Dual-Time Point [68Ga]Ga-PSMA-11 PET/CT Hybrid Imaging for Staging and Restaging of Prostate Cancer
Simple Summary
Abstract
1. Introduction
2. Results
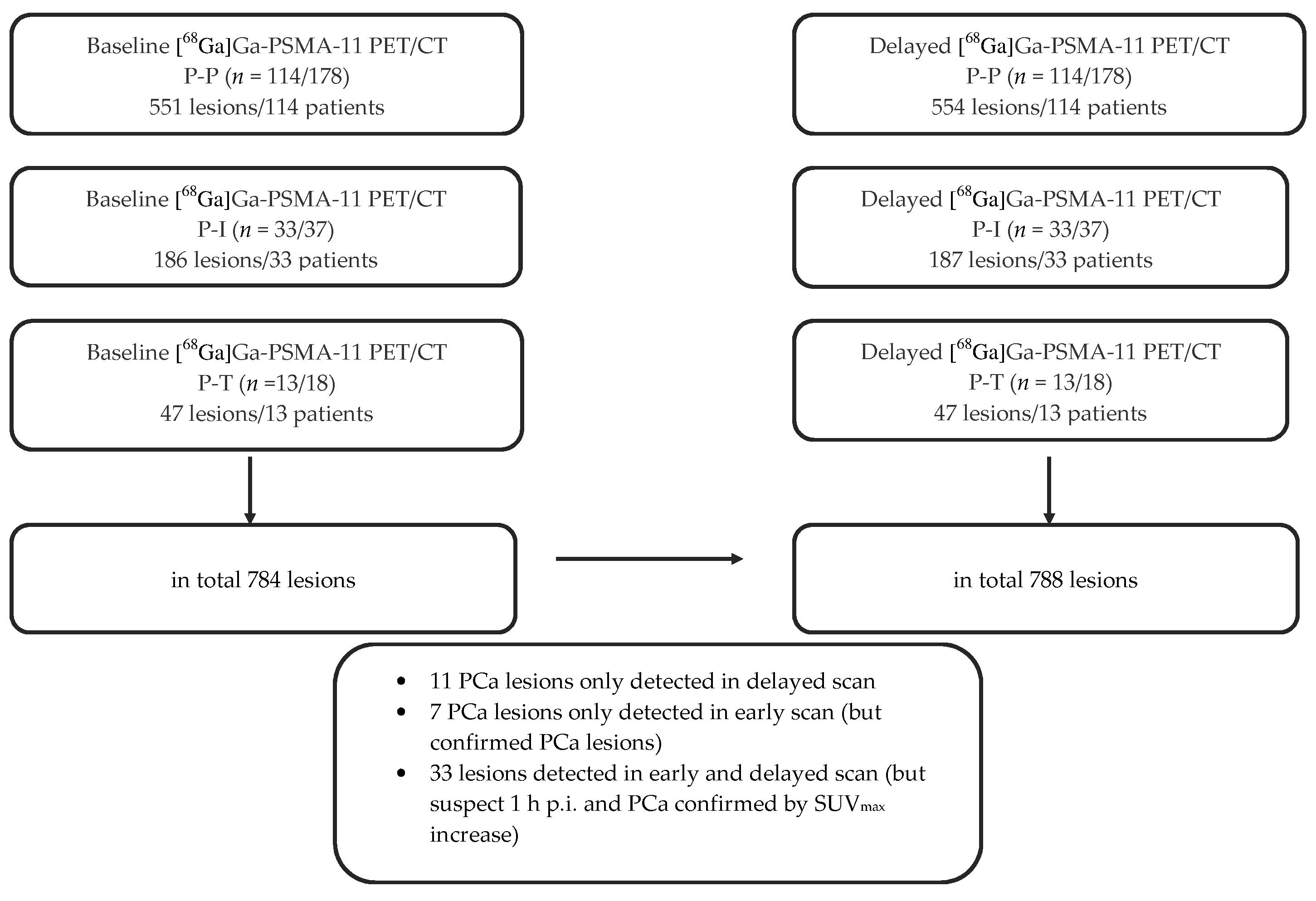
2.1. Overall Lesion Positivity Rate
2.1.1. Lesion Positivity Rate Post-Prostatectomized (P-P)
- Baseline: 551 lesions in 114 patients
- Delayed: 554 lesions in 114 patients
2.1.2. Lesion Positivity Rate Post-Irradiated (P-I)
- Baseline: 186 lesions in 33 patients
- Delayed: 187 lesions in 33 patients
2.1.3. Lesion Positivity Rate Pre-Therapy (P-T)
- Baseline: 47 lesions in 13 patients
- Delayed: 47 lesions in 13 patients
2.2. Impact of Delayed Imaging on Lesion Positivity Rate
2.2.1. Impact of Delayed Imaging on Lesion Positivity Rate P-P
2.2.2. Impact of Delayed Imaging on Lesion Positivity Rate P-I
2.2.3. Impact of Delayed Imaging on Lesion Positivity Rate P-T
2.2.4. Total Comparison of Biphasic Lesion Detection
- LPR on PET, but not on CT:
- Lesions only detected on early imaging:
- Lesions only detected on delayed imaging:
- Additional impact of delayed imaging:
- No additional impact of delayed imaging/concordant lesions:
- Time dependency of LPR:
2.3. SUVmax
2.3.1. SUVmax of Malignant Lesions (P-P)
2.3.2. SUVmax of Malignant Lesions (P-I)
2.3.3. SUVmax of Malignant Lesions (P-T)
2.4. Gleason Score
2.5. Subpopulation
3. Discussion
4. Materials and Methods
4.1. Patient Characteristics
4.2. Imaging Protocol and Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Wibmer, A.G.; Burger, I.A.; Sala, E.; Hricak, H.; Weber, W.A.; Vargas, H.A. Molecular Imaging of Prostate Cancer. Radiographics 2016, 36, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.M.; Joniau, S.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Heston, W.D. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell. Biochem. 2004, 91, 528–539. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef]
- Coenen, H.H.; Gee, A.D.; Adam, M.; Antoni, G.; Cutler, C.S.; Fujibayashi, Y.; Jeong, J.M.; Mach, R.H.; Mindt, T.L.; Pike, V.W.; et al. Open letter to journal editors on: International Consensus Radiochemistry Nomenclature Guidelines. EJNMMI Radiopharm. Chem. 2019, 4, 7. [Google Scholar] [CrossRef]
- Sanli, Y.; Sanli, O.; Has Simsek, D.; Subramaniam, R.M. 68Ga-PSMA PET/CT and PET/MRI in high-risk prostate cancer patients. Nucl. Med. Commun. 2018, 39, 871–880. [Google Scholar] [CrossRef]
