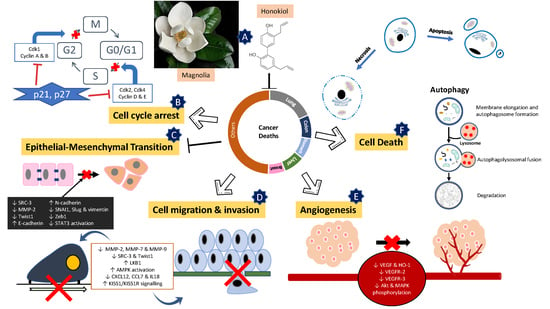
Honokiol: A Review of Its Anticancer Potential and Mechanisms
Abstract
1. Introduction
2. Research Methodology
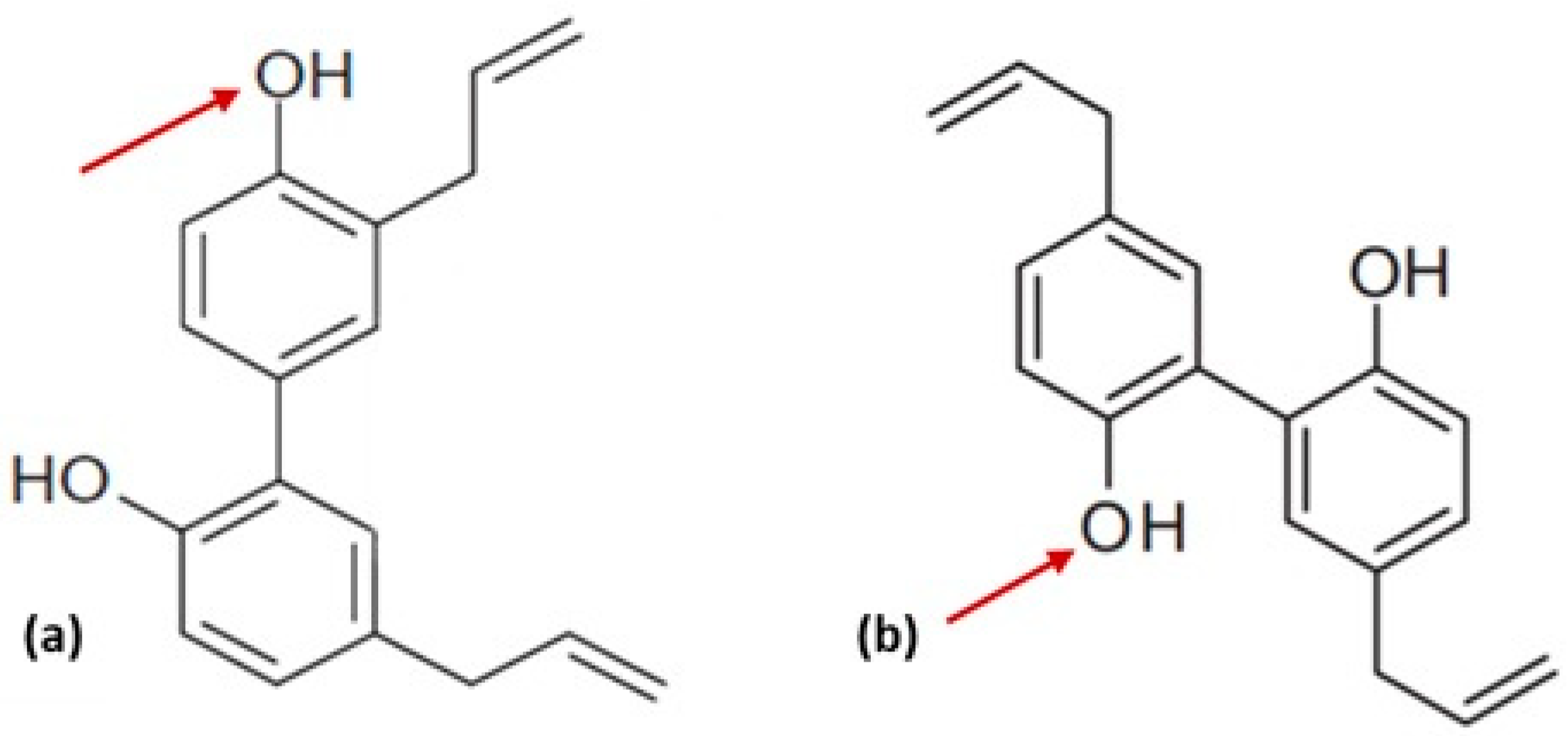
3. Structure Activity Relationship and Its Derivatives
4. Anticancer Properties of Honokiol
4.1. In Vitro Studies
| Cell Lines | Mechanism of Action | Concentration Used | Efficacy/IC50 (Exposure Time) | References | |
|---|---|---|---|---|---|
| Colorectal cancer | RKO | Inhibit cell proliferation Induce G1 phase cell cycle arrest Induce apoptosis ↓ Bcl-xL; ↑ Caspase-3 & caspase-9 | 0–150 μM | 46.76 μM (68 h) | [33] |
| HCT116, HCT116-CH2, HCT116-CH3 | Inhibit cell proliferation Induce G0/G1 & G2/M phase cell cycle arrest: ↓ cyclin D1 & A1; ↑ p53 phosphorylation Induce apoptosis: ↓ Caspase-3; ↓ Bcl-2; ↑ Bax protein | 25 μM Honokiol with 2.5 or 5.0 Gy IR | N/A | [34] | |
| HT-29 | Inhibit cell growth & proliferation Induce G1 phase cell cycle arrest: ↓ Cdk1 & cyclin B1 | 0–50 μM followed by 0–5 Gy IR | 23.05 μM (24 h) 13.24 μM (72 h) | [24] | |
| HCT116 & SW480 | Inhibit cell proliferation via Inhibition of Notch signalling: ↓ Notch1 & Jagged-1; ↓ Hey-1 & Hes1; ↓ γ-secretase complex; ↓ Skip1 Induce apoptosis: ↑ caspase-3/-7 activity; ↓ Bcl-2 & Bcl-xL; ↑ Bax protein; ↓ cyclin D1 & c-Myc; ↑ p21WAF1 protein Inhibit primary and secondary colonosphere formation | 0–50 μM | N/A | [35] | |
| RKO & HCT116 | Inhibit cell viability Induce apoptosis: ↑ caspase-3, caspase-8 & caspase-9 activation; ↑ DR5 & cleaved PARP proteins; ↑ survivin protein; ↑ phosphorylated p53 & p53 proteins; ↓ PUMA protein | 0–60 μM | RKO: 38.25 μM (24 h) HCT116: 39.64 μM (24 h) | [36] | |
| Blood cancer | B-CLL | Inhibit cell viability Induce apoptosis: ↑ caspase-3 activity; ↑ caspase-8 & caspase-9 activation; ↓ caspase-9; ↑ Bax protein; ↓ Mcl-1 protein | 0–100 μM | 49 μM (6 h) 38 μM (24 h) | [37] |
| Raji, Molt-4 | Inhibit cell growth: ↓ p65; ↓ NF-κB Induce apoptosis: ↑ JNK activation Increase ROS activity: ↑ Nrf2 & c-Jun protein activation | 0–2.5 μM | Raji: 3.500 μM (24 h) 0.092 μM (72 h) Molt-4: 0.521 μM (24 h) | [38] | |
| Breast cancer | MCF-7, MDA-MB-231, SKBR-3, ZR-75-1, BT-474 | Inhibit cell viability and growth: ↓ EFGR; ↓ MAPK/PI3K pathway activity Induce apoptosis: ↑ PARP protein degradation; ↓ caspase-8; ↑ Bax proteins Induce G1 phase cell cycle arrest: ↓ cyclin D1; ↑ p21 & p27 | 0–100 μM | MCF-7: 40 μM (24 h) MDA-MB-231: 33 μM (24 h) SKBR-3: 29 μM (24 h) ZR-75-1: 39 μM (24 h) BT-474: 50 μM (24 h) | [39] |
| MCF-7, MDA-MB-231 | Inhibit cell clonogenicity Inhibit cell anchorage-dependent colony formation Inhibit cell growth, migration & invasion: ↓ pS6K & 4EBP1 phosphorylation; ↑ AMPK activation; ↓ mTORC1 function; ↑ LKB1 & cytosolic localisation | 1–25 μM | N/A | [40] | |
| MCF-7, MDA-MB-231, SUM149, SUM159 | Inhibit cell migration & invasion: ↑ AMPK phosphorylation; ↑ LKB1 Inhibit stem-like characteristics: ↓ Oct4, Nanog & Sox4 protein; ↓ STAT3; ↓ iPSC inducer mRNA | 5 μM | N/A | [41] | |
| MCF-7, MDA-MB-231, T47D, SKBR-3, Zr-75, BT-474 | Inhibit cell growth: ↓ PI3K/Akt/mTOR signalling Inhibit cell invasion Induce G0/G1 phase cell cycle arrest: ↓ cyclin D1 & cyclin E; ↓ Cdk2 & c-myc; ↑ PTEN Induce apoptosis: ↑ caspase-3, caspase-6 & caspase-9 activation | 0–40 μM | MCF7: 34.9 μM (24 h) 13.7 μM (48 h) 13.5 μM (72 h) 10.5 μM (96 h) MDA-MB-231: 56.9 μM (24 h) 44.4 μM (48 h) 16.0 μM (72 h) 12.0 μM (96 h) T47D: 47.7 μM (24 h) 41.6 μM (48 h) 17.6 μM (72 h) 7.1 μM (96 h) SKBR-3: 76.1 μM (24 h) 68.1 μM (48 h) 62.7 μM (72 h) 15.7 μM (96 h) ZR-75: 71.1 μM (24 h) 58.1 μM (48 h) 28.7 μM (72 h) 14.5 μM (96 h) BT-474: 80.2 μM (24 h) 65.6 μM (48 h) 39.5 μM (72 h) 15.1 μM (96 h) | [42] | |
| MDA-MB-231 | Inhibit cell proliferation: ↓ c-Src/EGFR-mediated signalling pathway; ↓ c-Myc protein Induce G0/G1 phase cell cycle arrest: ↓ cyclin A, cyclin D1 & cyclin E; ↓ Cdk2, Cdk4 & p-pRbSer780; ↑ p27Kip−1 Induce apoptosis: ↑ caspase-3, caspase-8 & caspase-9 cascade; ↓ Bcl-2 & Bid protein; ↑ PARP cleavage | 0–100 μM | 59.5 μM (72 h) | [43] | |
| Lung cancer | A549 | Inhibit cell growth & proliferation Induce G0/G1 phase cell cycle arrest: ↓ Cdk1 & cyclin B1 | 0–50 μM | 12.51 μM (24 h) 7.75 μM (72 h) | [24] |
| A549, H460, H226, H1299 | Reduce invasive potential Inhibit PGE2-induced cell migration: ↓ PGE2 production ↓ COX-2 ↑ β-catenin degradation ↓ NF-κB/p65 activity ↓ IKKα | 0–20 μM | N/A | [44] | |
| A549, H1299 | Inhibit cell viability and growth: ↓ class I HDAC proteins; ↓ HDAC activity; ↑ histone acetyltransferase (HAT) activity; ↑ histone H3 & H4 Induce G1 phase cell cycle arrest: ↓ cyclin D1 & cyclin D2; ↓ Cdk2, Cdk4 & Cdk6 | 0–60 μM | N/A | [45] | |
| H460 & A549 | Inhibit cell proliferation Induce apoptosis: ↑ cathepsin D; ↑ cleaved PARP; ↑ caspase-3 Inhibit autophagy: ↑ p62; ↑ LC3-II | 0–60 μM | H460: ~30 μM (48 h) A549: ~40 μM (48 h) | [46] | |
| Pc9-BrM3 & H2030-BrM3 (brain metastatic) | Inhibit cell proliferation and cell invasion: ↓ STAT3 protein phosphorylation; ↓ STAT-3 mediated mitochondrial respiratory function | 0–50 μM | PC9-BrM3: 28.4 μM (48 h) H2030-BrM3: 25.7 μM (48 h) | [47] | |
| H23, A549 & HCC827 | Inhibit cell growth Induce G1 phase cell cycle arrest: ↓EGFR; ↓ class I HDAC; ↓ class IIb HDAC6 activity; ↑ Hsp90 acetylation & EGFR degradation | 0–40 μM | A549: 23.55 μM (24h) | [48] | |
| H460, A549, H358 | Inhibit cell growth: ↓ c-RAF, ERK & AKT phosphorylation Inhibit colony formation capacity Induce apoptosis: ↑ Bax protein; ↓ Bcl-2 protein; ↑ PARP cleavage Induce G1 phase cell cycle arrest: ↓ cyclin D1; ↑ p21 & p27; ↓ P70S6k kinase activity Induce autophagy: ↑ LC3-I conversion to LC3-II; ↑ Sirt3 mRNA & protein; ↓ Hif-1α protein | 0–80 μM | H460: 30.42 μM (72 h) A549: 50.58 μM (72 h) H358: 59.38 μM (72 h) | [49] | |
| A549 & 95-D | Inhibit cell viability Induce apoptosis: ↑ ER stress signalling pathway activation; ↑ GRP78, phosphorylation PERK & phosphorylated IRE1α; ↑ cleaved caspase-9 & CHOP; ↓ Bcl-2 protein; ↑ Bax, caspase-3 & caspase-9 Inhibit cell migration | 0–60 μM | N/A | [50] | |
| CH27, H460 & H1299 | Inhibit cell growth Induce apoptosis: ↓ Bcl-XL; ↑ mitochondrial cytochrome c release; ↑ BAD protein; ↑ caspase-1, caspase-2, caspase-3, caspase-6, caspase-8 & caspase-9 activity; ↑ PARP cleavage | 0–100 μM | CH27: 40.9 μM (24 h) H460: 41.4 μM (24 h) H1299: 34.7 μM (24 h) | [25] | |
| MSTO-211H | Inhibit cell viability Induce apoptosis: ↑ PARP cleavage; ↑ caspase-3 activation; ↓ Bid & Bcl-xL protein; ↑ Bax protein; ↓ Mcl-1 & survivin protein; ↓ Sp1 Induce G1 phase cell cycle arrest: ↓ cyclin D1 | 0–22.5 μM | N/A | [51] | |
| Skin cancer | SK-MEL2 & MeWo | Inhibit cell growth & cell proliferation Induce apoptosis via DNA degradation Induce cell death via mitochondrial depolarization | 0–100 μM | N/A | [52] |
| A431 | Inhibit cell viability & proliferation Induce G0/G1 phase cell cycle arrest: ↓ cyclin A, cyclin D1, cyclin D2 & cyclin E; ↓ Cdk2, Cdk4 & Cdk6; ↑ p21 & p27 Induce cell apoptosis: ↑ PARP | 0–75 μM | N/A | [53] | |
| B16-F10 | Inhibit cell proliferation Induce cell death: ↑ Autophagosome (vacuoles) formation; ↓ cyclin D1; ↓ AKT/mTOR & Notch signalling | 0–50 μM | N/A | [54] | |
| B16/F-10 & SKMEL-28 | Inhibit cell proliferation & viability: ↓ Notch signalling; ↓ TACE & γ-secretase complex proteins Inhibit clonogenicity Induce G0/G1 phase cell cycle arrest Induce autophagy: ↑ autophagosome formation; ↑ LC3B cleavage Inhibit cell stemness: ↓ CD271, CD166, Jarid1B & ABCB5 | 0–60 μM | N/A | [55] | |
| UACC903 | Inhibit cell growth & proliferation | 0–50 μM | 7.45 μM (24 h) 5.10 μM (72 h) | [24] | |
| SKMEL-2 | Inhibit cell proliferation & viability Induce apoptotic death: ↑ caspase-3, caspase-6, caspase-8 & caspase-9; ↑ PARP cleavage; ↓ procaspase-3, procaspase-8 & procaspase-9 Induce G2/M phase cell cycle arrest: ↓ cyclin B1, cyclin D1, cyclin D2 & PCNA; ↓ Cdk2 & Cdk4; ↑ p21 & p53 | 0–100 μM | N/A | [56] | |
| UACC-62 | Inhibit cell proliferation & viability Induce apoptotic death: ↑ caspase-3, caspase-6, caspase-8 & caspase-9; ↑ cleaved PARP; ↓ procaspase-3, procaspase-8 & procaspase-9 Induce G0/G1 phase cell cycle arrest: ↓ cyclin B1, cyclin D1 & cyclin D2; ↓ Cdk2, Cdk4 & Cdc2p34; ↓ p21 & p27 | 0–100 μM | N/A | [56] | |
| Renal cancer | A498 | Inhibit cell proliferation Inhibit colony formation capability Inhibit cell migration and invasion: ↓ Epithelial-mesenchymal transition (EMT); ↓ cancer stem cells (CSC) properties; ↑ miR-141; ↓ ZEB2 Inhibit tumoursphere formation | 0–80 μM | ~12 μM (72 h) | [57] |
| Cervix cancer | KB-3-1, KB-8-5, KB-C1, KB-V1 | Inhibit cell viability: ↓ EGFR-STAT3 signalling Induce mitochondria-dependent & death receptor-dependent apoptosis: ↓ Bcl-2, Mcl-1 & survivin; ↑ PARP & caspase-3 cleavage; ↑ mitochondrial release of cytochrome c; ↑ DR5 Enhances in vitro cytotoxicity of Paclitaxel | 0–75 μM | KB-3-1: 12.56 μM (72 h) KB-8-5: 12.08 μM (72 h) KB-C1: 11.40 μM (72 h) KB-V1: 10.39 μM (72 h) | [31] |
| Pancreatic cancer | MiaPaCa & Colo-357 | Suppress plating efficiency of cells Reduce anchorage-independent clonogenicity growth Suppress migration and invasion ability | 0–5 μM | N/A | [58] |
| MiaPaCa & Panc1 | Inhibit cell growth Induce G1 phase cell cycle arrest: ↓ cyclin D1 & cyclin E; ↓ Cdk2 & Cdk4; ↑ p21 & p27 Induce apoptosis: ↓ Bcl-2 & Bcl-xL proteins; ↑ Bax protein; ↓ IKB-α phosphorylation; ↓ NF-κB constitutive activation | 0–60 μM | MiaPaCa: 43.25 μM (24 h) 31.08 μM (48 h) 18.54 μM (72 h) Panc1: 47.44 μM (24 h) 34.17 μM (48 h) 21.86 μM (72 h) | [59] | |
| Thyroid cancer | ARO, WRO | Inhibit cell growth & proliferation: ↓ ERK, JNK & p37 activation and expression; ↓ mTOR & p70S6K Inhibit colony formation Induce apoptosis: ↑ PARP cleavage; ↑ caspase-3, caspase-8 & PARP activation; ↓ PI3K/AKT & MAPK pathways Induce G0/G1 cell cycle arrest: ↓ cyclin D1; ↓ Cdk2 & Cdk4; ↑ p21 & p27 Induce autophagy & autophagy flux: ↑ LC3-II | ARO & WRO: 0–60 μM SW579: 0–40 μM | ARO: 36.3 μM (24 h) 40.1 μM (48 h) 44.8 μM (72 h) WRO: 37.7 μM (24 h) 31.8 μM (48 h) 30.7 μM (72 h) SW579: 19.9 μM (24 h) 10.5 μM (48 h) 8.8 μM (72 h) | [60] |
