Conversion Surgery in Metastatic Gastric Cancer and Cancer Dormancy as a Prognostic Biomarker
Abstract
1. Introduction
2. Results
2.1. Patients’ Demographic Data
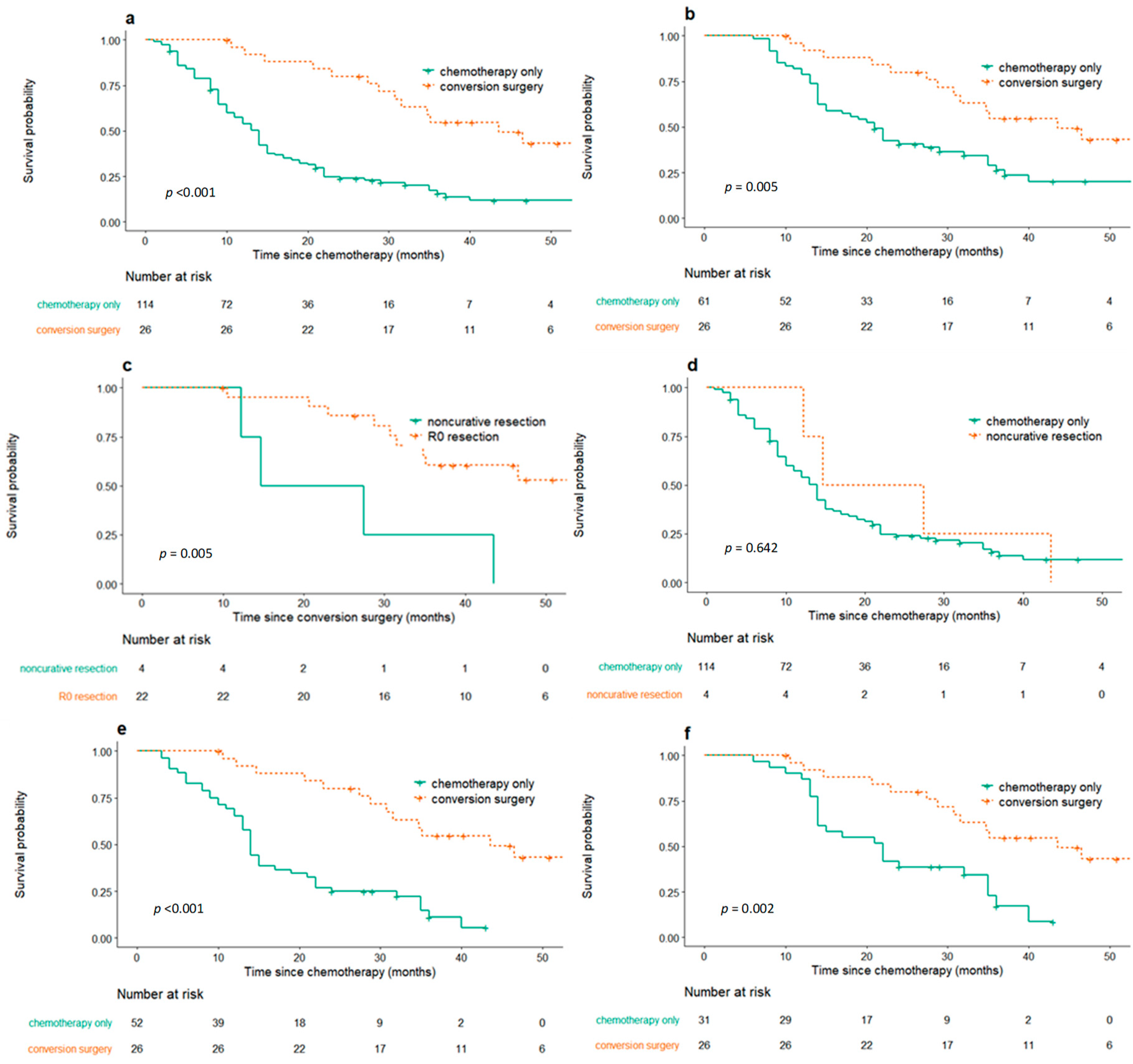
2.2. Survival Outcomes of Conversion Surgery and Palliative Chemotherapy Only Group
2.3. Propensity Score Matching Analysis
2.4. Mortality and Morbidity of Conversion Surgery
2.5. Cancer Dormancy Marker Expression
3. Discussion
4. Patients and Methods
4.1. Patients with Conversion Surgery
4.2. Control Patients for Propensity Score Analysis
4.3. Data Collection
4.4. Expression of Cancer Dormancy Marker
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Lee, E.S. Prediction of Cancer Incidence and Mortality in Korea, 2019. Cancer Res. Treat. 2019, 51, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean Practice Guideline for Gastric Cancer 2018: An Evidence-based, Multi-disciplinary Approach. J. Gastric Cancer 2019, 19, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, K.; Yang, H.K.; Mizusawa, J.; Kim, Y.W.; Terashima, M.; Han, S.U.; Iwasaki, Y.; Hyung, W.J.; Takagane, A.; Park, D.J.; et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): A phase 3, randomised controlled trial. Lancet Oncol. 2016, 17, 309–318. [Google Scholar] [CrossRef]
- Du, R.; Hu, P.; Liu, Q.; Zhang, J. Conversion Surgery for Unresectable Advanced Gastric Cancer: A Systematic Review and Meta-Analysis. Cancer Investig. 2019, 37, 16–28. [Google Scholar] [CrossRef]
- Einama, T.; Abe, H.; Shichi, S.; Matsui, H.; Kanazawa, R.; Shibuya, K.; Suzuki, T.; Matsuzawa, F.; Hashimoto, T.; Kohei, N.; et al. Long-term survival and prognosis associated with conversion surgery in patients with metastatic gastric cancer. Mol. Clin. Oncol. 2017, 6, 163–166. [Google Scholar] [CrossRef][Green Version]
- Fukuchi, M.; Mochiki, E.; Ishiguro, T.; Kumagai, Y.; Ishibashi, K.; Ishida, H. Prognostic Significance of Conversion Surgery Following First- or Second-line Chemotherapy for Unresectable Gastric Cancer. Anticancer Res. 2018, 38, 6473–6478. [Google Scholar] [CrossRef]
- Fukuchi, M.; Mochiki, E.; Ishiguro, T.; Ogura, T.; Sobajima, J.; Kumagai, Y.; Ishibashi, K.; Ishida, H. Efficacy of Conversion Surgery Following S-1 plus Cisplatin or Oxaliplatin Chemotherapy for Unresectable Gastric Cancer. Anticancer Res. 2017, 37, 1343–1347. [Google Scholar]
- Kim, K.H.; Lee, K.W.; Baek, S.K.; Chang, H.J.; Kim, Y.J.; Park, D.J.; Kim, J.H.; Kim, H.H.; Lee, J.S. Survival benefit of gastrectomy ± metastasectomy in patients with metastatic gastric cancer receiving chemotherapy. Gastric Cancer 2011, 14, 130–138. [Google Scholar] [CrossRef][Green Version]