- Grubnic, S.; Vinnicombe, S.J.; Norman, A.R.; Husband, J.E. MR evaluation of normal retroperitoneal and pelvic lymph nodes. Clin. Radiol. 2002, 57, 193–200. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Hövels, A.M.; Heesakkers, R.A.; Adang, E.M.; Jager, G.J.; Strum, S.; Hoogeveen, Y.L.; Severens, J.L.; Barentsz, J.O. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin. Radiol. 2008, 63, 387–395. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Wieler, H.J.; Baues, C.; Kuntz, N.J.; Richardsen, I.; Schreckenberger, M. The Impact of 68Ga-PSMA PET/CT and PET/MRI on the Management of Prostate Cancer. Urology 2019, 130, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Maurer, T.; Gschwend, J.E.; Rauscher, I.; Souvatzoglou, M.; Haller, B.; Weirich, G.; Wester, H.J.; Heck, M.; Kübler, H.; Beer, A.J.; et al. Diagnostic Efficacy of (68)Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J. Urol. 2016, 195, 1436–1443. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Buchholz, H.G.; Wieler, H.J.; Miederer, M.; Rosar, F.; Fischer, N.; Müller-Hübenthal, J.; Trampert, L.; Pektor, S.; Schreckenberger, M. PSA and PSA Kinetics Thresholds for the Presence of 68Ga-PSMA-11 PET/CT-Detectable Lesions in Patients with Biochemical Recurrent Prostate Cancer. Cancers 2020, 12, 398. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Bluemel, C.; Weineisen, M.; Schottelius, M.; Wester, H.J.; Czernin, J.; Eberlein, U.; Beykan, S.; Lapa, C.; Riedmiller, H.; et al. Biodistribution and radiation dosimetry for a probe targeting prostate-specific membrane antigen for imaging and therapy. J. Nucl. Med. 2015, 56, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Miederer, M.; Wieler, H.J.; Ruf, C.; Jakobs, F.M.; Schreckenberger, M. Diagnostic performance of 68Gallium-PSMA-11 PET/CT to detect significant prostate cancer and comparison with 18FEC PET/CT. Oncotarget 2017, 14, 111073–111083. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading Committee. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- Alberts, I.; Sachpekidis, C.; Dijkstra, L.; Prenosil, G.; Gourni, E.; Boxler, S.; Gross, T.; Thalmann, G.; Rahbar, K.; Rominger, A.; et al. The role of additional late PSMA-ligand PET/CT in the differentiation between lymph node metastases and ganglia. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 642–651. [Google Scholar] [CrossRef]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Ceci, F.; Cho, S.; Giesel, F.; Haberkorn, U.; Hope, T.A.; Kopka, K.; et al. 68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1014–1024. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Sattler, L.P.; Mier, W.; Hadaschik, B.A.; Debus, J.; Holland-Letz, T.; Kopka, K.; Haberkorn, U. The Clinical Impact of Additional Late PET/CT Imaging with 68Ga-PSMA-11 (HBED-CC) in the Diagnosis of Prostate Cancer. J. Nucl. Med. 2017, 58, 750–755. [Google Scholar] [CrossRef]