| Nasopharyngeal cancer | HNE-1 | Inhibit cell growth Induce apoptosis Induce G1 phase cell cycle arrest | 0–150 μM (Honokiol & ATNH—Active targeting nanoparticles-loaded honokiol) | Honokiol: 144.71 μM (24 h) ATNH: 69.04 μM (24 h) | [30] |
| Brain cancer | U251 | Inhibit cell growth Inhibit cell proliferation Induce apoptosis | 0–120 μM | 61.43 μM (24 h) | [61] |
| T98G | Inhibit cell viability Inhibit cell invasion Induce cell apoptosis: ↑ Bax protein; ↓ Bcl-2; ↑ Bax/Bcl-2 ratio | 0–50 μM | N/A | [62] | |
| GBM8401 (Parental) & GBM8401 SP | Inhibit cell proliferation & viability Induce sub-G1 phase cell cycle arrest Induce apoptosis: ↓ Notch3/Hes1 pathway | 0–20 μM | GBM8401 (Parental): 5.30 μM (48 h) GBM8401 SP: 11.20 μM (48 h) | [36] | |
| U251 & U-87 MG | Inhibit cell viability & proliferation: ↓ PI3K/Akt & MAPK/Erk signalling pathways Inhibit cell invasion & migration: ↓ MMP2 & MMP9; ↓ NF-κB-mediated E-cadherin pathway Inhibit colony formation Induce apoptosis: ↓ Bcl-2, p-AKT & p-ERK; ↑ Bax protein; ↑ caspase-3 cleavage; ↓ EGFR-STAT3 signalling Reduce spheroid formation: ↓ CD133 & Nestin protein | 0–60 μM | U251: 54.00 μM (24 h) U-87 MG: 62.50 μM (24 h) | [63] | |
| DBTRG-05MG | Inhibit cell growth Induce apoptosis: ↓ Rb protein; ↑ PARP & Bcl-x(S/L) cleavage Induce autophagy: ↑ Beclin-1 & LC3-II | 0–50 μM | ~30 μM | [64] | |
| U87 MG (Human) BMEC (Mouse) | Inhibit cell viability Inhibit epithelial-mesenchymal transition (EMT): ↓ Snail, β-catenin & N-cadherin; ↑ E-cadherin Inhibit cell adhesion & invasion: ↓ VCAM-1; ↓ phosphor-VE-cadherin-mediated BMEC permeability | 0–20 μM | U87MG: 22.66 μM (24 h) BMEC: 13.09 μM (24 h) | [65] | |
| U87 MG | Inhibit cell viability Induce G1 phase cell cycle arrest: ↑ p21 & p53; ↓ cyclin D1; ↓ Cdk4 & Cdk6; ↓ p-Rb protein; ↓ E2F1 Induce apoptosis: ↓ procaspase-3; ↑ caspase-8 & caspase-9 activity | 0–100 μM | 52.70 μM | [66] | |
| Bone cancer | HOS & U20S | Inhibit cell proliferation Inhibit colony formation Induce G0/G1 phase cell cycle arrest: ↓ cyclin D1 & cyclin E; ↓ Cdk4 Induce mitochondria-mediated apoptosis: ↑ caspase-3 & caspase-9 activation; ↑ PARP cleavage; ↓ Bcl-2, Bcl-xL & survivin; ↑ ERK activation; ↓ proteasome activity; ↑ ER stress and subsequent ROS overgeneration; ↑ GRP78 Induce autophagy: ↑ Atg7 protein activation; ↑ Atg5; ↑ LC3B-II | 0–30 μM | HOS: 17.70 μM (24 h) U20S: 21.50 μM (24 h) | [67] |
| SAOS-2, HOS, 143B, MG-63 M8, HU09, HU09 M132 Dunn, LM5, LM8 & LM8-LacZ (Mouse) | Inhibit cell metabolic activity Inhibit cell proliferation Inhibit cell migration Induce rapid cell death via Honokiol-provoked vacuolation | 0–150 μM | (72 h) SAOS-2: 48.38 μM HOS: 51.38 μM 143B: 41.63 μM MG-63M8: 34.88 μM HU09: 59.25 μM HU09M132: 31.88 μM (72 h) Dunn: 36.00 μM LM5: 30.00 μM LM8: 31.13 μM | [68] | |
| Saos-2 & MG-63 | Inhibit cell viability Induce apoptosis: ↑ caspase-3 & PARP cleavage; ↑ Bax protein; ↓ Bcl-2; ↓ PI3K/AKT signalling pathway; ↓ miR-21 | 0–100 μM | Saos-2: 37.85 μM (24 h) MG-63: 38.24 μM (24h) | [69] | |
| Oral cancer | OC2 & OCSL | Inhibit cell growth Induce G0/G1 phase cell cycle arrest: ↑ cyclin E accumulation; ↑ p21 & p27; ↓ cyclin D1, ↓ Cdk2 & Cdk4 Induce apoptosis: ↓ caspase-8 & caspase-9; ↑ caspase-3 cleavage; ↓ Bid protein Induce autophagy and autophagic flux: ↑ LC3-II; ↓ Akt/mTORC1 pathway; ↑ AMPK signalling pathway; ↑ p62 | 0–60 μM | OC2: 35.00 μM (24 h) 22.00 μM (48 h) OCSL: 33 μM (24 h) 13 μM (48 h) | [26] |
| HN-22 & HSC-4 | Inhibit cell viability Induce apoptosis: ↓ Sp1 protein; ↑ p21 & p27; ↑ PARP & caspase-3 activation; ↓ Mcl-1 & survivin protein Induce G1 phase cell cycle arrest: ↓ cyclin D1 | 0–37.5 μM | HN-22: 26.63 μM (48 h) HSC-4: 30.00 μM (48 h) | [70] | |
| Liver cancer | HepG2 | Inhibit cell growth & proliferation: ↓ β-catenin protein Induce apoptosis: ↑ BAD protein; ↓ Bcl-2 protein Upregulation of BAD protein expression Downregulation of Bcl-2 protein level | 0–2 μM | N/A | [71] |
| SMMC-7721 | Inhibit cell growth Induce G0/G1 phase cell cycle arrest Induce apoptosis: ↓ mitochondrial potential; ↑ ROS production; ↓ Bcl-2 protein; ↑ Bax protein | 0–37.5 μM | N/A | [72] | |
| HepG2, HUH7, PLC/PRF5, Hep3B | Inhibit cell proliferation: ↓ STAT3 activation; ↓ IL-induced Akt phosphorylation; ↓ c-Src activation; ↓ JAK1 & JAK2; ↑ SHP-1 protein Induce sub-G1 phase cell cycle arrest: ↓ cyclin D1 Downregulation of cyclin D1 level Induce apoptosis: ↓ Bcl-2 & Bcl-xL; ↓ survivin & Mcl-1 protein; ↑ caspase-3 activation; ↑ PARP cleavage Enhance apoptotic effect of doxorubicin & paclitaxel | 0–100 μM | N/A | [32] | |
| Ovarian cancer | A2780s & A2780cp | Inhibit cell growth Induce apoptosis | 0–100 μM | A2780s: 36.00 μM (48 h) A2780cp: 34.70 μM (48 h) | [73] |
| SKOV3 & Caov-3 | Inhibit cell proliferation and growth Inhibit colony formation Induce apoptosis: ↑ AMPK pathway activation; ↑ caspase-3, caspase-7 & caspase-9 activation; ↑ PARP cleavage Induce G0/G1 phase cell cycle arrest Inhibit cell migration and invasion | 0–100 μM | SKOV: 48.71 μM (24 h) Caov-3: 46.42 μM (24 h) | [28] | |
| SKOV3, COC1, Angelen & A2780 | Inhibit cell proliferation Induce cell apoptosis: ↓ Bcl-xL; ↑ BAD protein; ↑ caspase-3 activation Induce G1 phase cell cycle arrest | 0–93.75 μM | SKOV3: 62.63 μM (24 h) COC1: 73.50 μM (24 h) Angelen: 61.50 μM (24 h) A2780: 55.85 μM (24 h) | [74] | |
| Prostate cancer | PC-3 & LNCaP | Inhibit cell viability Induce G0/G1 phase cell cycle arrest: ↓ cyclin D1 & cyclin E; ↓ Cdk2, Cdk4 & Cdk6; ↑ p21 & p53; ↓ Rb & E2F1 proteins; ↓ Rb phosphorylation at Ser807/811; ↑ ROS generation | 0–60 μM | N/A | [75] |
| PC-3, LNCaP & C4-2 | Inhibit cell growth Induce apoptosis: ↑ caspase-3, caspase-8 & caspase-9 activation; ↑ PARP cleavage Induce apoptosis via DNA fragmentation: ↑ Bax & Bak proteins; ↓ Mcl-1 protein | 0–75 μM | 18.75–37.50 μM (24 h) | [76] | |
| PC-3, LNCaP | Inhibit cell viability Induce autophagy: ↑ LC3-BII protein; ↓ mTOR pathway Induce apoptosis via DNA fragmentation: ↑ ROS generation | 0–40 μM | N/A | [77] | |
| Head & neck squamous cancer | Cal-33 & MD-1483 | Inhibit cell growth Induce cell apoptosis and cell cycle arrest: ↓ EGFR signalling pathway; ↓ STAT3 signalling pathway; ↓ Bcl-xL & cyclin D1; ↓ phosphorylation p42/p44 MAPK & phosphorylated Akt | 0–100 μM | Cal-33: 3.80 μM (72 h) 1483: 7.44 μM (72 h) | [78] |
| Neuroblastoma | Neuro-2a | Induce apoptosis via DNA fragmentation: ↑ caspase-3, caspase-6 & caspase-9 activation; ↑ Bax protein; ↓ mitochondrial membrane potential; ↑ cytochrome c releaseInduce sub-G1 phase cell cycle arrest | 0–100 μM | 63.3 μM (72 h) | [79] |
| Neuro-2a & NB41A3 | Inhibit cell viability Induce autophagy: ↑ LC3-II; ↑ PI3K/Akt/mTOR signalling pathway; ↑ Grp78; ↑ ROS generation; ↑ ERK1/2; ↑ p-ERK1Induce apoptosis via DNA fragmentation Inhibit cell migration | 0–100 μM | Neuro-2a: ~50 μM (72 h) | [80] | |
| Bladder cancer | T24 & 5637 | Inhibit cell viability and induce apoptosis: ↑ Bax protein; ↑ PARP cleavage; ↓ Bcl-2 protein Inhibit clonogenicity Induce G1 phase cell cycle arrest: ↓ cyclin D1; ↑ p21 & p27 Inhibit sphere formation capacity Inhibit cell migration & invasion: ↓ EZH2 gene expression; ↓ MMP9 Inhibit cell stemness: ↓ EZH2 gene expression; ↓ CD44 & Sox2; ↑ miR-143 overexpression | 0–72 μM | N/A | [81] |
4.2. In Vivo Studies
| Cancer Cell Line | Animal Model & Site of Tumour Xenograft | Dose, Duration & Route of Administration | Observation & Mechanism of Action | Efficacy on Tumour Inhibition | References |
|---|---|---|---|---|---|
| Breast cancer | |||||
| MDA-MB-231 cells | Both flanks of athymic nude mice | 100 mg/kg/day 28 days IP | Induce tumour growth arrest | Complete arrest of tumour growth from week 2 onwards | [39] |
| MDA-MB-231 cells | Right gluteal region of athymic nude mice | 3 mg/mouse/day Three times a week 28 days IP | Inhibit tumour progression: ↓ Ki-67; ↑ LKB1 & pAMPK; ↑ ACC phosphorylation, ↓ pS6K & 4EBP1 phosphorylation | Tumour weight of honokiol-treated group was 0.22 g compared to control group which was 1.58 g | [40] |
| MDA-MB-231-pLKO.1 & MDA-MB-231-LKB1shRNA cells | Right gluteal region of athymic nude mice | 3 mg/mouse/day Three times a week 42 days Oral gavage | Inhibit cell stemness: ↓ Oct4, Nanog & Sox2; ↓ pSTAT3 & Ki-67 Inhibit mammosphere formation | Decreased expression of Oct4, Nanog, Sox2 Reduce number of tumour cells showing Ki-67 & pStat3 expression | [41] |
| Colorectal cancer | |||||
| RKO cells | Axilla of BALB/c nude mice | 80 mg/kg/day Treatment on days 8–11, 14–17, 21–24, 28–31 51 days IP | Inhibit tumour growth Prolong survival of mice | 709.9% increase in tumour growth rate in honokiol-treated group compared to 1627.6% and 1408.2% in control and vehicle groups respectively | [33] |
| HCT116 cells | Flank of athymic nude mice | 200 μg/kg/day + 5 Gy irradiation Once a week 21 days IP | Inhibit tumour growth: ↓ CSC proteins → ↓ DCLK1, Sox-9, CD133 & CD44 | Significantly lower tumour weight (<800 mg) in honokiol-IR combination, (~1500 mg) in honokiol treatment group compared to (~3300 mg) in control group | [35] |
| Cervical cancer | |||||
| KB-8-5 cells | Athymic nu/nu nude mice (site of xenograft not stated) | 50 mg/kg Honokiol Three times a week + 20 mg/kg Paclitaxel Once a week 28 days IP (honokiol) Tail vein injection (paclitaxel) | Suppress tumour growth: ↓ Ki-67 tissue level Induce apoptosis | Significantly lower average tumour volume for honokiol-paclitaxel combination treatment (573.9 mm3) compared to control (2585.4 mm3) | [31] |
| Lung cancer | |||||
| H2030-BrM3 cells | Left ventricle of NOD/SCID mice | 2 or 10 mg/kg/day 28 days Oral gavage | Prevent metastasis of lung cancer cells to brain | 10 mg/kg: Decrease brain metastasis for >70% | [47] |
| H2030-BrM3 cells | Left lung via left ribcage of athymic nude mice | 2 or 10 mg/kg/day Five days a week 28 days Oral gavage | Decrease lung tumour growth Inhibit metastasis to lymph node | 10 mg/kg: Significantly reduce incidence of mediastinal adenopathy, decrement of weight of mediastinal lymph node for >80%, only 2/6 mice have lymphatic metastasis | [47] |
| Blood cancer | |||||