- Mieno, H.; Yamashita, K.; Hosoda, K.; Moriya, H.; Higuchi, K.; Azuma, M.; Komori, S.; Yoshida, T.; Tanabe, S.; Koizumi, W.; et al. Conversion surgery after combination chemotherapy of docetaxel, cisplatin and S-1 (DCS) for far-advanced gastric cancer. Surg. Today 2017, 47, 1249–1258. [Google Scholar] [CrossRef]
- Morgagni, P.; Solaini, L.; Framarini, M.; Vittimberga, G.; Gardini, A.; Tringali, D.; Valgiusti, M.; Monti, M.; Ercolani, G. Conversion surgery for gastric cancer: A cohort study from a western center. Int. J. Surg. 2018, 53, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yamaguchi, K.; Okumura, N.; Tanahashi, T.; Kodera, Y. Is conversion therapy possible in stage IV gastric cancer: The proposal of new biological categories of classification. Gastric Cancer 2016, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Paez, D.; Labonte, M.J.; Bohanes, P.; Zhang, W.; Benhanim, L.; Ning, Y.; Wakatsuki, T.; Loupakis, F.; Lenz, H.J. Cancer dormancy: A model of early dissemination and late cancer recurrence. Clin. Cancer Res. 2012, 18, 645–653. [Google Scholar] [CrossRef]
- Katayama, H.; Kurokawa, Y.; Nakamura, K.; Ito, H.; Kanemitsu, Y.; Masuda, N.; Tsubosa, Y.; Satoh, T.; Yokomizo, A.; Fukuda, H.; et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg. Today 2016, 46, 668–685. [Google Scholar] [CrossRef]
- Cook, J.A. The challenges faced in the design, conduct and analysis of surgical randomised controlled trials. Trials 2009, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W. The result of conversion surgery in gastric cancer patients with peritoneal seeding. J. Gastric Cancer 2014, 14, 266–270. [Google Scholar] [CrossRef]
- Fukuchi, M.; Ishiguro, T.; Ogata, K.; Kimura, A.; Kumagai, Y.; Ishibashi, K.; Ishida, H.; Kuwano, H.; Mochiki, E. Risk Factors for Recurrence After Curative Conversion Surgery for Unresectable Gastric Cancer. Anticancer Res. 2015, 35, 6183–6187. [Google Scholar]
- Fukuchi, M.; Ishiguro, T.; Ogata, K.; Suzuki, O.; Kumagai, Y.; Ishibashi, K.; Ishida, H.; Kuwano, H.; Mochiki, E. Prognostic Role of Conversion Surgery for Unresectable Gastric Cancer. Ann. Surg. Oncol. 2015, 22, 3618–3624. [Google Scholar] [CrossRef]
- Nakamura, M.; Ojima, T.; Nakamori, M.; Katsuda, M.; Tsuji, T.; Hayata, K.; Kato, T.; Yamaue, H. Conversion Surgery for Gastric Cancer with Peritoneal Metastasis Based on the Diagnosis of Second-Look Staging Laparoscopy. J. Gastrointest. Surg. 2019, 23, 1758–1766. [Google Scholar] [CrossRef]
- Beom, S.H.; Choi, Y.Y.; Baek, S.E.; Li, S.X.; Lim, J.S.; Son, T.; Kim, H.I.; Cheong, J.H.; Hyung, W.J.; Choi, S.H.; et al. Multidisciplinary treatment for patients with stage IV gastric cancer: The role of conversion surgery following chemotherapy. BMC Cancer 2018, 18, 1116. [Google Scholar] [CrossRef]