- Schmuck, S.; Nordlohne, S.; von Klot, C.A.; Henkenberens, C.; Sohns, J.M.; Christiansen, H.; Wester, H.J.; Ross, T.L.; Bengel, F.M.; Derlin, T. Comparison of standard and delayed imaging to improve the detection rate of [68Ga]PSMA I&T PET/CT in patients with biochemical recurrence or prostate-specific antigen persistence after primary therapy for prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Schmuck, S.; Mamach, M.; Wilke, F.; von Klot, C.A.; Henkenberens, C.; Thackeray, J.T.; Sohns, J.M.; Geworski, L.; Ross, T.L.; Wester, H.J.; et al. Multiple Time-Point 68Ga-PSMA I&T PET/CT for Characterization of Primary Prostate Cancer: Value of Early Dynamic and Delayed Imaging. Clin. Nucl. Med. 2017, 42, 286–293. [Google Scholar] [CrossRef]
- Beheshti, M.; Paymani, Z.; Brilhante, J.; Geinitz, H.; Gehring, D.; Leopoldseder, T.; Wouters, L.; Pirich, C.; Loidl, W.; Langsteger, W. Optimal time-point for 68Ga-PSMA-11 PET/CT imaging in assessment of prostate cancer: Feasibility of sterile cold-kit tracer preparation? Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Sahlmann, C.O.; Meller, B.; Bouter, C.; Ritter, C.O.; Ströbel, P.; Lotz, J.; Trojan, L.; Meller, J.; Hijazi, S. Biphasic 68Ga-PSMA-HBED-CC-PET/CT in patients with recurrent and high-risk prostate carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 898–905. [Google Scholar] [CrossRef]
- Freitag, M.T.; Radtke, J.P.; Hadaschik, B.A.; Kopp-Schneider, A.; Eder, M.; Kopka, K.; Haberkorn, U.; Roethke, M.; Schlemmer, H.P.; Afshar-Oromieh, A. Comparison of hybrid (68)Ga-PSMA PET/MRI and (68)Ga-PSMA PET/CT in the evaluation of lymph node and bone metastases of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 70–83. [Google Scholar] [CrossRef]
- Kunikowska, J.; Kujda, S.; Królicki, L. 68Ga-PSMA PET/CT in Recurrence Prostate Cancer. Should We Perform Delayed Image in Cases of Negative 60 Minutes Postinjection Examination? Clin. Nucl. Med. 2020, 45, e213–e214. [Google Scholar] [CrossRef]
- Rosar, F.; Buchholz, H.G.; Michels, S.; Hoffmann, M.A.; Piel, M.; Waldmann, C.M.; Rösch, F.; Reuss, S.; Schreckenberger, M. Image quality analysis of 44Sc on two preclinical PET scanners: A comparison to 68Ga. EJNMMI Phys. 2020, 7, 16. [Google Scholar] [CrossRef]
- Luiting, H.B.; van Leeuwen, P.J.; Busstra, M.B.; Brabander, T.; van der Poel, H.G.; Donswijk, M.L.; Vis, A.N.; Emmett, L.; Stricker, P.D.; Roobol, M.J. Use of gallium-68 prostate-specific membrane antigen positron-emission tomography for detecting lymph node metastases in primary and recurrent prostate cancer and location of recurrence after radical prostatectomy: An overview of the current literature. BJU Int. 2020, 125, 206–214. [Google Scholar] [CrossRef]
