, Seliger B, Möller M. High PD-L1/CD274 Expression of Monocytes and Blood Dendritic Cells Is a Risk Factor in Lung Cancer Patients Undergoing Treatment with PD1 Inhibitor Therapy. Cancers. 2020; 12(10):2966. https://doi.org/10.3390/cancers12102966
Chicago/Turabian StyleRiemann, Dagmar, Wolfgang Schütte, Steffi Turzer, Barbara Seliger, and Miriam Möller. 2020. "High PD-L1/CD274 Expression of Monocytes and Blood Dendritic Cells Is a Risk Factor in Lung Cancer Patients Undergoing Treatment with PD1 Inhibitor Therapy" Cancers 12, no. 10: 2966. https://doi.org/10.3390/cancers12102966
APA StyleRiemann, D., Schütte, W., Turzer, S., Seliger, B., & Möller, M. (2020). High PD-L1/CD274 Expression of Monocytes and Blood Dendritic Cells Is a Risk Factor in Lung Cancer Patients Undergoing Treatment with PD1 Inhibitor Therapy. Cancers, 12(10), 2966. https://doi.org/10.3390/cancers12102966





