Three-Dimensional Cell Culture Systems in Radiopharmaceutical Cancer Research
Simple Summary
Abstract
1. Introduction
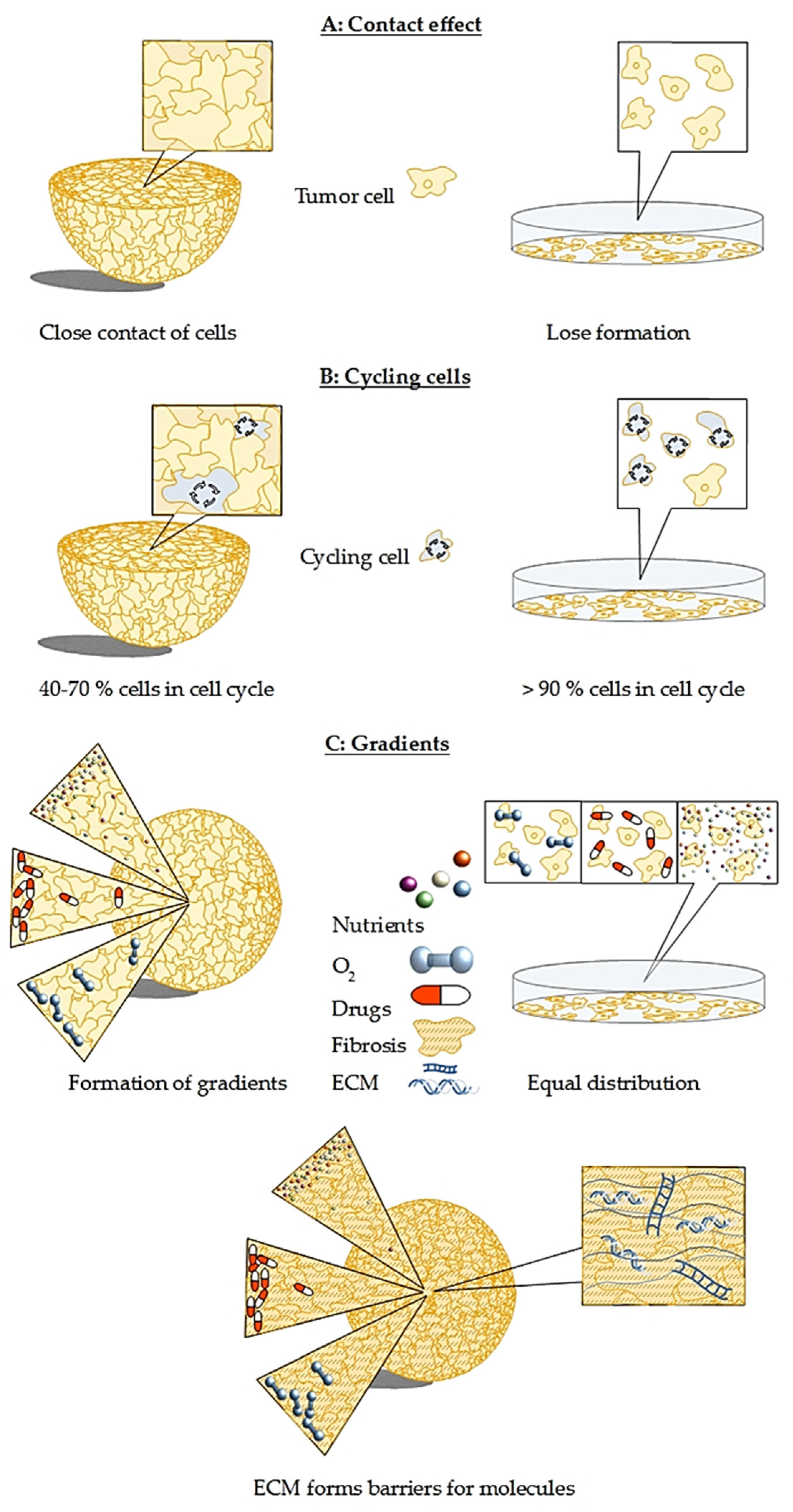
2. In Vitro 3D Models Are Superior to Classical Monolayer Cultures
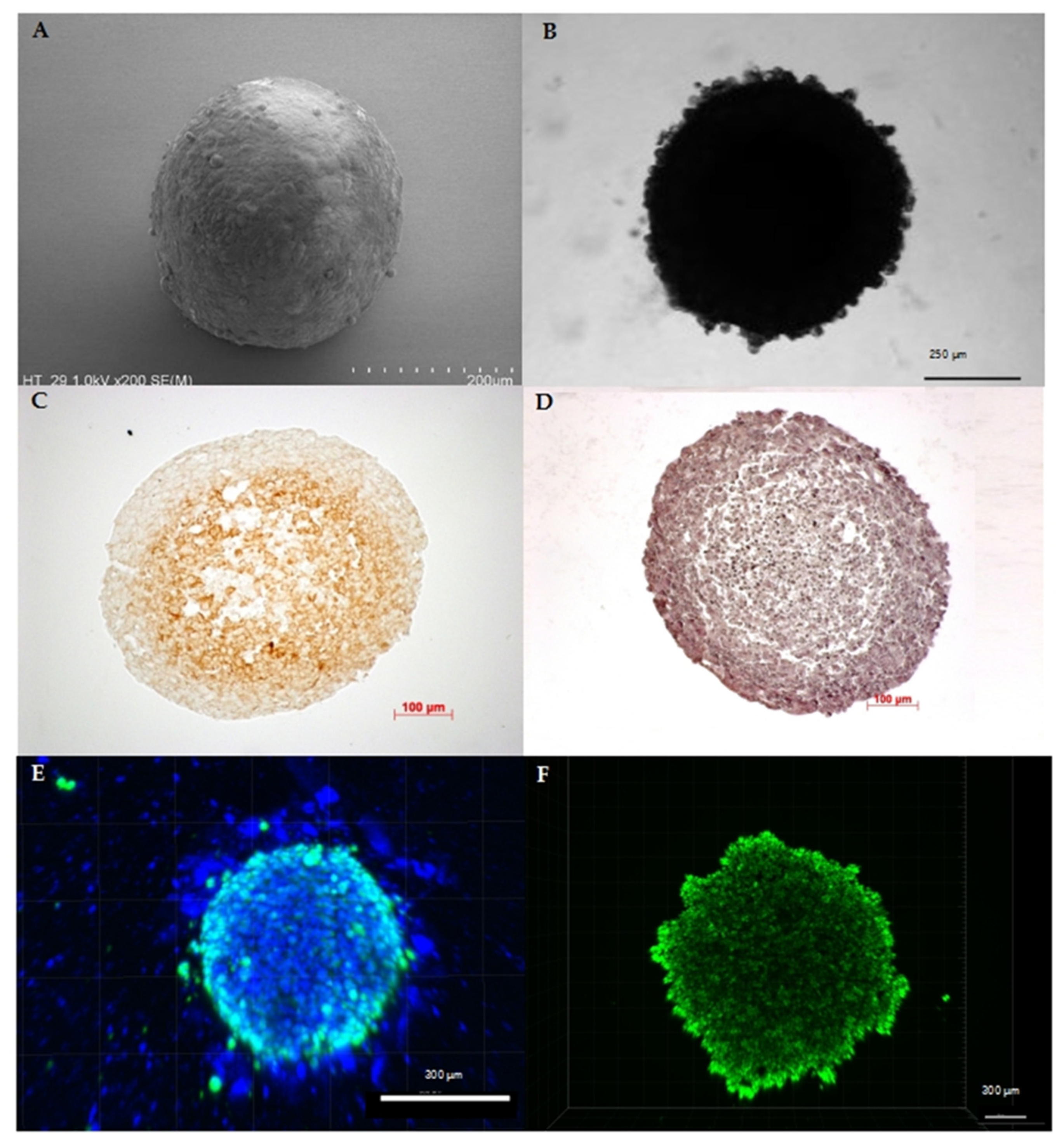
3. Current Methods and Challenges in Cultivation of Multicellular Spheroids
4. Organoids as a Step Forward to Personalized Medicine
5. Co-Cultures Visualize Challenges Associated with Tumor–Stroma Interactions
6. Biotechnical Microsystems Enable New Design of 3D Models
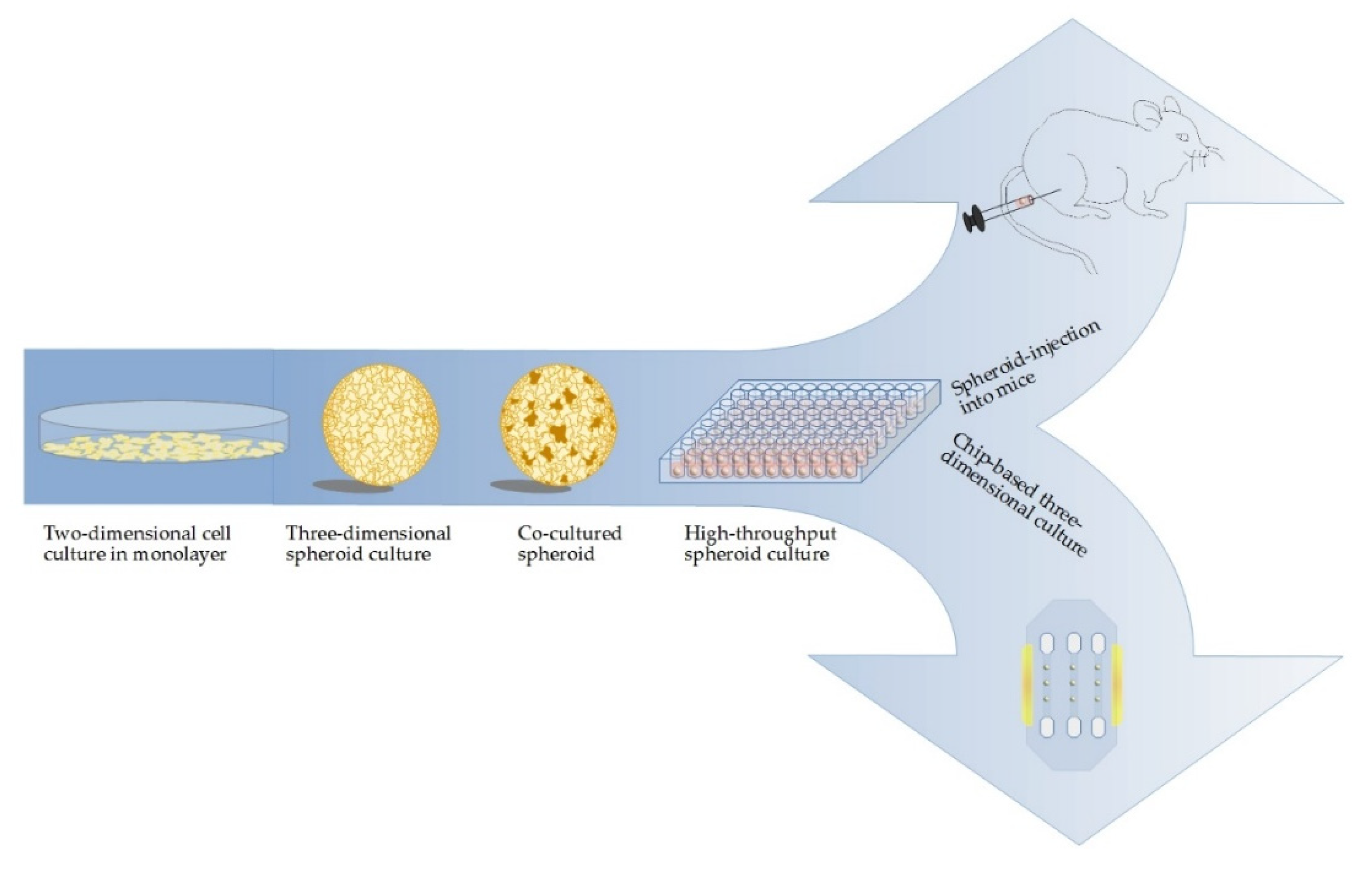
7. Translation of 3D Cell Culture Models from In Vitro to In Vivo
8. 3D Models as Pharmacists’ Tool for Drug Development
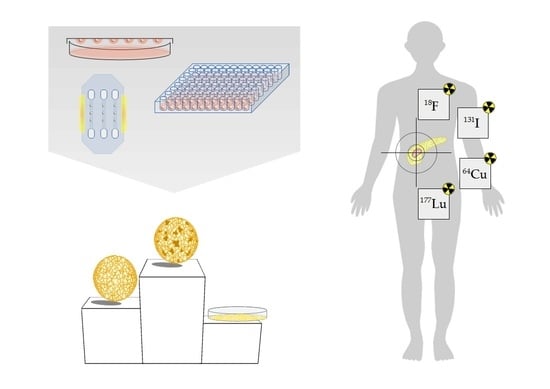
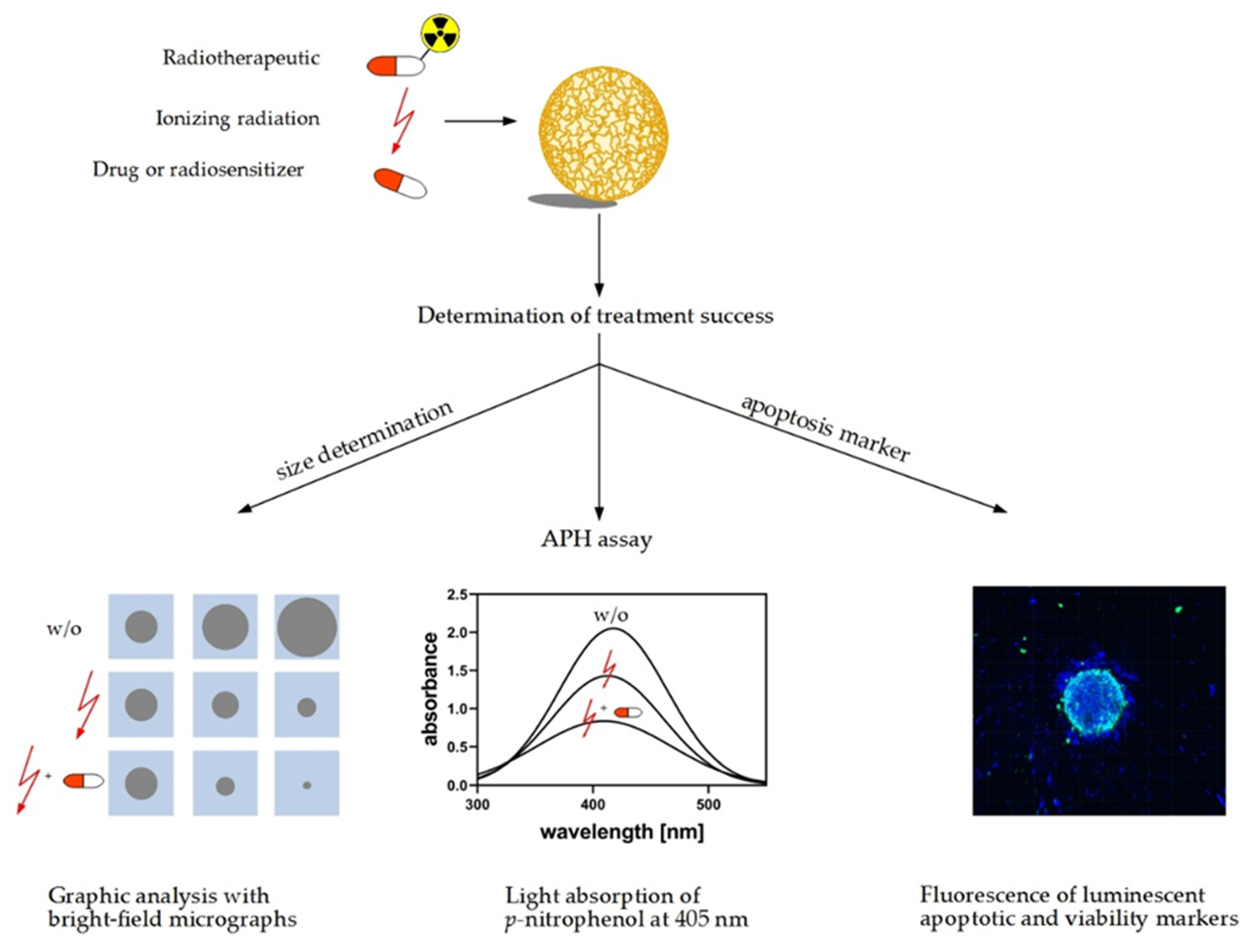
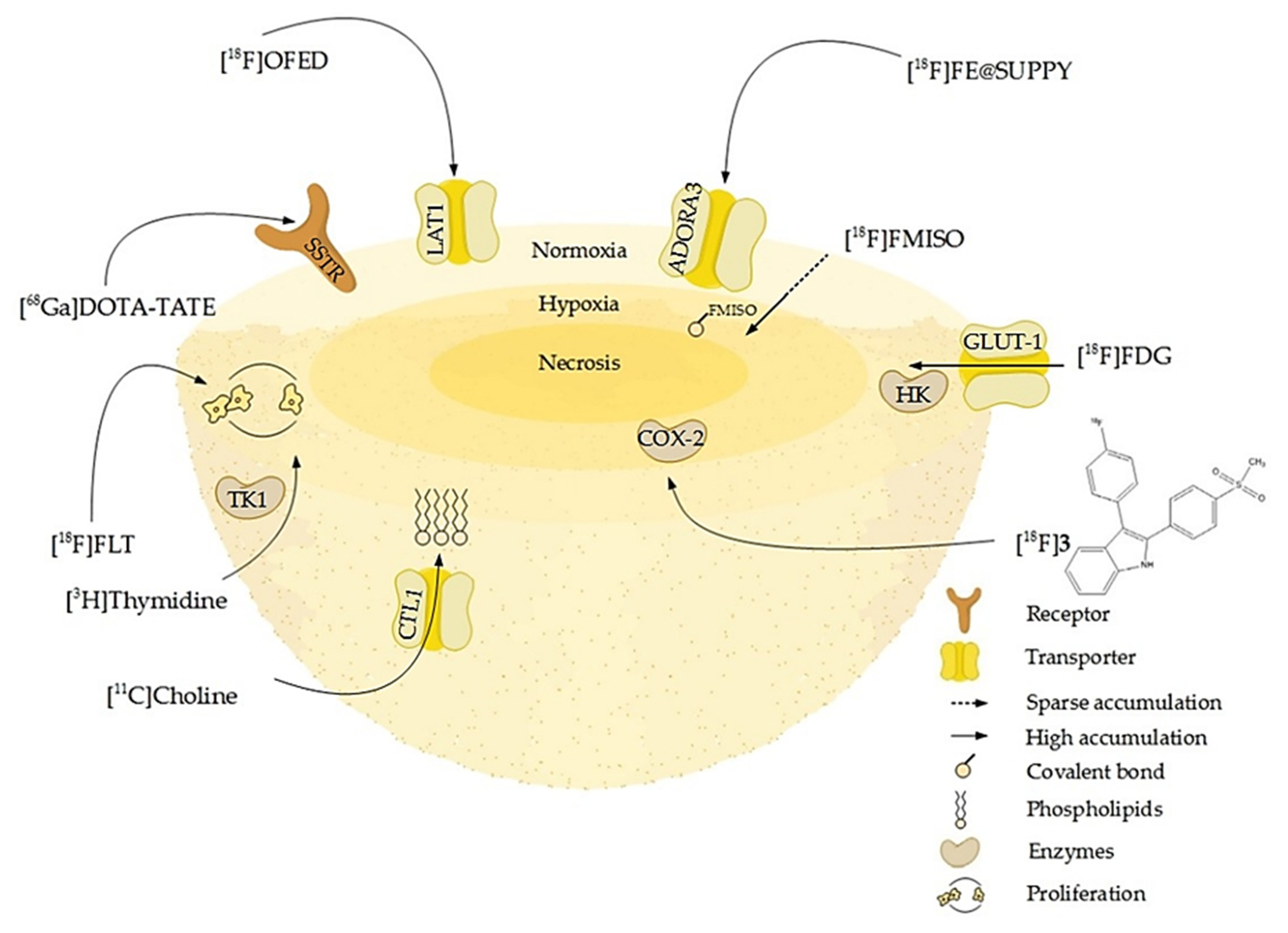
9. 3D Models as Radiopharmacists’ Tool for Development of Radiotheranostics
10. Conclusions and Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friedrich, J.; Ebner, R.; Kunz-Schughart, L.A. Experimental anti-tumor therapy in 3-D: Spheroids—Old hat or new challenge? Int. J. Radiat. Biol. 2007, 83, 849–871. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Devi, G.R. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef]
- Large, T.Y.S.L.; Bijlsma, M.F.; Kazemier, G.; van Laarhoven, H.W.M.; Giovannetti, E.; Jimenez, C.R. Key biological processes driving metastatic spread of pancreatic cancer as identified by multi-omics studies. Semin. Cancer Biol. 2017, 44, 153–169. [Google Scholar] [CrossRef]
- Guo, X.-Z.; Cui, Z.-M.; Liu, X. Current developments, problems and solutions in the non-surgical treatment of pancreatic cancer. World J. Gastrointest. Oncol. 2013, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, S.; Guillaumond, F. LDL Receptor: An open route to feed pancreatic tumor cells. Mol. Cell. Oncol. 2015, 3, e1033586. [Google Scholar] [CrossRef]
- Ghaneh, P.; Costello, E.; Neoptolemos, J.P. Biology and management of pancreatic cancer. Postgrad. Med. J. 2008, 84, 478–497. [Google Scholar] [CrossRef]
- Phillips, P. Pancreatic stellate cells and fibrosis. In Pancreatic Cancer and Tumor Microenvironment; Grippo, P.J., Munshi, H.G., Eds.; Transworld Research Network: Trivandrum, India, 2012; ISBN 978-81-7895-548-3. [Google Scholar]
- Apte, M.V.; Pirola, R.C.; Wilson, J.S. Pancreatic stellate cells: A starring role in normal and diseased pancreas. Front. Physiol. 2012, 3, 344. [Google Scholar] [CrossRef]
- Dubessy, C. Spheroids in radiobiology and photodynamic therapy. Crit. Rev. Oncol. Hematol. 2000, 36, 179–192. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- De Witt Hamer, P.C.; Van Tilborg, A.A.G.; Eijk, P.P.; Sminia, P.; Troost, D.; Van Noorden, C.J.F.; Ylstra, B.; Leenstra, S. The genomic profile of human malignant glioma is altered early in primary cell culture and preserved in spheroids. Oncogene 2008, 27, 2091–2096. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-Z.; Chang, H.-Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. ASSAY Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-dimensional cell culture matrices: State of the art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef] [PubMed]
- Kimlin, L.C.; Casagrande, G.; Virador, V.M. In vitro three-dimensional (3D) models in cancer research: An update. Mol. Carcinog. 2013, 52, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Rimann, M.; Graf-Hausner, U. Synthetic 3D multicellular systems for drug development. Curr. Opin. Biotechnol. 2012, 23, 803–809. [Google Scholar] [CrossRef]
- Casciari, J.J.; Sotirchos, S.V.; Sutherland, R.M. Glucose diffusivity in multicellular tumor spheroids. Cancer Res. 1988, 48, 3905–3909. [Google Scholar]
- Dardousis, K.; Voolstra, C.; Roengvoraphoj, M.; Sekandarzad, A.; Mesghenna, S.; Winkler, J.; Ko, Y.; Hescheler, J.; Sachinidis, A. Identification of differentially expressed genes involved in the formation of multicellular tumor spheroids by HT-29 colon carcinoma cells. Mol. Ther. J. Am. Soc. Gene Ther. 2007, 15, 94–102. [Google Scholar] [CrossRef]
- Kim, J.B. Three-dimensional tissue culture models in cancer biology. Semin. Cancer Biol. 2005, 15, 365–377. [Google Scholar] [CrossRef]
- Fang, Y.; Eglen, R.M. Three-dimensional cell cultures in drug discovery and development. Slas Discov. Adv. Life Sci. R D 2017, 22, 456–472. [Google Scholar] [CrossRef]
- Riffle, S.; Hegde, R.S. Modeling tumor cell adaptations to hypoxia in multicellular tumor spheroids. J. Exp. Clin. Cancer Res. 2017, 36, 102. [Google Scholar] [CrossRef] [PubMed]
- Kunz-Schughart, L.A.; Freyer, J.P.; Hofstaedter, F.; Ebner, R. The use of 3-D cultures for high-throughput screening: The multicellular spheroid model. J. Biomol. Screen. 2004, 9, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Seifert, V.; Liers, J.; Kniess, T.; Richter, S.; Bechmann, N.; Feldmann, A.; Bachmann, M.; Eisenhofer, G.; Pietzsch, J.; Ullrich, M. Fluorescent mouse pheochromocytoma spheroids expressing hypoxia-inducible factor 2 alpha: Morphologic and radiopharmacologic characterization. J. Cell. Biotechnol. 2019, 5, 135–151. [Google Scholar] [CrossRef]
- Mueller Klieser, W. Tumor biology and experimental therapeutics. Crit. Rev. Oncol. Hematol. 2000, 36, 123–139. [Google Scholar] [CrossRef]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist, 7th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; ISBN 978-1-60831-193-4. [Google Scholar]
- Forster, J.; Harriss-Phillips, W.; Douglass, M.; Bezak, E. A review of the development of tumor vasculature and its effects on the tumor microenvironment. Hypoxia 2017, 5, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Beasley, N.J.; Wykoff, C.C.; Watson, P.H.; Leek, R.; Turley, H.; Gatter, K.; Pastorek, J.; Cox, G.J.; Ratcliffe, P.; Harris, A.L. Carbonic anhydrase IX, an endogenous hypoxia marker, expression in head and neck squamous cell carcinoma and its relationship to hypoxia, necrosis, and microvessel density. Cancer Res. 2001, 61, 5262–5267. [Google Scholar] [PubMed]