| Raji cells | Back of BALB/c nude mice | 5 mg/20 g & 10 mg/20 g Treatment on days 8–12 & 15–19 20 days (Route of administration not specified) | Inhibit cell proliferation Inhibit tumour growth | Tumour growth of honokiol-treated mice was significantly lower (~90 cm3) compared to control mice (~270 cm3) | [38] |
| HL60 cells | Inoculated intraperitoneally into SCID mice | 100 mg/kg/day Treatment on Day 1–6 47 days IP | Prolong survival of mice | Median survival time of honokiol-treated mice are longer (37.5 days) compared to vehicle-treated mice (24.5 days) | [85] |
| Pancreatic cancer | |||||
| MiaPaCa cells | Pancreas of immunocompromised mice | 150 mg/kg/day 28 days IP | Suppress tumour growth Inhibit metastasis: ↓ CXCR & SHH; ↓ NF-κB & downstream pathway Inhibit desmoplastic reaction: ↓ ECM protein; ↓ collagen I | Significant decrease in tumour growth for honokiol-treated mice (99.6 mm3) compared to vehicle-treated mice (1361.0 mm3) | [58] |
| Skin cancer | |||||
| SKMEL-2 or UACC-62 cells | Right flank of athymic nude mice | 50 mg/kg Three times a week 14–54 days IP | Decrease tumour growth | SKMEL-2: 40% reduction in tumour volume UACC-62: 50% reduction in tumour volume | [56] |
| Thyroid cancer | |||||
| ARO cells | BALB/cAnN.Cg-Foxn1nu/CrlNarl mice (site of xenograft not stated) | 5 or 15 mg/kg/mouse Every three days 21 days Oral gavage | Decrease tumour volume & tumour weight Induce apoptosis & autophagy | Control: ~1000 mm3; 700 mg 5 mg/kg Honokiol: ~600 mm3; 400 mg 15 mg/kg Honokiol: ~400 mm3; 200 mg | [60] |
| Nasopharyngeal cancer | |||||
| HNE-1 cells | Right dorsal aspect of right foot of BALB/c athymic nude mice | Active-targeting nanoparticles-loaded HK (ATNH), Non-active-targeting nanoparticles-loaded HK (NATNH), Free Honokiol (HK) 3 mg/mouse/day Every three days Euthanise 50% mice after 12 days, rest are left to observe tumour growth & survival time up to 60 days; IV | Inhibit tumour progression, Induce apoptosis Potential inhibitor of angiogenesis & proliferation | Efficiency in tumour delay: ATNH > NATNH > Free HK Median survival time: Control: 28.5 days Free HK: 34 days NATNH: 42.5 days ATNH: 57.5 days | [30] |
| Brain cancer | |||||
| U21 cells | Right flank of athymic nude mice | 20 mg/kg Twice a week 27 days Caudal vein injection | Inhibit tumour growth Inhibit angiogenesis | Honokiol-treated mice have significant inhibition of tumour volume by 50.21% compared to vehicle Significantly lower microvessel present in honokiol-treated cells | [61] |
| U-87 MG cell suspension pre-treated with honokiol or vehicle for 48h | Yolk sac of Zebrafish larvae | (Concentration N/A) 3 days Injection of cells into zebrafish | Inhibit cell proliferation Inhibit cell migration | Reduced number of cell mass compared to vehicle-treated cells | [63] |
| U-87 MG cells | Right flank near upper extremity of nude mice | 100 mg/kg/day Treatment at days 1–7 21 days IP | Reduce tumour growth: ↓ EGFR, pSTAT3, CD133 & Nestin | Increased number of apoptotic cells in honokiol-treated tissue, Significantly lower tumour volume & tumour weight in honokiol-treated mice | [63] |
| Bone cancer | |||||
| HOS cells | Dorsal area of BALB/c-nu mice | 40 mg/kg/day 7 days IP | Reduce tumour growth Induce apoptosis & autophagy: ↑ cleaved caspase-3; ↑ LC3B-II & phosphor-ERK (ROS/ERK1/2 signalling pathway) | Significant decrease in tumour volume & weight of honokiol-treated mice (200 mm3; 0.2 g) compared to control group (~500 mm3; 0.5 g) Increased number of TUNEL-positive cells | [26] |
| LM8-LacZ cells | Left flank of C3H/HeNCrl mice | 150 mg/kg/day 25 days; IP | Inhibit metastasis | Mean number of micrometastases decreased significantly by 41.4% in honokiol-treated mice compared to control mice | [68] |
| Oral cancer | |||||
| SAS cells | Right flank of BALB/cAnN.Cg-Foxn1nu.CrlNarl nude mice | 5 mg/kg or 15 mg/kg Treatment on day 1, 4, 7, 10, 13, 16, 19, 22 35 days Oral | Reduce tumour growth & volume | Significantly reduction in tumour growth in honokiol-treated mice 29% reduction (5 mg/kg; 21 days), 40% reduction (15 mg/kg; 21 days) 41% reduction (5 mg/kg; 35 days), 56% reduction (15 mg/kg; 35 days) | [26] |
| Prostate cancer | |||||
| C4-2 cells | Bilateral tibia of BALB/c nu/nu athymic nude mice | 100 mg/kg/day 42 days IP | Inhibit cell proliferation: ↑ Ki-67 Induce apoptosis: ↑ M-31 Inhibit angiogenesis: ↑ CD-31 | Lower PSA value in honokiol-treated mice compared to control group | [76] |
| PC-3 cells | Left & right flanks above hind limb of nude mice | 1 or 2 mg/mice Monday, Wednesday & Friday two weeks before tumour implantation and duration of experiment after implantation 77 days Oral gavage | Inhibit tumour growth Inhibit cell proliferation Inhibit neovascularisation Induce apoptosis | Tumour volume of honokiol-treated mice are significantly lower (~330 mm3; 1 mg), (~50 mm3; 2 mg) compared to control (~400 mm3) | [18] |
| Gastric cancer | |||||
| MKN45 cells | Dorsal side of BALB/c nude mice (nu/nu) | 0.5 mg/kg/day & 1.5 mg/kg/day 10 days Injection (route not stated) | Inhibit tumour growth: ↓ GRP94 overexpression | 30% reduction in tumour volume (0.5 mg/kg) 60% reduction in tumour volume (1.5 mg/kg) Decreased accumulation of GRP94 | [86] |
| MKN45 & SCM-1 cells | Peritoneal cavity of BALB/c nude mice | 5 mg/kg Twice a week 28 days IP | Inhibit metastasis Inhibit angiogenesis | Honokiol inhibited STAT-3 signalling and VEGF signalling induced by calpain/SHP-1 | [87] |
| Ovarian cancer | |||||
| SKOV3 cells | Right axilla of BALB/c nude mice | 1 mg liposome-encapsulated honokiol/day 48 days IP | Inhibit tumour growth Inhibit angiogenesis | Reduction in tumour growth rate in liposome-encapsulated honokiol-treated mice by 67–70% compared to control | [73,88] |
| A2780s cells | Right flank of athymic BALB/c nude mice | 10 mg/kg Lipo-Honokiol Twice a week 21 days IV | Inhibit cancer growth Prolong survival of mice Increase intra-tumoural apoptosis Inhibit intra-tumoural angiogenesis | Lipo-HNK treated mice have significantly smaller tumour volume (222 ± 71 mm3) compared to liposome-treated mice (1823 ± 606 mm3) and control mice (3921 ± 235 mm3) | [73] |
| A2780cp cells | Right flank of athymic BALB/c nude mice | 10 mg/kg Lipo-Honokiol Twice a week 21 days IV | Inhibit cancer growth Prolong survival Increase intra-tumoural apoptosis Inhibit intra-tumoural angiogenesis | Lipo-HNK treated mice have significantly smaller tumour volume (408 ± 165 mm3) compared to liposome-treated mice (2575 ± 701 mm3) and control mice (2828 ± 796 mm3) | [73] |
5. Mechanism of Action of Honokiol
5.1. Dual Induction of Apoptotic and Necrotic Cell Death
5.2. Cell Cycle Arrest
5.3. Autophagy
5.4. Epithelial-Mesenchymal Transition (EMT)
5.5. Suppression of Migration, Invasion and Angiogenesis of Cancer Cells
6. Effect of Honokiol on Various Signalling Pathways
6.1. Nuclear Factor Kappa B (NF-κB)
6.2. Signal Transducers and Activators of Transcription (STATs)
6.3. Epidermal Growth Factor Receptor (EGFR)
6.4. Mammalian Target of Rapamycin (mTOR)
6.5. Hypoxia-Inducible-Factor (HIF) Pathway
6.6. Notch Signalling Pathway
6.7. Downregulation of P-Glycoprotein
7. Metabolism, Bioavailability, and Pharmacological Relevance of Honokiol
8. Potential Drug Delivery of Honokiol
9. Future Perspective
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Foster, I. Cancer: A cell cycle defect. Radiography 2008, 14, 144–149. [Google Scholar] [CrossRef]
- Cabral, C.; Efferth, T.; Pires, I.M.; Severino, P.; Lemos, M.F.L. Natural Products as a Source for New Leads in Cancer Research and Treatment. Evid.-Based Complement. Altern. Med. 2018, 2018, 8243680. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhu, W.; Thompson, P.; Hannun, Y.A. Evaluating intrinsic and non-intrinsic cancer risk factors. Nat. Commun. 2018, 9, 3490. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- DeVita, V.T.; Canellos, G.P. New therapies and standard of care in oncology. Nat. Rev. Clin. Oncol. 2011, 8, 67–68. [Google Scholar] [CrossRef]
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the use of therapeutic peptides for cancer treatment. J. Biomed. Sci. 2017, 24, 21. [Google Scholar] [CrossRef]
- Mitra, S.; Dash, R. Natural Products for the Management and Prevention of Breast Cancer. Evid.-Based Complement. Altern. Med. 2018, 2018, 8324696. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Robinson, M.M.Z.; Zhang, X. The World Medicines Situation 2011. Traditional Medicines: Global Situation, Issues and Challenges; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Seelinger, M.; Popescu, R.; Giessrigl, B.; Jarukamjorn, K.; Unger, C.; Wallnöfer, B.; Fritzer-Szekeres, M.; Szekeres, T.; Diaz, R.; Jäger, W.; et al. Methanol extract of the ethnopharmaceutical remedy Smilax spinosa exhibits anti-neoplastic activity. Int. J. Oncol. 2012, 41, 1164–1172. [Google Scholar] [CrossRef]
- Amaral, R.G.; dos Santos, S.A.; Andrade, L.N.; Severino, P.; Carvalho, A.A. Natural Products as Treatment against Cancer: A Historical and Current Vision. Clin. Oncol. 2019, 4, 1562. [Google Scholar]
- Arora, S.; Singh, S.; Piazza, G.A.; Contreras, C.M.; Panyam, J.; Singh, A.P. Honokiol: A novel natural agent for cancer prevention and therapy. Curr. Mol. Med. 2012, 12, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Wu, C.L.; Liu, J.F.; Fong, Y.C.; Hsu, S.F.; Li, T.M.; Su, Y.C.; Liu, S.H.; Tang, C.H. Honokiol induces cell apoptosis in human chondrosarcoma cells through mitochondrial dysfunction and endoplasmic reticulum stress. Cancer Lett. 2010, 291, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Lee, J.Y.; Baek, B.J.; Lee, B.D.; Koh, Y.W.; Lee, W.-S.; Lee, Y.-J.; Kwon, B.-M. The inhibitory effect of honokiol, a natural plant product, on vestibular schwannoma cells. Laryngoscope 2012, 122, 162–166. [Google Scholar] [CrossRef]
- Amblard, F.; Delinsky, D.; Arbiser, J.L.; Schinazi, R.F. Facile purification of honokiol and its antiviral and cytotoxic properties. J. Med. Chem. 2006, 49, 3426–3427. [Google Scholar] [CrossRef]
- Woodbury, A.; Yu, S.P.; Wei, L.; García, P. Neuro-modulating effects of honokiol: A review. Front. Neurol. 2013, 4, 130. [Google Scholar] [CrossRef]
- Hahm, E.R.; Arlotti, J.A.; Marynowski, S.W.; Singh, S.V. Honokiol, a constituent of oriental medicinal herb magnolia officinalis, inhibits growth of PC-3 xenografts in vivo in association with apoptosis induction. Clin. Cancer Res. 2008, 14, 1248–1257. [Google Scholar] [CrossRef]