- Han, S.; Woo, S.; Kim, Y.J.; Suh, C.H. Impact of 68Ga-PSMA PET on the Management of Patients with Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2018, 74, 179–190. [Google Scholar] [CrossRef]
- Krohn, T.; Verburg, F.A.; Pufe, T.; Neuhuber, W.; Vogg, A.; Heinzel, A.; Mottaghy, F.M.; Behrendt, F.F. [(68)Ga]PSMA-HBED uptake mimicking lymph node metastasis in coeliac ganglia: An important pitfall in clinical practice. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 210–214. [Google Scholar] [CrossRef]
- Derlin, T.; Schmuck, S.; Juhl, C.; Zörgiebel, J.; Schneefeld, S.M.; Walte, A.C.A.; Hueper, K.; von Klot, C.A.; Henkenberens, C.; Christiansen, H.; et al. PSA-stratified detection rates for [68Ga]THP-PSMA, a novel probe for rapid kit-based 68Ga-labeling and PET imaging, in patients with biochemical recurrence after primary therapy for prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Hetzheim, H.; Kübler, W.; Kratochwil, C.; Giesel, F.L.; Hope, T.A.; Eder, M.; Eisenhut, M.; Kopka, K.; Haberkorn, U. Radiation dosimetry of (68)Ga-PSMA-11 (HBED-CC) and preliminary evaluation of optimal imaging timing. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Haupt, F.; Dijkstra, L.; Alberts, I.; Sachpekidis, C.; Fech, V.; Boxler, S.; Gross, T.; Holland-Letz, T.; Zacho, H.D.; Haberkorn, U.; et al. 68Ga-PSMA-11 PET/CT in patients with recurrent prostate cancer-a modified protocol compared with the common protocol. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 624–631. [Google Scholar] [CrossRef]
- Annunziata, S.; Pizzuto, D.A.; Treglia, G. Diagnostic Performance of PET Imaging Using Different Radiopharmaceuticals in Prostate Cancer According to Published Meta-Analyses. Cancers 2020, 12, 2153. [Google Scholar] [CrossRef]
- Werner, R.A.; Derlin, T.; Lapa, C.; Sheikbahaei, S.; Higuchi, T.; Giesel, F.L.; Behr, S.; Drzezga, A.; Kimura, H.; Buck, A.K.; et al. 18F-Labeled, PSMA-Targeted Radiotracers: Leveraging the Advantages of Radiofluorination for Prostate Cancer Molecular Imaging. Theranostics 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Eder, M.; Neels, O.; Müller, M.; Bauder-Wüst, U.; Remde, Y.; Schäfer, M.; Hennrich, U.; Eisenhut, M.; Afshar-Oromieh, A.; Haberkorn, U.; et al. Novel Preclinical and Radiopharmaceutical Aspects of [68Ga]Ga-PSMA-HBED-CC: A New PET Tracer for Imaging of Prostate Cancer. Pharmaceuticals 2014, 7, 779–796. [Google Scholar] [CrossRef]
- Strauss, L.G.; Conti, P.S. The applications of PET in clinical oncology. J. Nucl. Med. 1991, 32, 623–648. [Google Scholar]

| Characteristics (n) | Parameters |
|---|---|
| Number of patients | 233 |
| Age (y) (233) | |
| Median | 72 |