- Rodríguez-Enríquez, S.; Gallardo-Pérez, J.C.; Avilés-Salas, A.; Marín-Hernández, A.; Carreño-Fuentes, L.; Maldonado-Lagunas, V.; Moreno-Sánchez, R. Energy metabolism transition in multi-cellular human tumor spheroids. J. Cell. Physiol. 2008, 216, 189–197. [Google Scholar] [CrossRef]
- Cramer, G.M.; Jones, D.P.; El-Hamidi, H.; Celli, J.P. ECM composition and rheology regulate growth, motility, and response to photodynamic therapy in 3D models of pancreatic ductal adenocarcinoma. Mol. Cancer Res. 2017, 15, 15–25. [Google Scholar] [CrossRef]
- Senthebane, D.A.; Rowe, A.; Thomford, N.E.; Shipanga, H.; Munro, D.; Mazeedi, M.A.M.A.; Almazyadi, H.A.M.; Kallmeyer, K.; Dandara, C.; Pepper, M.S.; et al. The role of tumor microenvironment in chemoresistance: To survive, keep your enemies closer. Int. J. Mol. Sci. 2017, 18, 1586. [Google Scholar] [CrossRef]
- Nieskoski, M.D.; Marra, K.; Gunn, J.R.; Hoopes, P.J.; Doyley, M.M.; Hasan, T.; Trembly, B.S.; Pogue, B.W. Collagen complexity spatially defines microregions of total tissue pressure in pancreatic cancer. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.A.; Tiriac, H.; Clevers, H.; Tuveson, D.A. Modeling pancreatic cancer with organoids. Trends Cancer 2016, 2, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Barrera, A.M.; Menter, D.G.; Abbruzzese, J.L.; Reddy, S.A.G. Establishment of three-dimensional cultures of human pancreatic duct epithelial cells. Biochem. Biophys. Res. Commun. 2007, 358, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, J.J.; Bouvet, M. Determination of the ligand-binding specificities of the alpha2beta1 and alpha1beta1 integrins in a novel 3-dimensional in vitro model of pancreatic cancer. Pancreas 2007, 34, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Froeling, F.E.M.; Marshall, J.F.; Kocher, H.M. Pancreatic cancer organotypic cultures. J. Biotechnol. 2010, 148, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.J.; Colbert, K.; Keshishian, V.; Ho, J.; Corr, S.J.; Curley, S.A.; Godin, B. Generation of homogenous three-dimensional pancreatic cancer cell spheroids using an improved hanging drop technique. Tissue Eng. Part C Methods 2016, 22, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Ou, B.; Zhao, J.; Yin, S.; Lu, A.; Oechsle, E.; Thasler, W.E. Homogeneous pancreatic cancer spheroids mimic growth pattern of circulating tumor cell clusters and macrometastases: Displaying heterogeneity and crater-like structure on inner layer. J. Cancer Res. Clin. Oncol. 2017, 143, 1771–1786. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Ishiwata, T.; Kawamoto, Y.; Kawahara, K.; Peng, W.-X.; Yamamoto, T.; Naito, Z. Morphological and cytoskeletal changes of pancreatic cancer cells in three-dimensional spheroidal culture. Med. Mol. Morphol. 2010, 43, 211–217. [Google Scholar] [CrossRef]
- Wong, C.-W.; Han, H.-W.; Tien, Y.-W.; Hsu, S. Biomaterial substrate-derived compact cellular spheroids mimicking the behavior of pancreatic cancer and microenvironment. Biomaterials 2019, 213, 119202. [Google Scholar] [CrossRef]
- Meier-Hubberten, J.C.; Sanderson, M.P. Establishment and analysis of a 3D co-culture spheroid model of pancreatic adenocarcinoma for application in drug discovery. In Target Identification and Validation in Drug Discovery; Springer: New York, NY, USA, 2019; pp. 163–179. [Google Scholar]
- Yeon, S.-E.; No, D.Y.; Lee, S.-H.; Nam, S.W.; Oh, I.-H.; Lee, J.; Kuh, H.-J. Application of concave microwells to pancreatic tumor spheroids enabling anticancer drug evaluation in a clinically relevant drug resistance model. PLoS ONE 2013, 8, e73345. [Google Scholar] [CrossRef]
- Kelm, J.M.; Timmins, N.E.; Brown, C.J.; Fussenegger, M.; Nielsen, L.K. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol. Bioeng. 2003, 83, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, A.Y.; Tung, Y.-C.; Qu, X.; Patel, L.R.; Pienta, K.J.; Takayama, S. 384 hanging drop arrays give excellent Z-factors and allow versatile formation of co-culture spheroids. Biotechnol. Bioeng. 2012, 109, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Broutier, L.; Andersson-Rolf, A.; Hindley, C.J.; Boj, S.F.; Clevers, H.; Koo, B.-K.; Huch, M. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 2016, 11, 1724–1743. [Google Scholar] [CrossRef]
- Huch, M.; Bonfanti, P.; Boj, S.F.; Sato, T.; Loomans, C.J.M.; van de Wetering, M.; Sojoodi, M.; Li, V.S.W.; Schuijers, J.; Gracanin, A.; et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013, 32, 2708–2721. [Google Scholar] [CrossRef] [PubMed]
- Boj, S.F.; Hwang, C.-I.; Baker, L.A.; Chio, I.I.C.; Engle, D.D.; Corbo, V.; Jager, M.; Ponz-Sarvise, M.; Tiriac, H.; Spector, M.S.; et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 2015, 160, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Choudhury, D.; Fang, Y.; Hunziker, W. Towards manufacturing of human organoids. Biotechnol. Adv. 2020, 39, 107460. [Google Scholar] [CrossRef]
- Froeling, F.E.M.; Mirza, T.A.; Feakins, R.M.; Seedhar, A.; Elia, G.; Hart, I.R.; Kocher, H.M. Organotypic culture model of pancreatic cancer demonstrates that stromal cells modulate E-cadherin, beta-catenin, and Ezrin expression in tumor cells. Am. J. Pathol. 2009, 175, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.J.; Keshishian, V.; Law, J.J.; Ho, J.C.; Favela, C.A.; Rees, P.; Smith, B.; Mohammad, S.; Hwang, R.F.; Rajapakshe, K.; et al. Generation of an in vitro 3D PDAC stroma rich spheroid model. Biomaterials 2016, 108, 129–142. [Google Scholar] [CrossRef]
- Kuen, J.; Darowski, D.; Kluge, T.; Majety, M. Pancreatic cancer cell/fibroblast co-culture induces M2 like macrophages that influence therapeutic response in a 3D model. PLoS ONE 2017, 12, e0182039. [Google Scholar] [CrossRef]
- Goodwin, M.L.; Urs, S.K.; Simeone, D.M. Pancreatic microtumors: A novel 3D ex vivo testing platform. In Methods in Molecular Biology; Springer: New York, NY, USA, 2018; pp. 73–80. [Google Scholar]