- Chen, L. Rapid purification and scale-up of honokiol and magnolol using high-capacity high-speed counter-current chromatography A. J. Chromatogr. 2007, 1142, 115–122. [Google Scholar] [CrossRef]
- Chen, C.-M.; Liu, Y.-C. ChemInform Abstract: A Concise Synthesis of Honokiol. Tetrahedron Lett. 2009, 50, 1151–1152. [Google Scholar] [CrossRef]
- Gupta, M. Pharmacological Properties and Traditional Therapeutic Uses of Important Indian Spices: A Review. Int. J. Food Prop. 2010, 13, 1092–1116. [Google Scholar] [CrossRef]
- Anand, K.W.; Wakode, S. Development of drugs based on Benzimidazole Heterocycle: Recent advancement and insights. Int. J. Chem. Stud. 2017, 5, 350–362. [Google Scholar]
- Bohmdorfer, M.; Maier-Salamon, A.; Taferner, B.; Reznicek, G.; Thalhammer, T.; Hering, S.; Hufner, A.; Schuhly, W.; Jager, W. In vitro metabolism and disposition of honokiol in rat and human livers. J. Pharm. Sci. 2011, 100, 3506–3516. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.M.; Prakasha Gowda, A.S.; Sharma, A.K.; Amin, S. In vitro growth inhibition of human cancer cells by novel honokiol analogs. Bioorg. Med. Chem. 2012, 20, 3202–3211. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.E.; Hsieh, M.T.; Tsai, T.H.; Hsu, S.L. Down-modulation of Bcl-XL, release of cytochrome c and sequential activation of caspases during honokiol-induced apoptosis in human squamous lung cancer CH27 cells. Biochem. Pharmacol. 2002, 63, 1641–1651. [Google Scholar] [CrossRef]
- Huang, K.J.; Kuo, C.H.; Chen, S.H.; Lin, C.Y.; Lee, Y.R. Honokiol inhibits in vitro and in vivo growth of oral squamous cell carcinoma through induction of apoptosis, cell cycle arrest and autophagy. J. Cell. Mol. Med. 2018, 22, 1894–1908. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, K.; Guo, Y.; Huang, F.; Yang, K.; Chen, L.; Huang, K.; Zhang, F.; Long, Q. Honokiol protects against doxorubicin cardiotoxicity via improving mitochondrial function in mouse hearts. Sci. Rep. 2017, 7, 11989. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Sul, J.Y.; Park, J.B.; Lee, M.S.; Cha, E.Y.; Ko, Y.B. Honokiol induces apoptosis and suppresses migration and invasion of ovarian carcinoma cells via AMPK/mTOR signaling pathway. Int. J. Mol. Med. 2019, 43, 1969–1978. [Google Scholar] [CrossRef]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Yang, B.; Ni, X.; Chen, L.; Zhang, H.; Ren, P.; Feng, Y.; Chen, Y.; Fu, S.; Wu, J. Honokiol-loaded polymeric nanoparticles: An active targeting drug delivery system for the treatment of nasopharyngeal carcinoma. Drug Deliv. 2017, 24, 660–669. [Google Scholar] [CrossRef]
- Wang, X.; Beitler, J.J.; Wang, H.; Lee, M.J.; Huang, W.; Koenig, L.; Nannapaneni, S.; Amin, A.R.M.R.; Bonner, M.; Shin, H.J.C.; et al. Honokiol Enhances Paclitaxel Efficacy in Multi-Drug Resistant Human Cancer Model through the Induction of Apoptosis. PLoS ONE 2014, 9, e86369. [Google Scholar] [CrossRef]
- Rajendran, P.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1. J. Cell. Physiol. 2012, 227, 2184–2195. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, T.; Wu, Y.-F.; Gu, Y.; Xu, X.-L.; Zheng, S.; Hu, X. Honokiol: A potent chemotherapy candidate for human colorectal carcinoma. World J. Gastroenterol. 2004, 10, 3459–3463. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Subramaniam, D.; Ramalingam, S.; Dhar, A.; Postier, R.G.; Umar, S.; Zhang, Y.; Anant, S. Honokiol radiosensitizes colorectal cancer cells: Enhanced activity in cells with mismatch repair defects. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 301, G929–G937. [Google Scholar] [CrossRef] [PubMed]
- Ponnurangam, S.; Mammen, J.M.; Ramalingam, S.; He, Z.; Zhang, Y.; Umar, S.; Subramaniam, D.; Anant, S. Honokiol in combination with radiation targets notch signaling to inhibit colon cancer stem cells. Mol. Cancer Ther. 2012, 11, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Lai, I.C.; Shih, P.-H.; Yao, C.-J.; Yeh, C.-T.; Wang-Peng, J.; Lui, T.-N.; Chuang, S.-E.; Hu, T.-S.; Lai, T.-Y.; Lai, G.-M. Elimination of Cancer Stem-Like Cells and Potentiation of Temozolomide Sensitivity by Honokiol in Glioblastoma Multiforme Cells. PLoS ONE 2015, 10, e0114830. [Google Scholar] [CrossRef] [PubMed]
- Battle, T.E.; Arbiser, J.; Frank, D.A. The natural product honokiol induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia (B-CLL) cells. Blood 2005, 106, 690–697. [Google Scholar] [CrossRef]
- Gao, D.Q.; Qian, S.; Ju, T. Anticancer activity of Honokiol against lymphoid malignant cells via activation of ROS-JNK and attenuation of Nrf2 and NF-kappaB. J. BUON 2016, 21, 673–679. [Google Scholar]
- Wolf, I.; O’Kelly, J.; Wakimoto, N.; Nguyen, A.; Amblard, F.; Karlan, B.Y.; Arbiser, J.L.; Koeffler, H.P. Honokiol, a natural biphenyl, inhibits in vitro and in vivo growth of breast cancer through induction of apoptosis and cell cycle arrest. Int. J. Oncol. 2007, 30, 1529–1537. [Google Scholar] [CrossRef]
- Nagalingam, A.; Arbiser, J.L.; Bonner, M.Y.; Saxena, N.K.; Sharma, D. Honokiol activates AMP-activated protein kinase in breast cancer cells via an LKB1-dependent pathway and inhibits breast carcinogenesis. Breast Cancer Res. 2012, 14, R35. [Google Scholar] [CrossRef]
- Sengupta, S.; Nagalingam, A.; Muniraj, N.; Bonner, M.Y.; Mistriotis, P.; Afthinos, A.; Kuppusamy, P.; Lanoue, D.; Cho, S.; Korangath, P.; et al. Activation of tumor suppressor LKB1 by honokiol abrogates cancer stem-like phenotype in breast cancer via inhibition of oncogenic Stat3. Oncogene 2017, 36, 5709–5721. [Google Scholar] [CrossRef]
- Liu, H.; Zang, C.; Emde, A.; Planas-Silva, M.D.; Rosche, M.; Kuhnl, A.; Schulz, C.O.; Elstner, E.; Possinger, K.; Eucker, J. Anti-tumor effect of honokiol alone and in combination with other anti-cancer agents in breast cancer. Eur. J. Pharmacol. 2008, 591, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Min, H.Y.; Chung, H.J.; Hong, J.Y.; Kang, Y.J.; Hung, T.M.; Youn, U.J.; Kim, Y.S.; Bae, K.; Kang, S.S.; et al. Down-regulation of c-Src/EGFR-mediated signaling activation is involved in the honokiol-induced cell cycle arrest and apoptosis in MDA-MB-231 human breast cancer cells. Cancer Lett. 2009, 277, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Katiyar, S.K. Honokiol Inhibits Non-Small Cell Lung Cancer Cell Migration by Targeting PGE2-Mediated Activation of β-Catenin Signaling. PLoS ONE 2013, 8, e60749. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Prasad, R.; Katiyar, S.K. Inhibition of class I histone deacetylases in non-small cell lung cancer by honokiol leads to suppression of cancer cell growth and induction of cell death in vitro and in vivo. Epigenetics 2013, 8, 54–65. [Google Scholar] [CrossRef]
- Lv, X.; Liu, F.; Shang, Y.; Chen, S.Z. Honokiol exhibits enhanced antitumor effects with chloroquine by inducing cell death and inhibiting autophagy in human non-small cell lung cancer cells. Oncol. Rep. 2015, 34, 1289–1300. [Google Scholar] [CrossRef]
- Pan, J.; Lee, Y.; Zhang, Q.; Xiong, D.; Wan, T.C.; Wang, Y.; You, M. Honokiol Decreases Lung Cancer Metastasis through Inhibition of the STAT3 Signaling Pathway. Cancer Prev. Res. 2017, 10, 133–141. [Google Scholar] [CrossRef]
- Liou, S.-F.; Hua, K.-T.; Hsu, C.-Y.; Weng, M.-S. Honokiol from Magnolia spp. induces G1 arrest via disruption of EGFR stability through repressing HDAC6 deacetylated Hsp90 function in lung cancer cells. J. Funct. Foods 2015, 15, 84–96. [Google Scholar] [CrossRef]
- Luo, L.-X.; Li, Y.; Liu, Z.-Q.; Fan, X.-X.; Duan, F.-G.; Li, R.-Z.; Yao, X.-J.; Leung, E.L.-H.; Liu, L. Honokiol Induces Apoptosis, G1 Arrest, and Autophagy in KRAS Mutant Lung Cancer Cells. Front. Pharmacol. 2017, 8, 199. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, S.; Gao, W.; Feng, J.; Zhao, G. Honokiol induces endoplasmic reticulum stress-mediated apoptosis in human lung cancer cells. Life Sci. 2019, 221, 204–211. [Google Scholar] [CrossRef]
- Chae, J.I.; Jeon, Y.J.; Shim, J.H. Downregulation of Sp1 is involved in honokiol-induced cell cycle arrest and apoptosis in human malignant pleural mesothelioma cells. Oncol. Rep. 2013, 29, 2318–2324. [Google Scholar] [CrossRef]
- Mannal, P.W.; Schneider, J.; Tangada, A.; McDonald, D.; McFadden, D.W. Honokiol produces anti-neoplastic effects on melanoma cells in vitro. J. Surg. Oncol. 2011, 104, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Chilampalli, C.; Guillermo, R.; Kaushik, R.S.; Young, A.; Chandrasekher, G.; Fahmy, H.; Dwivedi, C. Honokiol, a chemopreventive agent against skin cancer, induces cell cycle arrest and apoptosis in human epidermoid A431 cells. Exp. Biol. Med. 2011, 236, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Ramalingam, S.; Subramaniam, D.; Rangarajan, P.; Protti, P.; Rammamoorthy, P.; Anant, S.; Mammen, J.M. Honokiol induces cytotoxic and cytostatic effects in malignant melanoma cancer cells. Am. J. Surg. 2012, 204, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Kwatra, D.; Subramaniam, D.; Jensen, R.A.; Anant, S.; Mammen, J.M.V. Honokiol affects melanoma cell growth by targeting the AMP-activated protein kinase signaling pathway. Am. J. Surg. 2014, 208, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Guillermo-Lagae, R.; Santha, S.; Thomas, M.; Zoelle, E.; Stevens, J.; Kaushik, R.S. Antineoplastic Effects of Honokiol on Melanoma. Biomed Res. Int. 2017, 2017, 5496398. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Q.; Su, Q.; Ma, D.; An, C.; Ma, L.; Liang, H. Honokiol suppresses renal cancer cells’ metastasis via dual-blocking epithelial-mesenchymal transition and cancer stem cell properties through modulating miR-141/ZEB2 signaling. Mol. Cells 2014, 37, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Averett, C.; Bhardwaj, A.; Arora, S.; Srivastava, S.K.; Khan, M.A.; Ahmad, A.; Singh, S.; Carter, J.E.; Khushman, M.D.; Singh, A.P. Honokiol suppresses pancreatic tumor growth, metastasis and desmoplasia by interfering with tumor–stromal cross-talk. Carcinogenesis 2016, 37, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Bhardwaj, A.; Srivastava, S.K.; Singh, S.; McClellan, S.; Wang, B.; Singh, A.P. Honokiol arrests cell cycle, induces apoptosis, and potentiates the cytotoxic effect of gemcitabine in human pancreatic cancer cells. PLoS ONE 2011, 6, e21573. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Chen, S.H.; Chang, Y.S.; Liu, Y.W.; Wu, J.Y.; Lim, Y.P.; Yu, H.I.; Lee, Y.R. Honokiol, a potential therapeutic agent, induces cell cycle arrest and program cell death in vitro and in vivo in human thyroid cancer cells. Pharmacol. Res. 2017, 115, 288–298. [Google Scholar] [CrossRef]