| Range | 47–85 |
| Mean ± SD | 70.3 ± 7.3 |
| Primary Gleason score (228) | |
| ≤6 (low risk + grade group 1) | 16 |
| 7a, 7b (intermediate risk + grade group 2 + 3) | 96 |
| 8 (high risk + grade group 4) | 33 |
| >8 (high risk + grade group 5) | 83 |
| PSA (ng/mL) (233) | |
| Median | 2.32 |
| Range | 0.2–960 |
| Mean ± SD | 15.1 ± 71.9 |
| Prior treatment of primary tumor (233) | |
| Surgery (radical prostatectomy) | 178 |
| Radiotherapy and other | 37 |
| Primary staging (pre-therapy) | 18 |
| Further treatment | |
| Anti-androgen therapy (x/233) | 101 |
| Lesion positivity rate (160/233) | 68.7% |
| Restaging (PET/CT-positive/total) | 147/215 |
| Primary staging (PET/CT-positive/total) | 13/18 |
| PSA (ng/mL) | 0.2–<0.5 | 0.5–<1.0 | 1.0–<2.0 | 2.0–<5.0 | ≥5.0 | Chi2, p |
|---|---|---|---|---|---|---|
| Number (x/178) post-prostatectomized patients | 33 | 25 | 27 | 43 | 50 | |
| PET/CT-positive (x/114) | 9 | 8 | 19 | 33 | 45 | r = 0.507; p < 0.001 |
| Lesion positivity rate | 27.3% | 32.0% | 70.4% | 76.6% | 90.0% | |
| Regions: | ||||||
| Local recurrence | 2 | 0 | 5 | 6 | 14 | r = 0.236; p = 0.01 |
| Metastases | 7 | 8 | 16 | 31 | 41 | r = 0.471; p < 0.001 |
| Site of metastases: | r = 0.459; p < 0.001 | |||||
| Local metastases | 4 | 4 | 9 | 11 | 12 | |
| Distant metastases | 0 | 4 | 4 | 10 | 13 | |
| Local + distant metastases | 3 | 0 | 3 | 10 | 16 | |
| Number of metastases: | r = 0.536; p < 0.001 | |||||
| Single metastases | 3 | 6 | 7 | 7 | 2 | |
| Multiple metastases | 4 | 2 | 9 | 24 | 39 | |
| Lymph node metastases (LNM) | 7 | 4 | 12 | 21 | 28 | r = 0.296; p = 0.001 |
| Site of LNM: | r = 0.297; p < 0.042 | |||||
| Pelvic LNM | 6 | 4 | 10 | 17 | 19 | |
| Extra-pelvic LNM | 0 | 0 | 1 | 1 | 2 | |
| Pelvic + extra-pelvic LNM | 1 | 0 | 1 | 3 | 7 | |
| Bone metastases | 2 | 4 | 5 | 18 | 24 | r = 0.355; p < 0.001 |
| Visceral metastases | 0 | 0 | 1 | 1 | 4 | r = 0.153; p = 0.352 * |
| Tumor Location | Number of Patients (x/178) | PET/CT-Positive Patients (x/114) | Number of PSMA-Positive Lesions | SUVmax Mean ± SD Range | Wilcoxon p |
|---|---|---|---|---|---|
| Local recurrence 1 h p.i. | 27 | 27 | 29 | 10.1 ± 9.5/2.1–45.2 | |
| Local recurrence 3 h p.i. | 27 | 27 | 29 | 11.7 ± 10.7/3.6–50.9 | p < 0.001 |
| LNM 1 h p.i. | 72 | 72 | 326 | 12.2 ± 13.6/1.1–87.2 | |
| LNM 3 h p.i. | 72 | 72 | 328 | 15.0 ± 15.1/1.2–89.2 | p < 0.001 |
| Pelvic LNM 1 h p.i. | 68 | 68 | 262 | 12.0 ± 13.7/0.9–87.2 | |
| Pelvic LNM 3 h p.i. | 68 | 68 | 262 | 15.2 ± 15.5/1.2–89.2 | p < 0.001 |
| Extra-pelvic LNM 1 h p.i. | 16 | 16 | 64 | 13.8 ± 13.8/1.2–44.2 | |
| Extra-pelvic LNM 3 h p.i. | 16 | 16 | 66 | 15.6 ± 15.6/2.0–55.5 | p < 0.005 |