- Lazzari, G.; Nicolas, V.; Matsusaki, M.; Akashi, M.; Couvreur, P.; Mura, S. Multicellular spheroid based on a triple co-culture: A novel 3D model to mimic pancreatic tumor complexity. Acta Biomater. 2018, 78, 296–307. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Lee, J.-H.; Shin, Y.; Chung, S.; Kuh, H.-J. Co-culture of tumor spheroids and fibroblasts in a collagen matrix-incorporated microfluidic chip mimics reciprocal activation in solid tumor microenvironment. PLoS ONE 2016, 11, e0159013. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, S.-K.; Khawar, I.A.; Jeong, S.-Y.; Chung, S.; Kuh, H.-J. Microfluidic co-culture of pancreatic tumor spheroids with stellate cells as a novel 3D model for investigation of stroma-mediated cell motility and drug resistance. J. Exp. Clin. Cancer Res. 2018, 37, 1–12. [Google Scholar] [CrossRef]
- Beer, M.; Kuppalu, N.; Stefanini, M.; Becker, H.; Schulz, I.; Manoli, S.; Schuette, J.; Schmees, C.; Casazza, A.; Stelzle, M.; et al. A novel microfluidic 3D platform for culturing pancreatic ductal adenocarcinoma cells: Comparison with in vitro cultures and in vivo xenografts. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ramappa, N.K. A System Biology Approach to Study Pancreatic Ductal Adenocarcinoma (PDAC) Cells in In Vitro Culture; Universita degli Studi di Firenze: Florence, Italy, 2017. [Google Scholar]
- Noel, P.; Muñoz, R.; Rogers, G.W.; Neilson, A.; Hoff, D.D.V.; Han, H. Preparation and metabolic assay of 3-dimensional spheroid co-cultures of pancreatic cancer cells and fibroblasts. J. Vis. Exp. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ivascu, A.; Kubbies, M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J. Biomol. Screen. 2006, 11, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.-C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.; Ho, M.; Takayama, S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef]
- Gurski, L.A.; Petrelli, N.J.; Jia, X.; Farach-Carson, M.C. 3D Matrices for anti-cancer drug testing and development. Oncol. Issues 2010, 25, 20–25. [Google Scholar] [CrossRef]
- Schmidt, J.J.; Rowley, J.; Kong, H.J. Hydrogels used for cell-based drug delivery. J. Biomed. Mater. Res. A 2008, 87, 1113–1122. [Google Scholar] [CrossRef]
- Norberg, K.J.; Liu, X.; Fernández Moro, C.; Strell, C.; Nania, S.; Blümel, M.; Balboni, A.; Bozóky, B.; Heuchel, R.L.; Löhr, J.M. A novel pancreatic tumour and stellate cell 3D co-culture spheroid model. BMC Cancer 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Cukierman, E.; Pankov, R.; Stevens, D.R.; Yamada, K.M. Taking cell-matrix adhesions to the third dimension. Science 2001, 294, 1708–1712. [Google Scholar] [CrossRef]
- Gagliano, N. Epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma: Characterization in a 3D-cell culture model. World J. Gastroenterol. 2016, 22, 4466. [Google Scholar] [CrossRef] [PubMed]
- Asthana, A.; Kisaalita, W.S. Microtissue size and hypoxia in HTS with 3D cultures. Drug Discov. Today 2012, 17, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Che, P.P.; Hassouni, B.E.; Buijs, M.; Coppola, S.; Löhr, M.; Funel, N.; Heuchel, R.; Carnevale, I.; Schmidt, T.; et al. Role of c-MET inhibitors in overcoming drug resistance in spheroid models of primary human pancreatic cancer and stellate cells. Cancers 2019, 11, 638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.C.; Kuo, C.J. Personalizing pancreatic cancer organoids with hPSCs. Nat. Med. 2015, 21, 1249–1251. [Google Scholar] [CrossRef] [PubMed]
- Seino, T.; Kawasaki, S.; Shimokawa, M.; Tamagawa, H.; Toshimitsu, K.; Fujii, M.; Ohta, Y.; Matano, M.; Nanki, K.; Kawasaki, K.; et al. Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression. Cell Stem Cell 2018, 22, 454–467. [Google Scholar] [CrossRef]
- Lee, J.; Snyder, E.R.; Liu, Y.; Gu, X.; Wang, J.; Flowers, B.M.; Kim, Y.J.; Park, S.; Szot, G.L.; Hruban, R.H.; et al. Reconstituting development of pancreatic intraepithelial neoplasia from primary human pancreas duct cells. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Yip, D.; Cho, C.H. A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochem. Biophys. Res. Commun. 2013, 433, 327–332. [Google Scholar] [CrossRef]
- Majety, M.; Pradel, L.P.; Gies, M.; Ries, C.H. Fibroblasts influence survival and therapeutic response in a 3D co-culture model. PLoS ONE 2015, 10, e0127948. [Google Scholar] [CrossRef]
- Kikuta, K.; Masamune, A.; Watanabe, T.; Ariga, H.; Itoh, H.; Hamada, S.; Satoh, K.; Egawa, S.; Unno, M.; Shimosegawa, T. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2010, 403, 380–384. [Google Scholar] [CrossRef]
- Rodenhizer, D.; Gaude, E.; Cojocari, D.; Mahadevan, R.; Frezza, C.; Wouters, B.G.; McGuigan, A.P. A three-dimensional engineered tumour for spatial snapshot analysis of cell metabolism and phenotype in hypoxic gradients. Nat. Mater. 2015, 15, 227–234. [Google Scholar] [CrossRef]
- Moreira, L.; Bakir, B.; Chatterji, P.; Dantes, Z.; Reichert, M.; Rustgi, A.K. Pancreas 3D organoids: Current and future aspects as a research platform for personalized medicine in pancreatic cancer. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.R.; Zhang, C.; Roche, S.; O’Neill, F.; Swan, N.; Luo, Y.; Larkin, A.; Crown, J.; Walsh, N. Modelling of pancreatic cancer biology: Transcriptomic signature for 3D PDX-derived organoids and primary cell line organoid development. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.-I.; Boj, S.F.; Clevers, H.; Tuveson, D.A. Preclinical models of pancreatic ductal adenocarcinoma: Pre-clinical models of pancreatic ductal adenocarcinoma. J. Pathol. 2016, 238, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Greggio, C.; De Franceschi, F.; Figueiredo-Larsen, M.; Gobaa, S.; Ranga, A.; Semb, H.; Lutolf, M.; Grapin-Botton, A. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development 2013, 140, 4452–4462. [Google Scholar] [CrossRef] [PubMed]
- Ferro, R.; Falasca, M. Emerging role of the KRAS-PDK1 axis in pancreatic cancer. World J. Gastroenterol. 2014, 20, 10752–10757. [Google Scholar] [CrossRef]