- Wang, X.; Duan, X.; Yang, G.; Zhang, X.; Deng, L.; Zheng, H.; Deng, C.; Wen, J.; Wang, N.; Peng, C.; et al. Honokiol crosses BBB and BCSFB, and inhibits brain tumor growth in rat 9L intracerebral gliosarcoma model and human U251 xenograft glioma model. PLoS ONE 2011, 6, e18490. [Google Scholar] [CrossRef]
- Jeong, J.J.; Lee, J.H.; Chang, K.C.; Kim, H.J. Honokiol exerts an anticancer effect in T98G human glioblastoma cells through the induction of apoptosis and the regulation of adhesion molecules. Int. J. Oncol. 2012, 41, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xue, W.; Schachner, M.; Zhao, W. Honokiol Eliminates Glioma/Glioblastoma Stem Cell-Like Cells Via JAK-STAT3 Signaling and Inhibits Tumor Progression by Targeting Epidermal Growth Factor Receptor. Cancers 2018, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Yan, M.D.; Yao, C.J.; Lin, P.C.; Lai, G.M. Honokiol-induced apoptosis and autophagy in glioblastoma multiforme cells. Oncol. Lett. 2013, 6, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.N.; Eun, S.Y.; Park, S.W.; Lee, J.H.; Chang, K.C.; Kim, H.J. Honokiol inhibits U87MG human glioblastoma cell invasion through endothelial cells by regulating membrane permeability and the epithelial-mesenchymal transition. Int. J. Oncol. 2014, 44, 187–194. [Google Scholar] [CrossRef]
- Lin, C.J.; Chen, T.L.; Tseng, Y.Y.; Wu, G.J.; Hsieh, M.H.; Lin, Y.W.; Chen, R.M. Honokiol induces autophagic cell death in malignant glioma through reactive oxygen species-mediated regulation of the p53/PI3K/Akt/mTOR signaling pathway. Toxicol. Appl. Pharmacol. 2016, 304, 59–69. [Google Scholar] [CrossRef]
- Huang, K.; Chen, Y.; Zhang, R.; Wu, Y.; Ma, Y.; Fang, X.; Shen, S. Honokiol induces apoptosis and autophagy via the ROS/ERK1/2 signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2018, 9, 157. [Google Scholar] [CrossRef]
- Steinmann, P.; Walters, D.K.; Arlt, M.J.; Banke, I.J.; Ziegler, U.; Langsam, B.; Arbiser, J.; Muff, R.; Born, W.; Fuchs, B. Antimetastatic activity of honokiol in osteosarcoma. Cancer 2012, 118, 2117–2127. [Google Scholar] [CrossRef]
- Yang, J.; Zou, Y.; Jiang, D. Honokiol suppresses proliferation and induces apoptosis via regulation of the miR21/PTEN/PI3K/AKT signaling pathway in human osteosarcoma cells. Int. J. Mol. Med. 2018, 41, 1845–1854. [Google Scholar] [CrossRef]
- Kim, D.W.; Ko, S.M.; Jeon, Y.J.; Noh, Y.W.; Choi, N.J.; Cho, S.D.; Moon, H.S.; Cho, Y.S.; Shin, J.C.; Park, S.M.; et al. Anti-proliferative effect of honokiol in oral squamous cancer through the regulation of specificity protein 1. Int. J. Oncol. 2013, 43, 1103–1110. [Google Scholar] [CrossRef]
- Xu, Q.; Tong, F.; He, C.; Song, P.; Xu, Q.; Chen, Z. The inhibition effect of Honokiol in liver cancer. Int. J. Clin. Exp. Med. 2018, 11, 10673–10678. [Google Scholar]
- Han, L.L.; Xie, L.P.; Li, L.H.; Zhang, X.W.; Zhang, R.Q.; Wang, H.Z. Reactive oxygen species production and Bax/Bcl-2 regulation in honokiol-induced apoptosis in human hepatocellular carcinoma SMMC-7721 cells. Environ. Toxicol. Pharmacol. 2009, 28, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhong, Q.; Chen, L.J.; Qi, X.R.; Fu, A.F.; Yang, H.S.; Yang, F.; Lin, H.G.; Wei, Y.Q.; Zhao, X. Liposomal honokiol, a promising agent for treatment of cisplatin-resistant human ovarian cancer. J. Cancer Res. Clin. Oncol. 2008, 134, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Y.; Zhao, X.; Pan, X.; Yin, R.; Huang, C.; Chen, L.; Wei, Y. Honokiol, a natural therapeutic candidate, induces apoptosis and inhibits angiogenesis of ovarian tumor cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 140, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.R.; Singh, S.V. Honokiol causes G0-G1 phase cell cycle arrest in human prostate cancer cells in association with suppression of retinoblastoma protein level/phosphorylation and inhibition of E2F1 transcriptional activity. Mol. Cancer Ther. 2007, 6, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, K.; Arbiser, J.L.; Sun, S.Y.; Zayzafoon, M.; Johnstone, P.A.; Fujisawa, M.; Gotoh, A.; Weksler, B.; Zhau, H.E.; Chung, L.W. Honokiol, a natural plant product, inhibits the bone metastatic growth of human prostate cancer cells. Cancer 2007, 109, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.R.; Sakao, K.; Singh, S.V. Honokiol activates reactive oxygen species-mediated cytoprotective autophagy in human prostate cancer cells. Prostate 2014, 74, 1209–1221. [Google Scholar] [CrossRef]
- Leeman-Neill, R.J.; Cai, Q.; Joyce, S.C.; Thomas, S.M.; Bhola, N.E.; Neill, D.B.; Arbiser, J.L.; Grandis, J.R. Honokiol inhibits epidermal growth factor receptor signaling and enhances the antitumor effects of epidermal growth factor receptor inhibitors. Clin. Cancer Res. 2010, 16, 2571–2579. [Google Scholar] [CrossRef]
- Lin, J.W.; Chen, J.T.; Hong, C.Y.; Lin, Y.L.; Wang, K.T.; Yao, C.J.; Lai, G.M.; Chen, R.M. Honokiol traverses the blood-brain barrier and induces apoptosis of neuroblastoma cells via an intrinsic bax-mitochondrion-cytochrome c-caspase protease pathway. Neuro-Oncology 2012, 14, 302–314. [Google Scholar] [CrossRef]
- Yeh, P.S.; Wang, W.; Chang, Y.A.; Lin, C.J.; Wang, J.J.; Chen, R.M. Honokiol induces autophagy of neuroblastoma cells through activating the PI3K/Akt/mTOR and endoplasmic reticular stress/ERK1/2 signaling pathways and suppressing cell migration. Cancer Lett. 2016, 370, 66–77. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, W.; Ye, C.; Zhuang, J.; Chang, C.; Li, Y.; Huang, X.; Shen, L.; Li, Y.; Cui, Y.; et al. Honokiol inhibits bladder tumor growth by suppressing EZH2/miR-143 axis. Oncotarget 2015, 6, 37335–37348. [Google Scholar] [CrossRef]
- Bao, L.; Jaramillo, M.C.; Zhang, Z.; Zheng, Y.; Yao, M.; Zhang, D.D.; Yi, X. Induction of autophagy contributes to cisplatin resistance in human ovarian cancer cells. Mol. Med. Rep. 2015, 11, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, J.; Xin, Q. Effects of tetracycline on developmental toxicity and molecular responses in zebrafish (Danio rerio) embryos. Ecotoxicology 2015, 24, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Chen, L.; Snaar-Jagalska, E.; Chaudhry, B. Embryonic zebrafish xenograft assay of human cancer metastasis. F1000Research 2018, 7, 1682. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Han, W.; Gu, Y.; Qiu, S.; Lu, Q.; Jin, J.; Luo, J.; Hu, X. Honokiol Induces a Necrotic Cell Death through the Mitochondrial Permeability Transition Pore. Cancer Res. 2007, 67, 4894. [Google Scholar] [CrossRef] [PubMed]
- Sheu, M.L.; Liu, S.H.; Lan, K.H. Honokiol induces calpain-mediated glucose-regulated protein-94 cleavage and apoptosis in human gastric cancer cells and reduces tumor growth. PLoS ONE 2007, 2, e1096. [Google Scholar] [CrossRef]
- Liu, S.H.; Wang, K.B.; Lan, K.H.; Lee, W.J.; Pan, H.C.; Wu, S.M.; Peng, Y.C.; Chen, Y.C.; Shen, C.C.; Cheng, H.C.; et al. Calpain/SHP-1 Interaction by Honokiol Dampening Peritoneal Dissemination of Gastric Cancer in nu/nu Mice. PLoS ONE 2012, 7, e43711. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; He, X.; Fan, L.; Yang, G.; Chen, X.; Lin, X.; Du, L.; Li, Z.; Ye, H.; et al. Enhancement of therapeutic effectiveness by combining liposomal honokiol with cisplatin in ovarian carcinoma. Int. J. Gynecol. Cancer 2008, 18, 652–659. [Google Scholar] [CrossRef]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. Biomed Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Gerl, R.; Vaux, D.L. Apoptosis in the development and treatment of cancer. Carcinogenesis 2005, 26, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Letai, A. Apoptosis and Cancer. Annu. Rev. Cancer Biol. 2017, 1, 275–294. [Google Scholar] [CrossRef]
- Jeong, Y.-H.; Hur, J.H.; Jeon, E.-J.; Park, S.-J.; Hwang, T.J.; Lee, S.A.; Lee, W.K.; Sung, J.M. Honokiol Improves Liver Steatosis in Ovariectomized Mice. Molecules 2018, 23, 194. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-S.; Yao, C.-J.; Chuang, S.-E.; Yeh, C.-T.; Lee, L.-M.; Chen, R.-M.; Chao, W.-J.; Whang-Peng, J.; Lai, G.-M. Honokiol inhibits sphere formation and xenograft growth of oral cancer side population cells accompanied with JAK/STAT signaling pathway suppression and apoptosis induction. BMC Cancer 2016, 16, 245. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Katiyar, S.K. Honokiol, an Active Compound of Magnolia Plant, Inhibits Growth, and Progression of Cancers of Different Organs. Adv. Exp. Med. Biol. 2016, 928, 245–265. [Google Scholar] [CrossRef] [PubMed]
- Banik, K.; Ranaware, A.M.; Deshpande, V.; Nalawade, S.P.; Padmavathi, G.; Bordoloi, D.; Sailo, B.L.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; et al. Honokiol for cancer therapeutics: A traditional medicine that can modulate multiple oncogenic targets. Pharmacol. Res. 2019, 144, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Beitler, J.J.; Huang, W.; Chen, G.; Qian, G.; Magliocca, K.; Patel, M.R.; Chen, A.Y.; Zhang, J.; Nannapaneni, S.; et al. Honokiol Radiosensitizes Squamous Cell Carcinoma of the Head and Neck by Downregulation of Survivin. Clin. Cancer Res. 2018, 24, 858–869. [Google Scholar] [CrossRef]
- Garcia, A.; Zheng, Y.; Zhao, C.; Toschi, A.; Fan, J.; Shraibman, N.; Brown, H.A.; Bar-Sagi, D.; Foster, D.A.; Arbiser, J.L. Honokiol Suppresses Survival Signals Mediated by Ras-Dependent Phospholipase D Activity in Human Cancer Cells. Clin. Cancer Res. 2008, 14, 4267. [Google Scholar] [CrossRef]
- Fried, L.E.; Arbiser, J.L. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid. Redox Signal. 2009, 11, 1139–1148. [Google Scholar] [CrossRef]
