Individualizing Follow-Up Strategies in High-Grade Soft Tissue Sarcoma with Flexible Parametric Competing Risk Regression Models
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Statistical Analysis
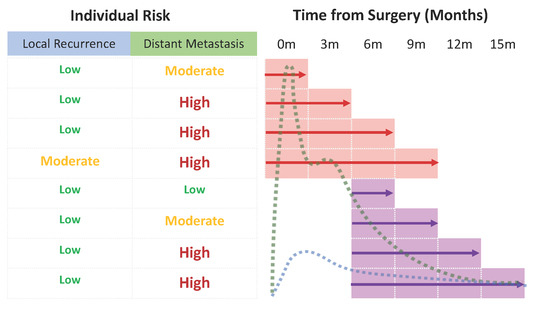
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Italiano, A.; Le Cesne, A.; Mendiboure, J.; Blay, J.Y.; Piperno-Neumann, S.; Chevreau, C.; Delcambre, C.; Penel, N.; Terrier, P.; Ranchere-Vince, D.; et al. Prognostic factors and impact of adjuvant treatments on local and metastatic relapse of soft-tissue sarcoma patients in the competing risks setting. Cancer 2014, 120, 3361–3369. [Google Scholar] [CrossRef] [PubMed]
- Maretty-Nielsen, K.; Aggerholm-Pedersen, N.; Safwat, A.; Jorgensen, P.H.; Hansen, B.H.; Baerentzen, S.; Pedersen, A.B.; Keller, J. Prognostic factors for local recurrence and mortality in adult soft tissue sarcoma of the extremities and trunk wall: A cohort study of 922 consecutive patients. Acta Orthop. 2014, 85, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Novais, E.N.; Demiralp, B.; Alderete, J.; Larson, M.C.; Rose, P.S.; Sim, F.H. Do surgical margin and local recurrence influence survival in soft tissue sarcomas? Clin. Orthop. Relat. Res. 2010, 468, 3003–3011. [Google Scholar] [CrossRef] [PubMed]
- Willeumier, J.; Fiocco, M.; Nout, R.; Dijkstra, S.; Aston, W.; Pollock, R.; Hartgrink, H.; Bovee, J.; van de Sande, M. High-grade soft tissue sarcomas of the extremities: Surgical margins influence only local recurrence not overall survival. Int. Orthop. 2015, 39, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.; Demetri, G.D.; Baldini, E.H.; Fletcher, C.D. Management of soft-tissue sarcomas: An overview and update. Lancet Oncol. 2000, 1, 75–85. [Google Scholar] [CrossRef]
- Fletcher, C.D.; Bridge, J.A.; Hodgendoorn, P.C.W.; Mertens, F. WHO Classification of Tumours of Soft Tissue and Bone; IARC: Lyon, France, 2013. [Google Scholar]
- Smolle, M.A.; van Praag, V.M.; Posch, F.; Bergovec, M.; Leitner, L.; Friesenbichler, J.; Heregger, R.; Riedl, J.M.; Pichler, M.; Gerger, A.; et al. Surgery for metachronous metastasis of soft tissue sarcoma—A magnitude of benefit analysis using propensity score methods. Eur. J. Surg. Oncol. 2018, 45, 242–248. [Google Scholar] [CrossRef]
- Casali, P.G.; Abecassis, N.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; Brodowicz, T.; Broto, J.M.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv51–iv67. [Google Scholar] [CrossRef]
- von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; Kane, J.M., 3rd; et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 536–563. [Google Scholar] [CrossRef]
- Gingrich, A.A.; Bateni, S.B.; Monjazeb, A.M.; Darrow, M.A.; Thorpe, S.W.; Kirane, A.R.; Bold, R.J.; Canter, R.J. Neoadjuvant Radiotherapy is Associated with R0 Resection and Improved Survival for Patients with Extremity Soft Tissue Sarcoma Undergoing Surgery: A National Cancer Database Analysis. Ann. Surg. Oncol. 2017, 24, 3252–3263. [Google Scholar] [CrossRef]
- Gronchi, A.; Ferrari, S.; Quagliuolo, V.; Broto, J.M.; Pousa, A.L.; Grignani, G.; Basso, U.; Blay, J.Y.; Tendero, O.; Beveridge, R.D.; et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): An international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017, 18, 812–822. [Google Scholar] [CrossRef]