- Cristobal, A.; van den Toorn, H.W.P.; van de Wetering, M.; Clevers, H.; Heck, A.J.R.; Mohammed, S. Personalized proteome profiles of healthy and tumor human colon organoids reveal both individual diversity and basic features of colorectal cancer. Cell Rep. 2017, 18, 263–274. [Google Scholar] [CrossRef]
- Walsh, A.J.; Castellanos, J.A.; Nagathihalli, N.S.; Merchant, N.B.; Skala, M.C. Optical imaging of drug-induced metabolism changes in murine and human pancreatic cancer organoids reveals heterogeneous drug response. Pancreas 2016, 45, 863–869. [Google Scholar] [CrossRef]
- Amrutkar, M.; Aasrum, M.; Verbeke, C.S.; Gladhaug, I.P. Secretion of fibronectin by human pancreatic stellate cells promotes chemoresistance to gemcitabine in pancreatic cancer cells. BMC Cancer 2019, 19, 596. [Google Scholar] [CrossRef]
- Huang, L.; Holtzinger, A.; Jagan, I.; BeGora, M.; Lohse, I.; Ngai, N.; Nostro, C.; Wang, R.; Muthuswamy, L.B.; Crawford, H.C.; et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell—And patient-derived tumor organoids. Nat. Med. 2015, 21, 1364–1371. [Google Scholar] [CrossRef]
- Durymanov, M.; Kroll, C.; Permyakova, A.; O’Neill, E.; Sulaiman, R.; Person, M.; Reineke, J. Subcutaneous inoculation of 3D pancreatic cancer spheroids results in development of reproducible stroma-rich tumors. Transl. Oncol. 2019, 12, 180–189. [Google Scholar] [CrossRef]
- Omary, M.B.; Lugea, A.; Lowe, A.W.; Pandol, S.J. The pancreatic stellate cell: A star on the rise in pancreatic diseases. J. Clin. Investig. 2007, 117, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Hwang, R.F.; Moore, T.; Arumugam, T.; Ramachandran, V.; Amos, K.D.; Rivera, A.; Ji, B.; Evans, D.B.; Logsdon, C.D. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008, 68, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Murakami, T.; Tsuchida, K.; Sugino, H.; Miyake, H.; Tashiro, S. Tumor-stroma interaction of human pancreatic cancer: Acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas 2004, 28, 38–44. [Google Scholar] [CrossRef]
- Uzunparmak, B.; Sahin, I.H. Pancreatic cancer microenvironment: A current dilemma. Clin. Transl. Med. 2019, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Gil, Z. Macrophages increase the resistance of pancreatic adenocarcinoma cells to gemcitabine by upregulating cytidine deaminase. OncoImmunology 2013, 2, e27231. [Google Scholar] [CrossRef]
- Al-Assar, O.; Demiciorglu, F.; Lunardi, S.; Gaspar-Carvalho, M.M.; McKenna, W.G.; Muschel, R.M.; Brunner, T.B. Contextual regulation of pancreatic cancer stem cell phenotype and radioresistance by pancreatic stellate cells. Radiother. Oncol. 2014, 111, 243–251. [Google Scholar] [CrossRef]
- Dauer, P.; Nomura, A.; Saluja, A.; Banerjee, S. Microenvironment in determining chemo-resistance in pancreatic cancer: Neighborhood matters. Pancreatology 2017, 17, 7–12. [Google Scholar] [CrossRef]
- Puls, T.J.; Tan, X.; Whittington, C.F.; Voytik-Harbin, S.L. 3D collagen fibrillar microstructure guides pancreatic cancer cell phenotype and serves as a critical design parameter for phenotypic models of EMT. PLoS ONE 2017, 12, e0188870. [Google Scholar] [CrossRef]
- Davies, C.d.L.; Berk, D.A.; Pluen, A.; Jain, R.K. Comparison of IgG diffusion and extracellular matrix composition in rhabdomyosarcomas grown in mice versus in vitro as spheroids reveals the role of host stromal cells. Br. J. Cancer 2002, 86, 1639–1644. [Google Scholar] [CrossRef]
- Young, E.W.K. Cells, tissues, and organs on chips: Challenges and opportunities for the cancer tumor microenvironment. Integr. Biol. Quant. Biosci. Nano Macro 2013, 5, 1096–1109. [Google Scholar] [CrossRef]
- Vamvakidou, A.P.; Mondrinos, M.J.; Petushi, S.P.; Garcia, F.U.; Lelkes, P.I.; Tozeren, A. Heterogeneous breast tumoroids: An in vitro assay for investigating cellular heterogeneity and drug delivery. J. Biomol. Screen. 2007, 12, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, B.; Lange, C.S. Hybrid spheroids as a tool for prediction of radiosensitivity in tumor therapy. Indian J. Exp. Biol. 2004, 42, 443–447. [Google Scholar] [PubMed]
- Djordjevic, B.; Lange, C.S. Cell-cell interactions in spheroids maintained in suspension. Acta Oncol. 2006, 45, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Kamb, A. What’s wrong with our cancer models? Nat. Rev. Drug Discov. 2005, 4, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; de Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef]
- Sato, N.; Maehara, N.; Goggins, M. Gene Expression Profiling of tumor—Stromal interactions between pancreatic cancer cells and stromal fibroblasts. Cancer Res. 2004, 64, 6950–6956. [Google Scholar] [CrossRef]
- Venter, C.; Niesler, C. A triple co-culture method to investigate the effect of macrophages and fibroblasts on myoblast proliferation and migration. BioTechniques 2018, 64, 52–58. [Google Scholar] [CrossRef]
- Bauleth-Ramos, T.; Feijão, T.; Gonçalves, A.; Shahbazi, M.-A.; Liu, Z.; Barrias, C.; Oliveira, M.J.; Granja, P.; Santos, H.A.; Sarmento, B. Colorectal cancer triple co-culture spheroid model to assess the biocompatibility and anticancer properties of polymeric nanoparticles. J. Controlled Release 2020, 323, 398–411. [Google Scholar] [CrossRef]
- Kunz-Schughart, L.A.; Heyder, P.; Schroeder, J.; Knuechel, R. A Heterologous 3-D coculture model of breast tumor cells and fibroblasts to study tumor-associated fibroblast differentiation. Exp. Cell Res. 2001, 266, 74–86. [Google Scholar] [CrossRef]
- Seidl, P.; Huettinger, R.; Knuechel, R.; Kunz-Schughart, L.A. Three-dimensional fibroblast-tumor cell interaction causes downregulation ofRACK1 mRNA expression in breast cancer cellsin vitro. Int. J. Cancer 2002, 102, 129–136. [Google Scholar] [CrossRef]
- Cavaco, A.C.M.; Rezaei, M.; Caliandro, M.F.; Lima, A.M.; Stehling, M.; Dhayat, S.A.; Haier, J.; Brakebusch, C.; Eble, J.A. The interaction between laminin-332 and α3β1 integrin determines differentiation and maintenance of CAFs, and supports invasion of pancreatic duct adenocarcinoma cells. Cancers 2018, 11, 14. [Google Scholar] [CrossRef]