| Bone metastases 1 h p.i. | 53 | 53 | 195 | 13.5 ± 18.0/1.3–100.1 | |
| Bone metastases 3 h p.i. | 53 | 53 | 196 | 17.0 ± 20.2/1.8–104.5 | p < 0.001 |
| Visceral metastases 1 h p.i. | 1 | 1 | 1 | 2.1 | |
| Visceral metastases 3 h p.i. | 1 | 1 | 1 | 3.2 |
| n = 178 | GS < 7 (11) | GS 7a (33) | GS 7b (51) | GS 8 (26) | GS > 8 (57) | Chi2 r, p Value |
|---|---|---|---|---|---|---|
| PSMA-positive (xx/114) | 1 | 11 | 38 | 25 | 39 | 0.326; p < 0.001 |
| Local recurrence (xx/27) | 1 | 4 | 5 | 6 | 11 | 0.112; p = 0.446 |
| Metastases (xx/103) | 0 | 9 | 34 | 23 | 37 | 0.346; p < 0.001 |
| LNM (xx/68) | 0 | 8 | 22 | 17 | 21 | 0.186; p = 0.029 |
| PSA Range (ng/mL) | Overall Positivity | Chi2 p/r Value | Single Metastases | Multiple Metastases | Chi2 p/r Value | |||
| 0.2 to <1 (58) | 17 (29.3%) | 9 (15.5%) | 6 (10.3%) | |||||
| <1.24 (71) | 24 (33.8%) | 12 (16.9%) | 9 (12.7%) | |||||
| ≥1.24 (107) | 90 (84.1%) | 13 (12.1%) | 69 (64.5%) | |||||
| Total (178) | 114 (64%) | p < 0.001 r 0.513 | 25 | 78 | p < 0.001 r 0.522 | |||
| PSA Range (ng/mL) | Local Recurrence | p/r Value | Local Metastases | Distant Metastases | Local + Distant Metastases | p/r Value | ||
| 0.2 to <1 (58) | 2 (3.4%) | 8 (13.8%) | 4 (6.9%) | 3 (5.2%) | ||||
| <1.24 (71) | 3 (4.2%) | 9 (12.7%) | 7 (9.9%) | 5 (7.0%) | ||||
| ≥1.24 (107) | 24 (22.4%) | 31 (29.0%) | 24 (22.4%) | 27 (25.2%) | ||||
| Total (178) | 27 | p = 0.001 r 0.249 | 40 | 31 | 32 | p < 0.001 r 0.412 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, M.A.; Buchholz, H.-G.; Wieler, H.J.; Rosar, F.; Miederer, M.; Fischer, N.; Schreckenberger, M. Dual-Time Point [68Ga]Ga-PSMA-11 PET/CT Hybrid Imaging for Staging and Restaging of Prostate Cancer. Cancers 2020, 12, 2788. https://doi.org/10.3390/cancers12102788
Hoffmann MA, Buchholz H-G, Wieler HJ, Rosar F, Miederer M, Fischer N, Schreckenberger M. Dual-Time Point [68Ga]Ga-PSMA-11 PET/CT Hybrid Imaging for Staging and Restaging of Prostate Cancer. Cancers. 2020; 12(10):2788. https://doi.org/10.3390/cancers12102788
Chicago/Turabian StyleHoffmann, Manuela A., Hans-Georg Buchholz, Helmut J Wieler, Florian Rosar, Matthias Miederer, Nicolas Fischer, and Mathias Schreckenberger. 2020. "Dual-Time Point [68Ga]Ga-PSMA-11 PET/CT Hybrid Imaging for Staging and Restaging of Prostate Cancer" Cancers 12, no. 10: 2788. https://doi.org/10.3390/cancers12102788
APA StyleHoffmann, M. A., Buchholz, H.-G., Wieler, H. J., Rosar, F., Miederer, M., Fischer, N., & Schreckenberger, M. (2020). Dual-Time Point [68Ga]Ga-PSMA-11 PET/CT Hybrid Imaging for Staging and Restaging of Prostate Cancer. Cancers, 12(10), 2788. https://doi.org/10.3390/cancers12102788