- Yuan, S.Q.; Nie, R.C.; Chen, S.; Chen, X.J.; Chen, Y.M.; Xu, L.P.; Yang, L.F.; Zhou, Z.W.; Peng, J.S.; Chen, Y.B. Selective Gastric Cancer Patients with Peritoneal Seeding Benefit from Gastrectomy after Palliative Chemotherapy: A Propensity Score Matching Analysis. J. Cancer 2017, 8, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Zurleni, T.; Gjoni, E.; Altomare, M.; Rausei, S. Conversion surgery for gastric cancer patients: A review. World J. Gastrointest. Oncol. 2018, 10, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Thompson, V.C.; Day, T.K.; Bianco-Miotto, T.; Selth, L.A.; Han, G.; Thomas, M.; Buchanan, G.; Scher, H.I.; Nelson, C.C.; Greenberg, N.M.; et al. A gene signature identified using a mouse model of androgen receptor-dependent prostate cancer predicts biochemical relapse in human disease. Int. J. Cancer 2012, 131, 662–672. [Google Scholar] [CrossRef]
- Borgen, E.; Rypdal, M.C.; Sosa, M.S.; Renolen, A.; Schlichting, E.; Lonning, P.E.; Synnestvedt, M.; Aguirre-Ghiso, J.A.; Naume, B. NR2F1 stratifies dormant disseminated tumor cells in breast cancer patients. Breast Cancer Res. 2018, 20, 120. [Google Scholar] [CrossRef]
- Miki, Y.; Tokunaga, M.; Tanizawa, Y.; Bando, E.; Kawamura, T.; Terashima, M. Staging Laparoscopy for Patients with cM0, Type 4, and Large Type 3 Gastric Cancer. World J. Surg. 2015, 39, 2742–2747. [Google Scholar] [CrossRef]
- Yasufuku, I.; Nunobe, S.; Ida, S.; Kumagai, K.; Ohashi, M.; Hiki, N.; Sano, T. Conversion therapy for peritoneal lavage cytology-positive type 4 and large type 3 gastric cancer patients selected as candidates for R0 resection by diagnostic staging laparoscopy. Gastric Cancer 2019. Epub ahead of print. [Google Scholar] [CrossRef]


| Variable | Conversion Surgery (n = 26) No. (%) | Chemotherapy Only | p-Value a | p-Value b | |
|---|---|---|---|---|---|
| Before Propensity Score Matching (n = 114) No. (%) | After Propensity Score Matching (n = 52) No. (%) | ||||
| Age (Median, Range) | 58 (39–78) | 61 (52–70) | 57 (52–68) | 0.855 | 1.000 |
| <70 years | 20 (76.9%) | 83 (72.8%) | 40 (76.9%) | ||
| ≥70 years | 6 (23.1%) | 31 (27.2%) | 12 (23.1%) | ||
| Sex | 0.829 | 0.514 | |||
| Male | 18 (69.2%) | 84 (73.7%) | 41 (78.8%) | ||
| Female | 8 (30.8%) | 30 (26.3%) | 11 (21.2%) | ||
| Metastatic site | 0.355 | 0.935 | |||
| Category 1–2 | 16 (61.5%) | 56 (49.1%) | 30 (57.7%) | ||
| Category 3–4 | 10 (38.5%) | 58 (50.9%) | 22 (42.3%) | ||
| 1st line palliative chemotherapy c | 0.011 | 0.180 | |||