- Bianco, M.; Zizzari, A.; Priore, P.; Moroni, L.; Metrangolo, P.; Frigione, M.; Rella, R.; Gaballo, A.; Arima, V. Lab-on-a-brane for spheroid formation. Biofabrication 2019, 11, 021002. [Google Scholar] [CrossRef] [PubMed]
- Lopa, S.; Piraino, F.; Kemp, R.J.; Di Caro, C.; Lovati, A.B.; Di Giancamillo, A.; Moroni, L.; Peretti, G.M.; Rasponi, M.; Moretti, M. Fabrication of multi-well chips for spheroid cultures and implantable constructs through rapid prototyping techniques: Multi-well PDMS chips and fibrin constructs. Biotechnol. Bioeng. 2015, 112, 1457–1471. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Sei, Y.; Jeon, N.; Kim, Y. Tumor microenvironment on a chip: The progress and future perspective. Bioengineering 2017, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Chiappalone, M.; Vato, A.; Tedesco, M.B.; Marcoli, M.; Davide, F.; Martinoia, S. Networks of neurons coupled to microelectrode arrays: A neuronal sensory system for pharmacological applications. Biosens. Bioelectron. 2003, 18, 627–634. [Google Scholar] [CrossRef]
- Manos, P.; Pancrazio, J.J.; Coulombe, M.G.; Ma, W.; Stenger, D.A. Characterization of rat spinal cord neurons cultured in defined media on microelectrode arrays. Neurosci. Lett. 1999, 271, 179–182. [Google Scholar] [CrossRef]
- Ben-Yoav, H.; Melamed, S.; Freeman, A.; Shacham-Diamand, Y.; Belkin, S. Whole-cell biochips for bio-sensing: Integration of live cells and inanimate surfaces. Crit. Rev. Biotechnol. 2011, 31, 337–353. [Google Scholar] [CrossRef]
- Stenger, D.A.; Gross, G.W.; Keefer, E.W.; Shaffer, K.M.; Andreadis, J.D.; Ma, W.; Pancrazio, J.J. Detection of physiologically active compounds using cell-based biosensors. Trends Biotechnol. 2001, 19, 304–309. [Google Scholar] [CrossRef]
- Kloss, D.; Fischer, M.; Rothermel, A.; Simon, J.C.; Robitzki, A.A. Drug testing on 3D in vitro tissues trapped on a microcavity chip. Lab Chip 2008, 8, 879–884. [Google Scholar] [CrossRef]
- Lin, S.-P.; Kyriakides, T.R.; Chen, J.-J.J. On-line observation of cell growth in a three-dimensional matrix on surface-modified microelectrode arrays. Biomaterials 2009, 30, 3110–3117. [Google Scholar] [CrossRef]
- Selimoglu, S.M.; Elibol, M. Alginate as an immobilization material for MAb production via encapsulated hybridoma cells. Crit. Rev. Biotechnol. 2010, 30, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Yi, S.H.; Jeong, S.H.; Ku, B.; Kim, J.; Lee, M.-Y. Plastic pillar inserts for three-dimensional (3D) cell cultures in 96-well plates. Sens. Actuators B Chem. 2013, 177, 78–85. [Google Scholar] [CrossRef]
- Kang, J.; Lee, D.W.; Hwang, H.J.; Yeon, S.-E.; Lee, M.-Y.; Kuh, H.-J. Mini-pillar array for hydrogel-supported 3D culture and high-content histologic analysis of human tumor spheroids. Lab Chip 2016, 16, 2265–2276. [Google Scholar] [CrossRef] [PubMed]
- Gui, Q.; Lawson, T.; Shan, S.; Yan, L.; Liu, Y. The application of whole cell-based biosensors for use in environmental analysis and in medical diagnostics. Sensors 2017, 17, 1623. [Google Scholar] [CrossRef]
- Van Duinen, V.; Trietsch, S.J.; Joore, J.; Vulto, P.; Hankemeier, T. Microfluidic 3D cell culture: From tools to tissue models. Curr. Opin. Biotechnol. 2015, 35, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Ott, C.; Heinmöller, E.; Gaumann, A.; Schölmerich, J.; Klebl, F. Intraepitheliale neoplasien (PanIN) und intraduktale papillär-muzinöse neoplasien (IPMN) des pankreas als vorläufer des pankreaskarzinoms. Med. Klin. 2007, 102, 127–135. [Google Scholar] [CrossRef]
- Xu, H.; Lyu, X.; Yi, M.; Zhao, W.; Song, Y.; Wu, K. Organoid technology and applications in cancer research. J. Hematol. Oncol. 2018, 11, 116. [Google Scholar] [CrossRef]
- Fujii, M.; Shimokawa, M.; Date, S.; Takano, A.; Matano, M.; Nanki, K.; Ohta, Y.; Toshimitsu, K.; Nakazato, Y.; Kawasaki, K.; et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 2016, 18, 827–838. [Google Scholar] [CrossRef]
- Barbone, D.; Yang, T.-M.; Morgan, J.R.; Gaudino, G.; Broaddus, V.C. Mammalian target of rapamycin contributes to the acquired apoptotic resistance of human mesothelioma multicellular spheroids. J. Biol. Chem. 2008, 283, 13021–13030. [Google Scholar] [CrossRef]
- Frankel, A.; Man, S.; Elliott, P.; Adams, J.; Kerbel, R.S. Lack of multicellular drug resistance observed in human ovarian and prostate carcinoma treated with the proteasome inhibitor PS-341. Clin. Cancer Res. 2000, 6, 3719–3728. [Google Scholar]
- Howes, A.L.; Chiang, G.G.; Lang, E.S.; Ho, C.B.; Powis, G.; Vuori, K.; Abraham, R.T. The phosphatidylinositol 3-kinase inhibitor, PX-866, is a potent inhibitor of cancer cell motility and growth in three-dimensional cultures. Mol. Cancer Ther. 2007, 6, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- De Witt Hamer, P.C.; Leenstra, S.; Van Noorden, C.J.F.; Zwinderman, A.H. Organotypic glioma spheroids for screening of experimental therapies: How many spheroids and sections are required? Cytometry A 2009, 75, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Laube, M.; Kniess, T.; Pietzsch, J. Development of antioxidant COX-2 inhibitors as radioprotective agents for radiation therapy—A hypothesis-driven review. Antioxidants 2016, 5, 14. [Google Scholar] [CrossRef]
- Kempf, H.; Bleicher, M.; Meyer-Hermann, M. Spatio-temporal cell dynamics in tumour spheroid irradiation. Eur. Phys. J. D 2010, 60, 177–193. [Google Scholar] [CrossRef]
- Sham, E.; Durand, R.E. Cell kinetics and repopulation mechanisms during multifraction irradiation of spheroids. Radiother. Oncol. 1998, 46, 201–207. [Google Scholar] [CrossRef]
- Azzam, E.I.; Little, J.B. The radiation-induced bystander effect: Evidence and significance. Hum. Exp. Toxicol. 2004, 23, 61–65. [Google Scholar] [CrossRef]
- Bishayee, A.; Rao, D.V.; Howell, R.W. Evidence for pronounced bystander effects caused by nonuniform distributions of radioactivity using a novel three-dimensional tissue culture model. Radiat. Res. 1999, 152, 88–97. [Google Scholar] [CrossRef]