- Li, H.Y.; Ye, H.G.; Chen, C.Q.; Yin, L.H.; Wu, J.B.; He, L.C.; Gao, S.M. Honokiol induces cell cycle arrest and apoptosis via inhibiting class I histone deacetylases in acute myeloid leukemia. J. Cell. Biochem. 2015, 116, 287–298. [Google Scholar] [CrossRef]
- Deng, J.; Qian, Y.; Geng, L.; Chen, J.; Wang, X.; Xie, H.; Yan, S.; Jiang, G.; Zhou, L.; Zheng, S. Involvement of p38 mitogen-activated protein kinase pathway in honokiol-induced apoptosis in a human hepatoma cell line (hepG2). Liver Int. 2008, 28, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Yonezawa, T.; Ahn, J.Y.; Cha, B.Y.; Teruya, T.; Takami, M.; Yagasaki, K.; Nagai, K.; Woo, J.T. Honokiol inhibits osteoclast differentiation and function in vitro. Biol. Pharm. Bull. 2010, 33, 487–492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tse, A.K.; Wan, C.K.; Shen, X.L.; Yang, M.; Fong, W.F. Honokiol inhibits TNF-alpha-stimulated NF-kappaB activation and NF-kappaB-regulated gene expression through suppression of IKK activation. Biochem. Pharmacol. 2005, 70, 1443–1457. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shao, X.; Wu, L.; Feng, T.; Jin, C.; Fang, M.; Wu, N.; Yao, H. Honokiol: An effective inhibitor of tumor necrosis factor-alpha-induced up-regulation of inflammatory cytokine and chemokine production in human synovial fibroblasts. Acta Biochim. Biophys. Sin. 2011, 43, 380–386. [Google Scholar] [CrossRef]
- Xu, H.L.; Tang, W.; Du, G.H.; Kokudo, N. Targeting apoptosis pathways in cancer with magnolol and honokiol, bioactive constituents of the bark of Magnolia officinalis. Drug Discov. Ther. 2011, 5, 202–210. [Google Scholar] [CrossRef]
- Raja, S.M.; Chen, S.; Yue, P.; Acker, T.M.; Lefkove, B.; Arbiser, J.L.; Khuri, F.R.; Sun, S.Y. The natural product honokiol preferentially inhibits cellular FLICE-inhibitory protein and augments death receptor-induced apoptosis. Mol. Cancer Ther. 2008, 7, 2212–2223. [Google Scholar] [CrossRef]
- Rauf, A.; Patel, S.; Imran, M.; Maalik, A.; Arshad, M.U.; Saeed, F.; Mabkhot, Y.N.; Al-Showiman, S.S.; Ahmad, N.; Elsharkawy, E. Honokiol: An anticancer lignan. Biomed. Pharmacother. 2018, 107, 555–562. [Google Scholar] [CrossRef]
- Schroder, M.; Kaufman, R.J. ER stress and the unfolded protein response. Mutat. Res. 2005, 569, 29–63. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Ferri, K.F.; Kroemer, G. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 2001, 3, E255–E263. [Google Scholar] [CrossRef]
- Chiu, C.-S.; Tsai, C.-H.; Hsieh, M.-S.; Tsai, S.-C.; Jan, Y.-J.; Lin, W.-Y.; Lai, D.-W.; Wu, S.-M.; Hsing, H.-Y.; Arbiser, J.L.; et al. Exploiting Honokiol-induced ER stress CHOP activation inhibits the growth and metastasis of melanoma by suppressing the MITF and β-catenin pathways. Cancer Lett. 2019, 442, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Shen, C.C.; Yi, Y.C.; Tsai, J.J.; Wang, C.C.; Chueh, J.T.; Lin, K.L.; Lee, T.C.; Pan, H.C.; Sheu, M.L. Honokiol inhibits gastric tumourigenesis by activation of 15-lipoxygenase-1 and consequent inhibition of peroxisome proliferator-activated receptor-γ and COX-2-dependent signals. Br. J. Pharmacol. 2010, 160, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Lee, W.J.; Lai, D.W.; Wu, S.M.; Liu, C.Y.; Tien, H.R.; Chiu, C.S.; Peng, Y.C.; Jan, Y.J.; Chao, T.H.; et al. Honokiol confers immunogenicity by dictating calreticulin exposure, activating ER stress and inhibiting epithelial-to-mesenchymal transition. Mol. Oncol. 2015, 9, 834–849. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Lamb, H.K.; Brady, C.; Lefkove, B.; Bonner, M.Y.; Thompson, P.; Lovat, P.E.; Arbiser, J.L.; Hawkins, A.R.; Redfern, C.P. Inducing apoptosis of cancer cells using small-molecule plant compounds that bind to GRP78. Br. J. Cancer 2013, 109, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Ju, M.K.; Jeon, H.M.; Jeong, E.K.; Lee, Y.J.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Regulation of Tumor Progression by Programmed Necrosis. Oxidative Med. Cell. Longev. 2018, 2018, 3537471. [Google Scholar] [CrossRef]
- Tian, W.; Xu, D.; Deng, Y.-C. Honokiol, a multifunctional tumor cell death inducer. Die Pharm. Int. J. Pharm. Sci. 2012, 67, 811–816. [Google Scholar] [CrossRef]
- Chen, G.; Izzo, J.; Demizu, Y.; Wang, F.; Guha, S.; Wu, X.; Hung, M.-C.; Ajani, J.A.; Huang, P. Different redox states in malignant and nonmalignant esophageal epithelial cells and differential cytotoxic responses to bile acid and honokiol. Antioxid. Redox Signal. 2009, 11, 1083–1095. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, Q.; Zhang, H.Y.; Zhang, X.; Huo, X.; Cheng, E.; Wang, D.H.; Arbiser, J.L.; Spechler, S.J.; Souza, R.F. Targeting the intrinsic inflammatory pathway: Honokiol exerts proapoptotic effects through STAT3 inhibition in transformed Barrett’s cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G561–G569. [Google Scholar] [CrossRef]
- Meier, J.A.; Hyun, M. Stress-induced dynamic regulation of mitochondrial STAT3 and its association with cyclophilin D reduce mitochondrial ROS production. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef]
- Cen, M.; Yao, Y.; Cui, L.; Yang, G.; Lu, G.; Fang, L.; Bao, Z.; Zhou, J. Honokiol induces apoptosis of lung squamous cell carcinoma by targeting FGF2-FGFR1 autocrine loop. Cancer Med. 2018, 7, 6205–6218. [Google Scholar] [CrossRef]
- Hahm, E.R.; Singh, K.B.; Singh, S.V. c-Myc is a novel target of cell cycle arrest by honokiol in prostate cancer cells. Cell Cycle 2016, 15, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Vaid, M.; Sharma, S.D.; Katiyar, S.K. Honokiol, a phytochemical from the Magnolia plant, inhibits photocarcinogenesis by targeting UVB-induced inflammatory mediators and cell cycle regulators: Development of topical formulation. Carcinogenesis 2010, 31, 2004–2011. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ma, L.; Zhao, Y.; Peng, A.; Cheng, B.; Zhou, Q.; Zheng, L.; Huang, K. Inhibitory effects of magnolol and honokiol on human calcitonin aggregation. Sci. Rep. 2015, 5, 13556. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Peng, Z.Y. Honokiol induces cell cycle arrest and apoptosis in human gastric carcinoma MGC-803 cell line. Int. J. Clin. Exp. Med. 2015, 8, 5454–5461. [Google Scholar] [PubMed]
- Grimmel, M.; Backhaus, C.; Proikas-Cezanne, T. WIPI-Mediated Autophagy and Longevity. Cells 2015, 4, 202–217. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef]
- Galluzzi, L.; Pietrocola, F.; Bravo-San Pedro, J.M.; Amaravadi, R.K.; Baehrecke, E.H.; Cecconi, F.; Codogno, P.; Debnath, J.; Gewirtz, D.A.; Karantza, V.; et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015, 34, 856–880. [Google Scholar] [CrossRef]
- Lin, L.; Baehrecke, E.H. Autophagy, cell death, and cancer. Mol. Cell Oncol. 2015, 2, e985913. [Google Scholar] [CrossRef]
- Paquette, M.; El-Houjeiri, L.; Pause, A. mTOR Pathways in Cancer and Autophagy. Cancers 2018, 10, 18. [Google Scholar] [CrossRef]
- Murray, J.T.; Tee, A.R. Mechanistic Target of Rapamycin (mTOR) in the Cancer Setting. Cancers 2018, 10, 168. [Google Scholar] [CrossRef]
- Itakura, E.; Mizushima, N. Atg14 and UVRAG: Mutually exclusive subunits of mammalian Beclin 1-PI3K complexes. Autophagy 2009, 5, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Torii, S.; Yoshida, T.; Arakawa, S.; Honda, S.; Nakanishi, A.; Shimizu, S. Identification of PPM1D as an essential Ulk1 phosphatase for genotoxic stress-induced autophagy. EMBO Rep. 2016, 17, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Criollo, A.; Kroemer, G. Crosstalk between apoptosis and autophagy within the Beclin 1 interactome. EMBO J. 2010, 29, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.R.; Simonsen, A. Membrane dynamics in autophagosome biogenesis. J. Cell Sci. 2015, 128, 193. [Google Scholar] [CrossRef] [PubMed]
- Mochida, K.; Oikawa, Y.; Kimura, Y.; Kirisako, H.; Hirano, H.; Ohsumi, Y.; Nakatogawa, H. Receptor-mediated selective autophagy degrades the endoplasmic reticulum and the nucleus. Nature 2015, 522, 359–362. [Google Scholar] [CrossRef]
- Wrighton, K.H. Selecting ER for eating. Nat. Rev. Mol. Cell Biol. 2015, 16, 389. [Google Scholar] [CrossRef]
- Chio, C.C.; Chen, K.Y.; Chang, C.K.; Chuang, J.Y.; Liu, C.C.; Liu, S.H.; Chen, R.M. Improved effects of honokiol on temozolomide-induced autophagy and apoptosis of drug-sensitive and -tolerant glioma cells. BMC Cancer 2018, 18, 379. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Roche, J. The Epithelial-to-Mesenchymal Transition in Cancer. Cancers 2018, 10, 52. [Google Scholar] [CrossRef]
- Røsland, G.V.; Dyrstad, S.E.; Tusubira, D.; Helwa, R.; Tan, T.Z.; Lotsberg, M.L.; Pettersen, I.K.N.; Berg, A.; Kindt, C.; Hoel, F.; et al. Epithelial to mesenchymal transition (EMT) is associated with attenuation of succinate dehydrogenase (SDH) in breast cancer through reduced expression of SDHC. Cancer Metab. 2019, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhang, F.; Huang, R.; Yan, J.; Shen, B. Honokiol inhibits bladder cancer cell invasion through repressing SRC-3 expression and epithelial-mesenchymal transition. Oncol. Lett. 2017, 14, 4294–4300. [Google Scholar] [CrossRef] [PubMed]
- Avtanski, D.B.; Nagalingam, A.; Bonner, M.Y.; Arbiser, J.L.; Saxena, N.K.; Sharma, D. Honokiol inhibits epithelial-mesenchymal transition in breast cancer cells by targeting signal transducer and activator of transcription 3/Zeb1/E-cadherin axis. Mol. Oncol. 2014, 8, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.-Q.; Qiao, X.-R.; Su, L.; Chen, S.-Z. Honokiol inhibits EMT-mediated motility and migration of human non-small cell lung cancer cells in vitro by targeting c-FLIP. Acta Pharmacol. Sin. 2016, 37, 1574–1586. [Google Scholar] [CrossRef]
- Qin, L.; Liu, Z.; Chen, H.; Xu, J. The steroid receptor coactivator-1 regulates twist expression and promotes breast cancer metastasis. Cancer Res. 2009, 69, 3819–3827. [Google Scholar] [CrossRef]
- Yao, C.-J.; Lai, G.-M.; Yeh, C.-T.; Lai, M.-T.; Shih, P.-H.; Chao, W.-J.; Whang-Peng, J.; Chuang, S.-E.; Lai, T.-Y. Honokiol Eliminates Human Oral Cancer Stem-Like Cells Accompanied with Suppression of Wnt/β-Catenin Signaling and Apoptosis Induction. Evid.-Based Complement. Altern. Med. 2013, 2013, 146136. [Google Scholar] [CrossRef]