| S1/capecitabine + cisplatin/oxaliplatin | 14 (53.8%) | 67 (58.8%) | 31 (59.6%) | ||
| FOLFOX | 5 (19.2%) | 36 (31.6%) | 15 (28.8%) | ||
| Herceptin + capecitabine + cisplatin | 7 (26.9%) | 6 (5.3%) | 4 (7.7%) | ||
| 5-fluorouracil + cisplatin | 0 (0.0%) | 4 (3.5%) | 1 (1.9%) | ||
| Docetaxel + 5-fluorouracil + cisplatin | 0 (0.0%) | 1 (0.9%) | 1 (1.9%) | ||
| Best tumor response | 0.298 | 1.000 | |||
| CR, PR | 17 (65.4%) | 59 (51.8%) | 34 (65.4%) | ||
| SD, NE | 9 (34.6%) | 55 (48.2%) | 18 (34.6%) | ||
| Variable | N (%) |
|---|---|
| Initial biological disease category before palliative chemotherapy | |
| Category 1 | 5 (19.2) |
| Category 2 | 11 (42.3) |
| Category 3 | 4 (15.4) |
| Category 4 | 6 (23.1) |
| Best tumor response before conversion surgery | |
| Complete response | 2 (7.7) |
| Partial response | 15 (57.7) |
| Stable disease | 3 (11.5) |
| Not evaluable | 6 (23.1) |
| Type of resection | |
| Subtotal gastrectomy | 11(42.3) |
| Total gastrectomy | 8 (30.8) |
| Extended total gastrectomy | 7 (26.9) |
| R0 resection | |
| R0 | 22 (84.6) |
| R2 | 4 (15.4) |
| Lymphatic invasion | |
| Not identified | 5 (19.2) |
| Present | 21 (80.8) |
| Vascular invasion | |
| Not identified | 15 (57.7) |
| Present | 11 (42.3) |
| Perineural invasion | |
| Not identified | 9 (34.6) |
| Present | 17 (65.4) |
| Lauren classification | |
| Intestinal | 13 (50.0) |
| Diffuse | 10 (38.5) |
| Indeterminate | 1 (3.8) |
| Others | 2 (7.7) |
| Histologic differentiation | |
| Tubular adenocarcionma | 16 (61.5) |
| Poorly cohesive carcinoma | 5 (16.2) |
| Papillary adenocarcinoma | 3 (11.5) |
| No tumor | 2 (7.7) |
| TNM a | |
| ypT | |
| 0 | 1 (3.8) |
| 1 | 2 (7.7) |
| 2 | 3 (11.5) |
| 3 | 12 (46.2) |
| 4 | 7 (26.9) |
| ypN | |
| 0 | 8 (30.8) |
| 1 | 2 (7.7) |
| 2 | 6 (23.1) |
| 3 | 10 (38.5) |
| Postoperative stage | |
| 0 | 2 (7.7) |
| I | 2 (7.7) |
| II | 3 (11.5) |
| III | 10 (38.5) |
| IV | 9 (34.6) |
| Case No. | Age (Years) | Sex | Initial Metastatic Sites | Initial Biological Category Before Palliative Chemotherapy | Initial Chemotherapy | Chemotherapy Duration Before Conversion Surgery (Months) | Chemotherapy Response | Operation | Curativity | TNM Stage | Maintenance Chemotherapy | Recur | Survival Status | Overall Survival (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | M | Liver | 2 | FOLFOX | 4.0 | PR | TG + D2 + intraoperative radiofrequency ablation (scar change) | R0 | pT2N1 | Yes | No | alive | 142.2 |
| 2 | 65 | F | Liver and pancreas invasion | 2 | FOLFOX | 3.8 | CR | STG + D2 | R0 | pT0N0 | No | No | alive | 91.2 |
| 3 | 45 | M | Peritoneal seeding, paraaortic LN | 4 | XELOX | 4.1 | PR | extended TG + D3 dissection | R0 | pT0N0 | Yes | No | alive | 66.5 |