- Durand, R.E.; Olive, P.L. Resistance of tumor cells to chemo- and radiotherapy modulated by the three-dimensional architecture of solid tumors and spheroids. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2001; Volume 64, pp. 211–233. ISBN 978-0-12-544167-4. [Google Scholar]
- Schwachöfer, J.H. Multicellular tumor spheroids in radiotherapy research (review). Anticancer Res. 1990, 10, 963–969. [Google Scholar]
- Zschenker, O.; Streichert, T.; Hehlgans, S.; Cordes, N. Genome-wide gene expression analysis in cancer cells reveals 3D growth to affect ECM and processes associated with cell adhesion but not DNA repair. PLoS ONE 2012, 7, e34279. [Google Scholar] [CrossRef] [PubMed]
- Neshasteh-Riz, A.; Angerson, W.; Reeves, J.; Smith, G.; Rampling, R.; Mairs, R. Incorporation of iododeoxyuridine in multicellular glioma spheroids: Implications for DNA-targeted radiotherapy using Auger electron emitters. Br. J. Cancer 1997, 75, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Keyomarsi, K. Role of cell cycle in mediating sensitivity to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 928–942. [Google Scholar] [CrossRef]
- Al-Ramadan, A.; Mortensen, A.; Carlsson, J.; Nestor, M. Analysis of radiation effects in two irradiated tumor spheroid models. Oncol. Lett. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kempf, H.; Hatzikirou, H.; Bleicher, M.; Meyer-Hermann, M. In silico analysis of cell cycle synchronisation effects in radiotherapy of tumour spheroids. PLoS Comput. Biol. 2013, 9, e1003295. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.; Senkowski, W.; Fryknäs, M.; Mansoori, S.; Linder, S.; Gullbo, J.; Larsson, R.; Nygren, P. A novel tumor spheroid model identifies selective enhancement of radiation by an inhibitor of oxidative phosphorylation. Oncotarget 2019, 10, 5372–5382. [Google Scholar] [CrossRef]
- Zoller, F.; Eisenhut, M.; Haberkorn, U.; Mier, W. Endoradiotherapy in cancer treatment—Basic concepts and future trends. Eur. J. Pharmacol. 2009, 625, 55–62. [Google Scholar] [CrossRef]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Controlled Release 2012, 164, 192–204. [Google Scholar] [CrossRef]
- Gaze, M.N.; Mairs, R.J.; Boyack, S.M.; Wheldon, T.E.; Barrett, A. 131I-meta-iodobenzylguanidine therapy in neuroblastoma spheroids of different sizes. Br. J. Cancer 1992, 66, 1048–1052. [Google Scholar] [CrossRef]
- Sihver, W.; Pietzsch, J.; Krause, M.; Baumann, M.; Steinbach, J.; Pietzsch, H.-J. Radiolabeled cetuximab conjugates for EGFR targeted cancer diagnostics and therapy. Pharmaceuticals 2014, 7, 311–338. [Google Scholar] [CrossRef]
- Volkert, W.A.; Goeckeler, W.F.; Ehrhardt, G.J.; Ketring, A.R. Therapeutic radionuclides: Production and decay property considerations. J. Nucl. Med. 1991, 32, 174–185. [Google Scholar] [PubMed]
- Kassis, A.I. Therapeutic radionuclides: Biophysical and radiobiologic principles. Semin. Nucl. Med. 2008, 38, 358–366. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.; Breeman, W.A.P.; Valkema, R.; Bernard, B.F.; Krenning, E.P. Combination radionuclide therapy using 177Lu- and 90Y-labeled somatostatin analogs. J. Nucl. Med. 2005, 46 (Suppl. S1), 13S–17S. [Google Scholar] [PubMed]
- McMillan, D.D.; Maeda, J.; Bell, J.J.; Genet, M.D.; Phoonswadi, G.; Mann, K.A.; Kraft, S.L.; Kitamura, H.; Fujimori, A.; Yoshii, Y.; et al. Validation of 64Cu-ATSM damaging DNA via high-LET Auger electron emission. J. Radiat. Res. 2015, 56, 784–791. [Google Scholar] [CrossRef]
- Mairs, R.J.; Fullerton, N.E.; Cosimo, E.; Boyd, M. Gene manipulation to enhance MIBG-targeted radionuclide therapy. Nucl. Med. Biol. 2005, 32, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Wheldon, T.E. Radiation physics and genetic targeting: New directions for radiotherapy. Phys. Med. Biol. 2000, 45, R77–R95. [Google Scholar] [CrossRef] [PubMed]
- Rae, C.; Tesson, M.; Babich, J.W.; Boyd, M.; Sorensen, A.; Mairs, R.J. The role of copper in disulfiram-induced toxicity and radiosensitization of cancer cells. J. Nucl. Med. 2013, 54, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Rae, C.; Mairs, R.J. Evaluation of the radiosensitizing potency of chemotherapeutic agents in prostate cancer cells. Int. J. Radiat. Biol. 2017, 93, 194–203. [Google Scholar] [CrossRef]
- Lundsten, S.; Spiegelberg, D.; Stenerlöw, B.; Nestor, M. The HSP90 inhibitor onalespib potentiates 177Lu-DOTATATE therapy in neuroendocrine tumor cells. Int. J. Oncol. 2019, 55, 1287–1295. [Google Scholar] [CrossRef]
- De Kruijff, R.M.; van der Meer, A.J.G.M.; Windmeijer, C.A.A.; Kouwenberg, J.J.M.; Morgenstern, A.; Bruchertseifer, F.; Sminia, P.; Denkova, A.G. The therapeutic potential of polymersomes loaded with 225Ac evaluated in 2D and 3D in vitro glioma models. Eur. J. Pharm. Biopharm. 2018, 127, 85–91. [Google Scholar] [CrossRef]
- Wang, J.; Abbas Rizvi, S.M.; Madigan, M.C.; Cozzi, P.J.; Power, C.A.; Qu, C.F.; Morgenstern, A.; Apostolidis, C.; Russell, P.J.; Allen, B.J.; et al. Control of prostate cancer spheroid growth using213Bi-labeled multiple targeted α radioimmunoconjugates. Prostate 2006, 66, 1753–1767. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.F.; Song, Y.J.; Rizvi, S.M.A.; Li, Y.; Smith, R.; Perkins, A.; Morgenstern, A.; Brechbiel, M.W.; Allen, B.J. In vivo and in vitro inhibition of pancreatic cancer growth by targeted alpha therapy using 213Bi-CHX.A”-C595. Cancer Biol. Ther. 2005, 4, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.J.; Rizvi, S.M.A.; Qu, C.F.; Smith, R.C. Targeted alpha therapy approach to the management of pancreatic cancer. Cancers 2011, 3, 1821–1843. [Google Scholar] [CrossRef]
- Song, H.; Shahverdi, K.; Huso, D.L.; Esaias, C.; Fox, J.; Liedy, A.; Zhang, Z.; Reilly, R.T.; Apostolidis, C.; Morgenstern, A.; et al. 213Bi (-Emitter)-Antibody Targeting of Breast Cancer Metastases in the neu-N Transgenic Mouse Model. Cancer Res. 2008, 68, 3873–3880. [Google Scholar] [CrossRef]