- Wang, W.D.; Shang, Y.; Li, Y.; Chen, S.Z. Honokiol inhibits breast cancer cell metastasis by blocking EMT through modulation of Snail/Slug protein translation. Acta Pharm. Sin. 2019, 40, 1219–1227. [Google Scholar] [CrossRef]
- Galichon, P.; Hertig, A. Epithelial to mesenchymal transition as a biomarker in renal fibrosis: Are we ready for the bedside? Fibrogenesis Tissue Repair 2011, 4, 11. [Google Scholar] [CrossRef]
- Conacci-Sorrell, M.; Ngouenet, C.; Anderson, S.; Brabletz, T.; Eisenman, R.N. Stress-induced cleavage of Myc promotes cancer cell survival. Genes Dev. 2014, 28, 689–707. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Wyckoff, J.; Condeelis, J. Cell migration in tumors. Curr. Opin. Cell Biol. 2005, 17, 559–564. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Tay, R.Y.; Fernández-Gutiérrez, F.; Foy, V.; Burns, K.; Pierce, J.; Morris, K.; Priest, L.; Tugwood, J.; Ashcroft, L.; Lindsay, C.R.; et al. Prognostic value of circulating tumour cells in limited-stage small-cell lung cancer: Analysis of the concurrent once-daily versus twice-daily radiotherapy (CONVERT) randomised controlled trial. Ann. Oncol. 2019, 30, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Katiyar, S.K. Honokiol, a phytochemical from Magnolia spp., inhibits breast cancer cell migration by targeting nitric oxide and cyclooxygenase-2. Int. J. Oncol. 2011, 38, 769–776. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Zhang, Y.; Shen, W.; Fu, R.; Ding, Z.; Zhen, Y.; Wan, Y. Cytological effects of honokiol treatment and its potential mechanism of action in non-small cell lung cancer. Biomed. Pharmacother. 2019, 117, 109058. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Castillo, V.; Welty, M.; Eliaz, I.; Sliva, D. Honokiol inhibits migration of renal cell carcinoma through activation of RhoA/ROCK/MLC signaling pathway. Int. J. Oncol. 2016, 49, 1525–1530. [Google Scholar] [CrossRef]
- Balan, M.; Chakraborty, S.; Flynn, E.; Zurakowski, D.; Pal, S. Honokiol inhibits c-Met-HO-1 tumor-promoting pathway and its cross-talk with calcineurin inhibitor-mediated renal cancer growth. Sci. Rep. 2017, 7, 5900. [Google Scholar] [CrossRef]
- Alizadeh, A.M.; Shiri, S.; Farsinejad, S. Metastasis review: From bench to bedside. Tumor Biol. 2014, 35, 8483–8523. [Google Scholar] [CrossRef]
- Klein, C.A. Cancer. The metastasis cascade. Science 2008, 321, 1785–1787. [Google Scholar] [CrossRef]
- Bai, X.; Cerimele, F.; Ushio-Fukai, M.; Waqas, M.; Campbell, P.M.; Govindarajan, B.; Der, C.J.; Battle, T.; Frank, D.A.; Ye, K.; et al. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J. Biol. Chem. 2003, 278, 35501–35507. [Google Scholar] [CrossRef]
- Kolligs, F.T.; Bommer, G.; Goke, B. Wnt/beta-catenin/tcf signaling: A critical pathway in gastrointestinal tumorigenesis. Digestion 2002, 66, 131–144. [Google Scholar] [CrossRef]
- Hlubek, F.; Spaderna, S.; Jung, A.; Kirchner, T.; Brabletz, T. Beta-catenin activates a coordinated expression of the proinvasive factors laminin-5 gamma2 chain and MT1-MMP in colorectal carcinomas. Int. J. Cancer 2004, 108, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Castillo, V.; Eliaz, I.; Sliva, D. Honokiol suppresses metastasis of renal cell carcinoma by targeting KISS1/KISS1R signaling. Int. J. Oncol. 2015, 46, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Tonini, T.; Rossi, F.; Claudio, P.P. Molecular basis of angiogenesis and cancer. Oncogene 2003, 22, 6549–6556. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Basu, A.; Arbiser, J.L.; Pal, S. The natural product honokiol inhibits calcineurin inhibitor-induced and Ras-mediated tumor promoting pathways. Cancer Lett. 2013, 338, 292–299. [Google Scholar] [CrossRef]
- Vavilala, D.T.; Ponnaluri, V.K.C.; Kanjilal, D.; Mukherji, M. Evaluation of anti-HIF and anti-angiogenic properties of honokiol for the treatment of ocular neovascular diseases. PLoS ONE 2014, 9, e113717. [Google Scholar] [CrossRef]
- Vavilala, D.T.; O’Bryhim, B.E.; Ponnaluri, V.K.; White, R.S.; Radel, J.; Symons, R.C.; Mukherji, M. Honokiol inhibits pathological retinal neovascularization in oxygen-induced retinopathy mouse model. Biochem. Biophys. Res. Commun. 2013, 438, 697–702. [Google Scholar] [CrossRef]
- Wen, J.; Wang, X.; Pei, H.; Xie, C.; Qiu, N.; Li, S.; Wang, W.; Cheng, X.; Chen, L. Anti-psoriatic effects of Honokiol through the inhibition of NF-kappaB and VEGFR-2 in animal model of K14-VEGF transgenic mouse. J. Pharmacol. Sci. 2015, 128, 116–124. [Google Scholar] [CrossRef][Green Version]
- Hu, J.; Chen, L.J.; Liu, L.; Chen, X.; Chen, P.L.; Yang, G.; Hou, W.L.; Tang, M.H.; Zhang, F.; Wang, X.H.; et al. Liposomal honokiol, a potent anti-angiogenesis agent, in combination with radiotherapy produces a synergistic antitumor efficacy without increasing toxicity. Exp. Mol. Med. 2008, 40, 617–628. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-signaling pathway in cancer. Onco Targets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Sethi, G.; Shishodia, S.; Sung, B.; Arbiser, J.L.; Aggarwal, B.B. Honokiol Potentiates Apoptosis, Suppresses Osteoclastogenesis, and Inhibits Invasion through Modulation of Nuclear Factor-κB Activation Pathway. Mol. Cancer Res. 2006, 4, 621. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X. Chemopreventive Activity of Honokiol against 7, 12—Dimethylbenz[a]anthracene-Induced Mammary Cancer in Female Sprague Dawley Rats. Front. Pharmacol. 2017, 8, 320. [Google Scholar] [CrossRef]
- Katiyar, S.K. Emerging Phytochemicals for the Prevention and Treatment of Head and Neck Cancer. Molecules 2016, 21, 1610. [Google Scholar] [CrossRef]
- Hua, H.; Chen, W.; Shen, L.; Sheng, Q.; Teng, L. Honokiol augments the anti-cancer effects of oxaliplatin in colon cancer cells. Acta Biochim. Biophys. Sin. 2013, 45, 773–779. [Google Scholar] [CrossRef][Green Version]
- Nabekura, T.; Hiroi, T.; Kawasaki, T.; Uwai, Y. Effects of natural nuclear factor-kappa B inhibitors on anticancer drug efflux transporter human P-glycoprotein. Biomed. Pharmacother. 2015, 70, 140–145. [Google Scholar] [CrossRef]
- Furqan, M.; Akinleye, A.; Mukhi, N.; Mittal, V.; Chen, Y.; Liu, D. STAT inhibitors for cancer therapy. J. Hematol. Oncol. 2013, 6, 90. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Pan, J.; Lee, Y.; Cheng, G.; Zielonka, J.; Zhang, Q.; Bajzikova, M.; Xiong, D.; Tsaih, S.-W.; Hardy, M.; Flister, M.; et al. Mitochondria-Targeted Honokiol Confers a Striking Inhibitory Effect on Lung Cancer via Inhibiting Complex I Activity. iScience 2018, 3, 192–207. [Google Scholar] [CrossRef]
- He, Z.; Subramaniam, D.; Zhang, Z.; Zhang, Y.; Anant, S. Honokiol as a Radiosensitizing Agent for Colorectal cancers. Curr. Colorectal Cancer Rep. 2013, 9, 358–364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bi, L.; Yu, Z.; Wu, J.; Yu, K.; Hong, G.; Lu, Z.; Gao, S. Honokiol Inhibits Constitutive and Inducible STAT3 Signaling via PU.1-Induced SHP1 Expression in Acute Myeloid Leukemia Cells. Tohoku J. Exp. Med. 2015, 237, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, C.; Arbiser, J.L.; Mori, N. Honokiol induces cell cycle arrest and apoptosis via inhibition of survival signals in adult T-cell leukemia. Biochim. Biophys. Acta 2012, 1820, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Hiroki, K.; Yamashita, Y. The role of epidermal growth factor receptor in cancer metastasis and microenvironment. BioMed Res. Int. 2013, 2013, 546318. [Google Scholar] [CrossRef] [PubMed]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, D.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Kari, C.; Chan, T.O.; Rocha de Quadros, M.; Rodeck, U. Targeting the Epidermal Growth Factor Receptor in Cancer. Cancer Res. 2003, 63, 1. [Google Scholar] [PubMed]
- Yewale, C.; Baradia, D.; Vhora, I.; Patil, S.; Misra, A. Epidermal growth factor receptor targeting in cancer: A review of trends and strategies. Biomaterials 2013, 34, 8690–8707. [Google Scholar] [CrossRef]
- Song, J.M.; Anandharaj, A.; Upadhyaya, P.; Kirtane, A.R.; Kim, J.-H.; Hong, K.H.; Panyam, J.; Kassie, F. Honokiol suppresses lung tumorigenesis by targeting EGFR and its downstream effectors. Oncotarget 2016, 7, 57752–57769. [Google Scholar] [CrossRef]
- Dai, X.; Li, R.-Z.; Jiang, Z.-B.; Wei, C.-L.; Luo, L.-X.; Yao, X.-J.; Li, G.-P.; Leung, E.L.-H. Honokiol Inhibits Proliferation, Invasion and Induces Apoptosis Through Targeting Lyn Kinase in Human Lung Adenocarcinoma Cells. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Biscardi, J.S.; Ishizawar, R.C.; Silva, C.M.; Parsons, S.J. Tyrosine kinase signalling in breast cancer: Epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res. 2000, 2, 203–210. [Google Scholar] [CrossRef]
- Singh, T.; Gupta, N.A.; Xu, S.; Prasad, R.; Velu, S.E.; Katiyar, S.K. Honokiol inhibits the growth of head and neck squamous cell carcinoma by targeting epidermal growth factor receptor. Oncotarget 2015, 6, 21268–21282. [Google Scholar] [CrossRef] [PubMed]
- Dufour, M.; Dormond-Meuwly, A.; Demartines, N.; Dormond, O. Targeting the Mammalian Target of Rapamycin (mTOR) in Cancer Therapy: Lessons from Past and Future Perspectives. Cancers 2011, 3, 2478–2500. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dong, H.; Li, M.; Wu, Y.; Liu, Y.; Zhao, Y.; Chen, X.; Ma, M. Honokiol induces autophagy and apoptosis of osteosarcoma through PI3K/Akt/mTOR signaling pathway. Mol. Med. Rep. 2018, 17, 2719–2723. [Google Scholar] [CrossRef]
- Pezzuto, A.; Carico, E. Role of HIF-1 in Cancer Progression: Novel Insights. A Review. Curr. Mol. Med. 2018, 18, 343–351. [Google Scholar] [CrossRef]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- Soni, S.; Padwad, Y.S. HIF-1 in cancer therapy: Two decade long story of a transcription factor. Acta Oncol. 2017, 56, 503–515. [Google Scholar] [CrossRef]
- Aster, J.C.; Pear, W.S.; Blacklow, S.C. The Varied Roles of Notch in Cancer. Annu. Rev. Pathol. 2017, 12, 245–275. [Google Scholar] [CrossRef]
- Venkatesh, V.; Nataraj, R.; Thangaraj, G.S.; Karthikeyan, M.; Gnanasekaran, A.; Kaginelli, S.B.; Kuppanna, G.; Kallappa, C.G.; Basalingappa, K.M. Targeting Notch signalling pathway of cancer stem cells. Stem Cell Investig. 2018, 5, 5. [Google Scholar] [CrossRef]