| 4 | 46 | F | Retroperitoneal LN | 2 | XP | 3.1 | PR | STG + D3 | R0 | pT1N2 | No | Yes | alive | 61.8 |
| 5 | 56 | F | Portocaval LN | 2 | XP + Herceptin | 3.8 | SD | STG + D3 | R0 | pT3N3a | Yes | No | alive | 56.2 |
| 6 | 47 | F | Peritoneal seeding | 3 | XELOX | 4.7 | NE | TG + D2 | R0 | pT2N0 | Yes | Yes | alive | 50.9 |
| 7 | 43 | M | Retroperitoneal LN | 1 | XP + Herceptin | 4.9 | PR | STG + D3 | R0 | pT1N0 | Yes | No | alive | 47.6 |
| 8 | 51 | M | Peritoneal seeding, Retroperitoneal LN | 4 | TS1 + Cisplatin | 10.1 | SD | TG + D3 | R0 | pT4aN3bM1(LN #16b1, #14) | Yes | Yes | expired | 46.5 |
| 9 | 73 | M | Peritoneal seeding, Colon and pancreas invasion | 4 | FOLFOX | 3.2 | CR | STG + D2 | R0 | pT2N0M1 (LN #13) | No | No | alive | 46.0 |
| 10 | 65 | M | Retroperitoneal LN | 2 | XP | 11.1 | PR | STG + D3 | R2 | T3N2M1(residual lesion at cardia) | Yes | Yes | expired | 43.6 |
| 11 | 57 | M | Peritoneal seeding | 3 | XELOX | 13.9 | SD | TG + D2 | R0 | pT3N2 | No | Yes | alive | 40.3 |
| 12 | 76 | M | Pancreas body, and gallbladder invasion | 2 | XP + Herceptin | 4.4 | PR | STG + D2 + cholecystectomy + LN dissection (#8) | R0 | pT3N2 | Yes | No | alive | 38.5 |
| 13 | 56 | M | Retroperitoneal LN | 2 | TS1 + Cisplatin | 5.0 | NE | STG + D2 (no visual retroperitoneal LN) | R0 | pT3N0 | Yes | No | alive | 37.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choe, H.J.; Kim, J.W.; Han, S.-H.; Lee, J.H.; Ahn, S.-H.; Park, D.J.; Kim, J.-W.; Kim, Y.J.; Lee, H.S.; Kim, J.H.; et al. Conversion Surgery in Metastatic Gastric Cancer and Cancer Dormancy as a Prognostic Biomarker. Cancers 2020, 12, 86. https://doi.org/10.3390/cancers12010086
Choe HJ, Kim JW, Han S-H, Lee JH, Ahn S-H, Park DJ, Kim J-W, Kim YJ, Lee HS, Kim JH, et al. Conversion Surgery in Metastatic Gastric Cancer and Cancer Dormancy as a Prognostic Biomarker. Cancers. 2020; 12(1):86. https://doi.org/10.3390/cancers12010086
Chicago/Turabian StyleChoe, Hun Jee, Jin Won Kim, Song-Hee Han, Ju Hyun Lee, Sang-Hoon Ahn, Do Joong Park, Ji-Won Kim, Yu Jung Kim, Hye Seung Lee, Jee Hyun Kim, and et al. 2020. "Conversion Surgery in Metastatic Gastric Cancer and Cancer Dormancy as a Prognostic Biomarker" Cancers 12, no. 1: 86. https://doi.org/10.3390/cancers12010086
APA StyleChoe, H. J., Kim, J. W., Han, S.-H., Lee, J. H., Ahn, S.-H., Park, D. J., Kim, J.-W., Kim, Y. J., Lee, H. S., Kim, J. H., Kim, H.-H., & Lee, K.-W. (2020). Conversion Surgery in Metastatic Gastric Cancer and Cancer Dormancy as a Prognostic Biomarker. Cancers, 12(1), 86. https://doi.org/10.3390/cancers12010086