- Kennel, S.J.; Stabin, M.; Roeske, J.C.; Foote, L.J.; Lankford, P.K.; Terzaghi-Howe, M.; Patterson, H.; Barkenbus, J.; Popp, D.M.; Boll, R.; et al. Radiotoxicity of bismuth-213 bound to membranes of monolayer and spheroid cultures of tumor cells. Radiat. Res. 1999, 151, 244. [Google Scholar] [CrossRef]
- Koi, L.; Bergmann, R.; Brüchner, K.; Pietzsch, J.; Pietzsch, H.-J.; Krause, M.; Steinbach, J.; Zips, D.; Baumann, M. Radiolabeled anti-EGFR-antibody improves local tumor control after external beam radiotherapy and offers theragnostic potential. Radiother. Oncol. 2014, 110, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Ingargiola, M.; Runge, R.; Heldt, J.-M.; Freudenberg, R.; Steinbach, J.; Cordes, N.; Baumann, M.; Kotzerke, J.; Brockhoff, G.; Kunz-Schughart, L.A. Potential of a Cetuximab-based radioimmunotherapy combined with external irradiation manifests in a 3-D cell assay: Potential of EGFR-based RIT with X-ray. Int. J. Cancer 2014, 135, 968–980. [Google Scholar] [CrossRef]
- Kasten, B.B.; Gangrade, A.; Kim, H.; Fan, J.; Ferrone, S.; Ferrone, C.R.; Zinn, K.R.; Buchsbaum, D.J. 212Pb-labeled B7-H3-targeting antibody for pancreatic cancer therapy in mouse models. Nucl. Med. Biol. 2018, 58, 67–73. [Google Scholar] [CrossRef]
- Monazzam, A.; Razifar, P.; Ide, S.; Rugaard Jensen, M.; Josephsson, R.; Blomqvist, C.; Langström, B.; Bergström, M. Evaluation of the Hsp90 inhibitor NVP-AUY922 in multicellular tumour spheroids with respect to effects on growth and PET tracer uptake. Nucl. Med. Biol. 2009, 36, 335–342. [Google Scholar] [CrossRef]
- Senavirathna, L.K.; Fernando, R.; Maples, D.; Zheng, Y.; Polf, J.C.; Ranjan, A. Tumor spheroids as an in vitro model for determining the therapeutic response to proton beam radiotherapy and thermally sensitive nanocarriers. Theranostics 2013, 3, 687–691. [Google Scholar] [CrossRef]
- Friedrich, J.; Eder, W.; Castaneda, J.; Doss, M.; Huber, E.; Ebner, R.; Kunz-Schughart, L.A. A reliable tool to determine cell viability in complex 3-D culture: The acid phosphatase assay. J. Biomol. Screen. 2007, 12, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Liao, Q.; Hu, Y.; You, L.; Zhou, L.; Zhao, Y. A spheroid-based 3-D culture model for pancreatic cancer drug testing, using the acid phosphatase assay. Braz. J. Med. Biol. Res. 2013, 46, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Qvarnström, O.F.; Simonsson, M.; Eriksson, V.; Turesson, I.; Carlsson, J. γH2AX and cleaved PARP-1 as apoptotic markers in irradiated breast cancer BT474 cellular spheroids. Int. J. Oncol. 2009, 35, 41–47. [Google Scholar] [CrossRef]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Furukawa, T.; Yuan, Q.; Jin, Z.-H.; Aung, W.; Yoshii, Y.; Hasegawa, S.; Endo, H.; Inoue, M.; Zhang, M.-R.; Fujibayashi, Y.; et al. A limited overlap between intratumoral distribution of 1-(5-fluoro-5-deoxy-α-D-arabinofuranosyl)-2-nitroimidazole and copper-diacetyl-bis[N(4)-methylthiosemicarbazone]. Oncol. Rep. 2015, 34, 1379–1387. [Google Scholar] [CrossRef]
- Litos, L.-M. The Evaluation of PET-Tracer Accumulation in Multicellular Tumor Spheroids. Ph.D. Thesis, University of Vienna, Vienna, Austria, 2017. [Google Scholar]
- Kniess, T.; Laube, M.; Bergmann, R.; Sehn, F.; Graf, F.; Steinbach, J.; Wuest, F.; Pietzsch, J. Radiosynthesis of a 18F-labeled 2,3-diarylsubstituted indole via McMurry coupling for functional characterization of cyclooxygenase-2 (COX-2) in vitro and in vivo. Bioorg. Med. Chem. 2012, 20, 3410–3421. [Google Scholar] [CrossRef]
- Reissenweber, B.; Mosch, B.; Pietzsch, J. Experimental hypoxia does not influence gene expression and protein synthesis of Eph receptors and ephrin ligands in human melanoma cells in vitro. Melanoma Res. 2013, 23, 85–95. [Google Scholar] [CrossRef]
- Rasey, J.S.; Nelson, N.J.; Chin, L.; Evans, M.L.; Grunbaum, Z. Characteristics of the binding of labeled fluoromisonidazole in cells in vitro. Radiat. Res. 1990, 122, 301. [Google Scholar] [CrossRef]
- Gross, M.W.; Karbach, U.; Groebe, K.; Franko, A.J.; Mueller-Klieser, W. Calibration of misonidazole labeling by simultaneous measurement of oxygen tension and labeling density in multicellular spheroids. Int. J. Cancer 1995, 61, 567–573. [Google Scholar] [CrossRef]
- Monazzam, A.; Razifar, P.; Simonsson, M.; Qvarnström, F.; Josephsson, R.; Blomqvist, C.; Långström, B.; Bergström, M. Multicellular tumour spheroid as a model for evaluation of [18F]FDG as biomarker for breast cancer treatment monitoring. Cancer Cell Int. 2006, 6, 6. [Google Scholar] [CrossRef]
- Senekowitsch-Schmidtke, R.; Matzen, K.; Truckenbrodt, R.; Mattes, J.; Heiss, P.; Schwaiger, M. Tumor cell spheroids as a model for evaluation of metabolic changes after irradiation. J. Nucl. Med. 1998, 39, 1762–1768. [Google Scholar] [PubMed]
- Mairs, R.; Angerson, W.; Gaze, M.; Murray, T.; Babich, J.; Reid, R.; McSharry, C. The distribution of alternative agents for targeted radiotherapy within human neuroblastoma spheroids. Br. J. Cancer 1991, 63, 404–409. [Google Scholar] [CrossRef]
- Monazzam, A.; Razifar, P.; Lindhe, Ö.; Josephsson, R.; Långström, B.; Bergström, M. A new, fast and semi-automated size determination method (SASDM) for studying multicellular tumor spheroids. Cancer Cell Int. 2005, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Monazzam, A.; Josephsson, R.; Blomqvist, C.; Carlsson, J.; Långström, B.; Bergström, M. Application of the multicellular tumour spheroid model to screen PET tracers for analysis of early response of chemotherapy in breast cancer. Breast Cancer Res. 2007, 9, R45. [Google Scholar] [CrossRef] [PubMed]
- Laube, M.; Kniess, T.; Pietzsch, J. Radiolabeled COX-2 inhibitors for non-invasive visualization of COX-2 expression and activity—A critical update. Molecules 2013, 18, 6311–6355. [Google Scholar] [CrossRef] [PubMed]
- Servente, L.; Bianco, C.; Gigirey, V.; Alonso, O. Imaging benign pathology and variants with uptake in 68ga-Dotatate PET/CT studies. Rev. Argent. Radiol. 2017, 81, 184–191. [Google Scholar] [CrossRef]