- Nowell, C.S.; Radtke, F. Notch as a tumour suppressor. Nat. Rev. Cancer 2017, 17, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Venugopal, A.; Ramamoorthy, P.; Standing, D.; Subramaniam, D.; Umar, S.; Jensen, R.A.; Anant, S.; Mammen, J.M. Honokiol inhibits melanoma stem cells by targeting notch signaling. Mol. Carcinog. 2015, 54, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Wynn, M.L.; Consul, N.; Merajver, S.D.; Schnell, S. Inferring the Effects of Honokiol on the Notch Signaling Pathway in SW480 Colon Cancer Cells. Cancer Inform. 2014, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, R.; Luk, F.; Bebawy, M. Inhibition of the multidrug resistance P-glycoprotein: Time for a change of strategy? Drug Metab. Dispos. 2014, 42, 623–631. [Google Scholar] [CrossRef]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2018, 61, 5108–5121. [Google Scholar] [CrossRef]
- Nanayakkara, A.K.; Follit, C.A.; Chen, G.; Williams, N.S.; Vogel, P.D.; Wise, J.G. Targeted inhibitors of P-glycoprotein increase chemotherapeutic-induced mortality of multidrug resistant tumor cells. Sci. Rep. 2018, 8, 967. [Google Scholar] [CrossRef]
- Han, H.K.; Van Anh, L.T. Modulation of P-glycoprotein expression by honokiol, magnolol and 4-O-methylhonokiol, the bioactive components of Magnolia officinalis. Anticancer Res. 2012, 32, 4445–4452. [Google Scholar]
- Wang, X.; Cho, K.; Chen, Z.; Arbiser, J.; Shin, D. Honokiol reduces drug resistance by inhibition of P-glycoprotein expression in multidrug resistant (MDR) squamous cell carcinoma of the head and neck (SCCHN). Cancer Res. 2007, 67, 2776. [Google Scholar]
- Xu, D.; Lu, Q.; Hu, X. Down-regulation of P-glycoprotein expression in MDR breast cancer cell MCF-7/ADR by honokiol. Cancer Lett. 2006, 243, 274–280. [Google Scholar] [CrossRef]
- Wang, H.; Liao, Z.; Sun, X.; Shi, Q.; Huo, G.; Xie, Y.; Tang, X.; Zhi, X.; Tang, Z. Intravenous administration of Honokiol provides neuroprotection and improves functional recovery after traumatic brain injury through cell cycle inhibition. Neuropharmacology 2014, 86, 9–21. [Google Scholar] [CrossRef]
- Godugu, C.; Doddapaneni, R.; Singh, M. Honokiol nanomicellar formulation produced increased oral bioavailability and anticancer effects in triple negative breast cancer (TNBC). Colloids Surf. B 2017, 153, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Chou, C.J.; Cheng, F.C.; Chen, C.F. Pharmacokinetics of honokiol after intravenous administration in rats assessed using high-performance liquid chromatography. J. Chromatogr. B 1994, 655, 41–45. [Google Scholar] [CrossRef]
- Liang, Y.; Cui, G.; Wang, X.; Zhang, W.; An, Q.; Lin, Z.; Wang, H.; Chen, S. Pharmacokinetics of honokiol after intravenous guttae in beagle dogs assessed using ultra-performance liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2014, 28, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Su, W.J.; Huang, X.; Qin, E.; Jiang, L.; Ren, P. Pharmacokinetics of honokiol in rat after oral administration of Cortex of Magnolia officinalis and its compound preparation Houpu Sanwu Decoction. J. Chin. Med. Mater. 2008, 31, 255–258. [Google Scholar]
- Wang, J.; Miao, X.L.; Chen, J.Y.; Chen, Y. The Pharmacokinetics and Tissue Distribution of Honokiol and its Metabolites in Rats. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 587–594. [Google Scholar] [CrossRef]
- Sheng, Y.L.; Xu, J.H.; Shi, C.H.; Li, W.; Xu, H.Y.; Li, N.; Zhao, Y.Q.; Zhang, X.R. UPLC-MS/MS-ESI assay for simultaneous determination of magnolol and honokiol in rat plasma: Application to pharmacokinetic study after administration emulsion of the isomer. J. Ethnopharmacol. 2014, 155, 1568–1574. [Google Scholar] [CrossRef]
- Han, M.; Yu, X.; Guo, Y.; Wang, Y.; Kuang, H.; Wang, X. Honokiol nanosuspensions: Preparation, increased oral bioavailability and dramatically enhanced biodistribution in the cardio-cerebro-vascular system. Colloids Surf. B 2014, 116, 114–120. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Hu, Z. High-performance liquid chromatographic method for simultaneous determination of honokiol and magnolol in rat plasma. Talanta 2003, 59, 115–121. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, T.; Katiyar, S.K. Honokiol inhibits ultraviolet radiation-induced immunosuppression through inhibition of ultraviolet-induced inflammation and DNA hypermethylation in mouse skin. Sci. Rep. 2017, 7, 1657. [Google Scholar] [CrossRef]
- Gao, X.; Patel, M.G.; Bakshi, P.; Sharma, D.; Banga, A.K. Enhancement in the Transdermal and Localized Delivery of Honokiol Through Breast Tissue. AAPS PharmSciTech 2018, 19, 3501–3511. [Google Scholar] [CrossRef]
- Wang, X.H.; Cai, L.L.; Zhang, X.Y.; Deng, L.Y.; Zheng, H.; Deng, C.Y.; Wen, J.L.; Zhao, X.; Wei, Y.Q.; Chen, L.J. Improved solubility and pharmacokinetics of PEGylated liposomal honokiol and human plasma protein binding ability of honokiol. Int. J. Pharm. 2011, 410, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.Q.; Fan, L.Y.; Yang, G.L.; Guo, W.H.; Hou, W.L.; Chen, L.J.; Wei, Y.Q. Improved therapeutic effectiveness by combining liposomal honokiol with cisplatin in lung cancer model. BMC Cancer 2008, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Sun, Q.; Yang, H.; Tang, B.; Pu, H.; Li, H. Honokiol nanoparticles based on epigallocatechin gallate functionalized chitin to enhance therapeutic effects against liver cancer. Int. J. Pharm. 2018, 545, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, L.; Wang, L.; Zu, Y.; Wang, S.; Liu, P.; Zhao, X. Preparation of honokiol nanoparticles by liquid antisolvent precipitation technique, characterization, pharmacokinetics, and evaluation of inhibitory effect on HepG2 cells. Int. J. Nanomed. 2018, 13, 5469–5483. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, Y.; Wang, T.; Zhao, S.; Qiu, H.; Han, M.; Wang, X. Honokiol nanoparticles stabilized by oligoethylene glycols codendrimer: In vitro and in vivo investigations. J. Mater. Chem. B 2017, 5, 697–706. [Google Scholar] [CrossRef]
- Li, X.; Hou, X.; Ding, W.; Cong, S.; Zhang, Y.; Chen, M.; Meng, Y.; Lei, J.; Liu, Y.; Li, G. Sirolimus-loaded polymeric micelles with honokiol for oral delivery. J. Pharm. Pharmacol. 2015, 67, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Z.; Nie, S.; Song, L.; He, T.; Yang, S.; Yang, X.; Yi, C.; Wu, Q.; Gong, C. Biodegradable polymeric micelles coencapsulating paclitaxel and honokiol: A strategy for breast cancer therapy in vitro and in vivo. Int. J. Nanomed. 2017, 12, 1499–1514. [Google Scholar] [CrossRef]
- Gong, C.; Wei, X.; Wang, X.; Wang, Y.; Guo, G.; Mao, Y.; Luo, F.; Qian, Z. Biodegradable self-assembled PEG–PCL–PEG micelles for hydrophobic honokiol delivery: I. Preparation and characterization. Nanotechnology 2010, 21, 215103. [Google Scholar] [CrossRef]
- Zheng, X.; Kan, B.; Gou, M.; Fu, S.; Zhang, J.; Men, K.; Chen, L.; Luo, F.; Zhao, Y.; Zhao, X.; et al. Preparation of MPEG–PLA nanoparticle for honokiol delivery in vitro. Int. J. Pharm. 2010, 386, 262–267. [Google Scholar] [CrossRef]
- Wang, B.; Gou, M.; Zheng, X.; Wei, X.; Gong, C.; Wang, X.; Zhao, Y.; Luo, F.; Chen, L.; Qian, Z.; et al. Co-delivery honokiol and doxorubicin in MPEG-PLA nanoparticles. J. Nanosci. Nanotechnol. 2010, 10, 4166–4172. [Google Scholar] [CrossRef]
- Yu, R.; Zou, Y.; Liu, B.; Guo, Y.; Wang, X.; Han, M. Surface modification of pH-sensitive honokiol nanoparticles based on dopamine coating for targeted therapy of breast cancer. Colloids Surf. B 2019, 177, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Shi, S.; Wang, X.; Wang, Y.; Fu, S.; Dong, P.; Chen, L.; Zhao, X.; Wei, Y.; Qian, Z. Novel composite drug delivery system for honokiol delivery: Self-assembled poly(ethylene glycol)-poly(epsilon-caprolactone)-poly(ethylene glycol) micelles in thermosensitive poly(ethylene glycol)-poly(epsilon-caprolactone)-poly(ethylene glycol) hydrogel. J. Phys. Chem. B 2009, 113, 10183–10188. [Google Scholar] [CrossRef] [PubMed]
- Qiu, N.; Cai, L.L.; Xie, D.; Wang, G.; Wu, W.; Zhang, Y.; Song, H.; Yin, H.; Chen, L. Synthesis, structural and in vitro studies of well-dispersed monomethoxy-poly(ethylene glycol)-honokiol conjugate micelles. Biomed. Mater. 2010, 5, 065006. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, X.; Wang, D.; Zou, Y.; Qu, X.; He, C.; Deng, Y.; Jin, Y.; Zhou, Y.; Zhou, Y.; et al. Concurrently suppressing multidrug resistance and metastasis of breast cancer by co-delivery of paclitaxel and honokiol with pH-sensitive polymeric micelles. Acta Biomater. 2017, 62, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, L.; Xie, H.-J.; Ju, R.-J.; Xiao, Y.; Fu, M.; Liu, J.-J.; Li, X.-T. Functional paclitaxel plus honokiol micelles destroying tumour metastasis in treatment of non-small-cell lung cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1154–1169. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Xia, T.; Han, Y.; He, Q.J.; Zhao, R.; Ma, J.R. Synergistic antitumor effects of liposomal honokiol combined with cisplatin in colon cancer models. Oncol. Lett. 2011, 2, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Ju, R.J.; Cheng, L.; Qiu, X.; Liu, S.; Song, X.L.; Peng, X.M.; Wang, T.; Li, C.Q.; Li, X.T. Hyaluronic acid modified daunorubicin plus honokiol cationic liposomes for the treatment of breast cancer along with the elimination vasculogenic mimicry channels. J. Drug Target. 2018, 26, 793–805. [Google Scholar] [CrossRef]
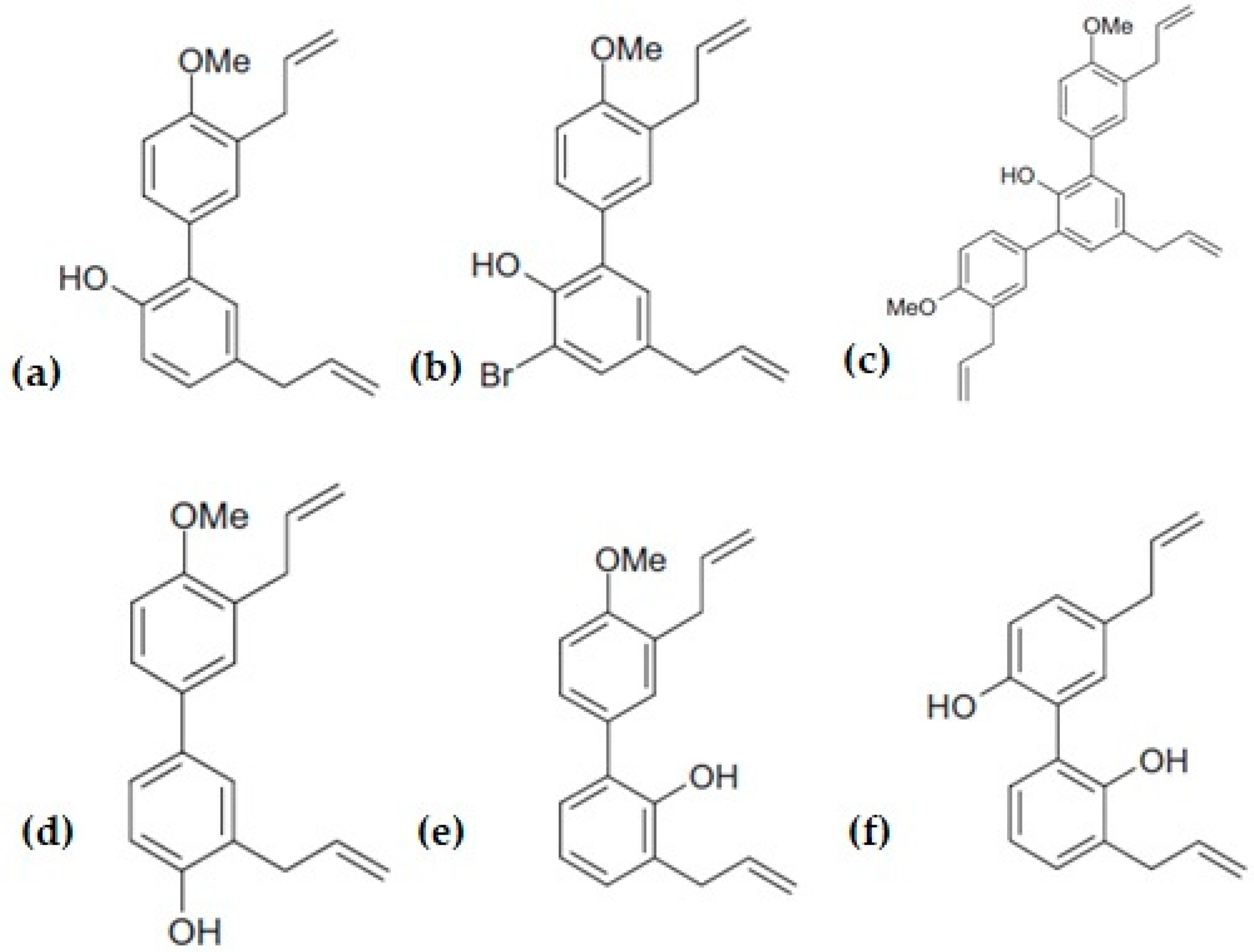
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, C.P.; Lee, W.L.; Tang, Y.Q.; Yap, W.H. Honokiol: A Review of Its Anticancer Potential and Mechanisms. Cancers 2020, 12, 48. https://doi.org/10.3390/cancers12010048
Ong CP, Lee WL, Tang YQ, Yap WH. Honokiol: A Review of Its Anticancer Potential and Mechanisms. Cancers. 2020; 12(1):48. https://doi.org/10.3390/cancers12010048
Chicago/Turabian StyleOng, Chon Phin, Wai Leong Lee, Yin Quan Tang, and Wei Hsum Yap. 2020. "Honokiol: A Review of Its Anticancer Potential and Mechanisms" Cancers 12, no. 1: 48. https://doi.org/10.3390/cancers12010048
APA StyleOng, C. P., Lee, W. L., Tang, Y. Q., & Yap, W. H. (2020). Honokiol: A Review of Its Anticancer Potential and Mechanisms. Cancers, 12(1), 48. https://doi.org/10.3390/cancers12010048