- Lakhotia, R.; Jhawar, S.; Malayeri, A.A.; Millo, C.; Del Rivero, J.; Ahlman, M.A. Incidental 68Ga-DOTATATE uptake in the pancreatic head: A case report and a unique opportunity to improve clinical care. Medicine 2020, 99, e20197. [Google Scholar] [CrossRef]
- Barrio, M.; Ceppa, E.P. Diagnosing microscopic pancreatic neuroendocrine tumor using 68-Ga-DOTATATE PET/CT: Case series. J. Surg. Case Rep. 2018, 2018, rjy237. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, P.; Ji, H.; Long, Y.; Jing, B.; Wan, L.; Xi, D.; An, R.; Lan, X. A mini-panel PET scanner-based microfluidic radiobioassay system allowing high-throughput imaging of real-time cellular pharmacokinetics. Lab Chip 2020, 20, 1110–1123. [Google Scholar] [CrossRef]
| 3D Model | Technique | Literature |
|---|---|---|
| Multicellular spheroids | Scaffold: Matrigel and collagen | [34,35,36,37] |
| Scaffold: Methylcellulose | [38,39] | |
| NanoCulture plates | [40] | |
| Hyaluronan/chitosan coated plates | [41] | |
| Ultralow attachment plates | [42] | |
| concave polydimethylsiloxane microwell plates | [43] | |
| Hanging drop | [38,44,45] | |
| Organoids | Mechanical and chemical dissociation | [46,47,48,49] |
| Co-cultures | Cultivation with stromal cells | [42,50] |
| Cultivation with stellate cells | [51] | |
| Triple culture with fibroblasts and monocytes | [52,53] | |
| Triple culture with fibroblasts and endothelial cells | [54] | |
| Biotechnical microsystems | Microfluidic chip | [55] |
| Microfluidic plate | [56] | |
| HepaChip® | [57,58] | |
| Magnetic bioprinting | [59] |
| 3D Model | Field of Interest | Literature |
|---|---|---|
| Multicellular spheroids | Protein levels | [40,65,66] |
| Hypoxia marker | [38] | |
| Drug screening | [42,43,67,68] | |
| MicroRNA expression | [43] | |
| qRT-PCR | [43] | |
| Organoids | KRAS or other genetic mutation | [46,69] |
| Orthotopic transplantation | [48,70,71] | |
| Subcutaneous transplantation | [70] | |
| PanIN development | [48] | |
| Co-cultures | Invasive behavior | [50,70] |
| Therapy resistance | [68,72,73] | |
| Migration | [41,74] | |
| EMT marker expression | [74] | |
| Proliferation | [73] | |
| T-cell inhibition | [52] | |
| Protein expression | [73] | |
| Signaling pathways | [37] | |
| Biotechnical microsystems | Drug response | [56,57,58] |
| Marker and factor expression | [56] | |
| Hypoxia | [75] |
| Therapeutics | Tumor Entity | Target | Treatment Combinations | Literature |
|---|---|---|---|---|
| [211At]MABG | neuroblastoma | norepinephrine receptor | [151] | |
| [131I]MIGB | glioma neuroblastoma | norepinephrine receptor | radiosensitizer Disulfiram | [145,152,153] |
| [131I]MIP-1145 | melanoma | melanin uptake | radiosensitizer topotecan, AG014699 | [154] |
| [125I]IUdR | glioblastoma | DNA (S-phase) | [138] | |
| [177Lu]Lu-DOTATATE | pancreatic neuroendocrine tumor, lung cancer | somatostatin analogue | radiosensitizer onalespib | [155] |
| [225Ac]DTPA | glioblastoma | DNA | [156] | |
| [213Bi]C595 AC | prostate cancer, pancreatic cancer | mucin1 | [157,158,159] | |
| [213Bi]BLCA-38 AC | prostate cancer | unknown glycoprotein | [157] | |
| [213Bi]PAI2 AC | prostate cancer | urokinase plasminogen activator | [157] | |
| [213Bi]7.16.4 | breast cancer | HER-2/neu | [160] | |
| [213Bi]Mab 13A | murine breast cancer | CD44 | [161] | |
| [90Y]Mab 13A | murine breast cancer | CD44 | [161] | |
| [90Y]cetuximab | human squamous cell carcinoma models | epidermal growth factor receptor | External radiation | [162] |
| [90Y]C225 | Head and neck squamous cell carcinoma | epidermal growth factor receptor | External radiation | [163] |
| [212Pb]mAb 376.96 | pancreatic ductal adenocarcinoma | B7-H3 (CD276) | [164] |
| Tracer | Tumor Entity | Application | Reference |
|---|---|---|---|
| [18F]FDG | pheochromocytoma, breast cancer, colorectal adenocarcinoma, colorectal carcinoma, glioblastoma, colon carcinoma | Glucose turnover, viability, metabolic activity | [24,165,172,177] |
| [18F]FMISO | colorectal adenocarcinoma, colorectal carcinoma, melanoma | Hypoxia | [172,173,174] |
| [18F]OFED | pheochromocytoma | Large neutral amino-acid transporter | [24] |
| [18F]FE@SUPPY | colorectal adenocarcinoma, colorectal carcinoma | A3-Adenosine receptor | [172] |
| [18F]3 | colorectal adenocarcinoma | Cyclooxygenase-2 | [173] |
| [18F]FLT | breast cancer, glioblastoma, colon carcinoma | Proliferation | [165] |
| [18F]FAZA | colon adenocarcinoma, lung squamous cell carcinoma, lung adenocarcinoma | Hypoxia | [171] |
| [68Ga]Ga-DOTATATE | pheochromocytoma | Somatostatin receptor 2/5 | [24] |
| [3H]Methionine | rectal adenocarcinoma | Protein synthesis | [178] |
| [3H]Thymidine | rectal adenocarcinoma | Proliferation | [178] |
| [14C]FDG | rectal adenocarcinoma | Glucose turnover, viability, metabolic activity | [178] |
| [11C]Methionine | breast cancer, glioblastoma, colon carcinoma | Protein synthesis | [165] |
| [11C]Choline | breast cancer, glioblastoma, colon carcinoma | Membrane lipid synthesis | [165] |
| [64Cu]ATSM | colon adenocarcinoma, lung squamous cell carcinoma, lung adenocarcinoma | Hypoxia | [171] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doctor, A.; Seifert, V.; Ullrich, M.; Hauser, S.; Pietzsch, J. Three-Dimensional Cell Culture Systems in Radiopharmaceutical Cancer Research. Cancers 2020, 12, 2765. https://doi.org/10.3390/cancers12102765
Doctor A, Seifert V, Ullrich M, Hauser S, Pietzsch J. Three-Dimensional Cell Culture Systems in Radiopharmaceutical Cancer Research. Cancers. 2020; 12(10):2765. https://doi.org/10.3390/cancers12102765
Chicago/Turabian StyleDoctor, Alina, Verena Seifert, Martin Ullrich, Sandra Hauser, and Jens Pietzsch. 2020. "Three-Dimensional Cell Culture Systems in Radiopharmaceutical Cancer Research" Cancers 12, no. 10: 2765. https://doi.org/10.3390/cancers12102765
APA StyleDoctor, A., Seifert, V., Ullrich, M., Hauser, S., & Pietzsch, J. (2020). Three-Dimensional Cell Culture Systems in Radiopharmaceutical Cancer Research. Cancers, 12(10), 2765. https://doi.org/10.3390/cancers12102765