- Posch, F.; Partl, R.; Doller, C.; Riedl, J.M.; Smolle, M.; Leitner, L.; Bergovec, M.; Liegl-Atzwanger, B.; Stotz, M.; Bezan, A.; et al. Benefit of Adjuvant Radiotherapy for Local Control, Distant Metastasis, and Survival Outcomes in Patients with Localized Soft Tissue Sarcoma: Comparative Effectiveness Analysis of an Observational Cohort Study. Ann. Surg. Oncol. 2018, 25, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Royce, T.J.; Punglia, R.S.; Chen, A.B.; Patel, S.A.; Thornton, K.A.; Raut, C.P.; Baldini, E.H. Cost-Effectiveness of Surveillance for Distant Recurrence in Extremity Soft Tissue Sarcoma. Ann. Surg. Oncol. 2017, 24, 3264–3270. [Google Scholar] [CrossRef] [PubMed]
- van Praag, V.M.; Rueten-Budde, A.J.; Jeys, L.M.; Laitinen, M.K.; Pollock, R.; Aston, W.; van der Hage, J.A.; Dijkstra, P.D.S.; Ferguson, P.C.; Griffin, A.M.; et al. A prediction model for treatment decisions in high-grade extremity soft-tissue sarcomas: Personalised sarcoma care (PERSARC). Eur. J. Cancer 2017, 83, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Royston, P.; Lambert, P.C. Flexible Parametric Survival Analysis Using Stata: Beyond the Cox Model; Stata Press: College Statoin, TX, USA, 2011. [Google Scholar]
- Callegaro, D.; Miceli, R.; Bonvalot, S.; Ferguson, P.; Strauss, D.C.; Levy, A.; Griffin, A.; Hayes, A.J.; Stacchiotti, S.; Pechoux, C.L.; et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: A retrospective analysis. Lancet Oncol. 2016, 17, 671–680. [Google Scholar] [CrossRef]
- Kang, H. The prevention and handling of the missing data. Korean J. Anesthesiol. 2013, 64, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Ranganathan, P.; Gulia, A.; Crasto, S.; Hawaldar, R.; Badwe, R.A. Does a less intensive surveillance protocol affect the survival of patients after treatment of a sarcoma of the limb? updated results of the randomized TOSS study. Bone Jt. J. 2018, 100-B, 262–268. [Google Scholar] [CrossRef]
- Hovgaard, T.B.; Nymark, T.; Skov, O.; Petersen, M.M. Follow-up after initial surgical treatment of soft tissue sarcomas in the extremities and trunk wall. Acta Oncol. 2017, 56, 1004–1012. [Google Scholar] [CrossRef]
- Rueten-Budde, A.J.; van Praag, V.M.; PERSARC studygroup; van de Sande, M.A.J.; Fiocco, M. Dynamic prediction of overall survival for patients with high-grade extremity soft tissue sarcoma. Surg. Oncol. 2018, 27, 695–701. [Google Scholar] [CrossRef]
- Kattan, M.W.; Leung, D.H.; Brennan, M.F. Postoperative nomogram for 12-year sarcoma-specific death. J. Clin. Oncol. 2002, 20, 791–796. [Google Scholar] [CrossRef]
- Lambert, P.C.; Royston, P. Further development of flexible parametric models for survival analysis. Stata J. 2009, 9, 265–290. [Google Scholar] [CrossRef]
- Wu, J.; Qian, S.; Jin, L. Prognostic factors of patients with extremity myxoid liposarcomas after surgery. J. Orthop. Surg. Res. 2019, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Trovik, C.S.; Bauer, H.C.; Alvegard, T.A.; Anderson, H.; Blomqvist, C.; Berlin, O.; Gustafson, P.; Saeter, G.; Walloe, A. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. Eur. J. Cancer 2000, 36, 710–716. [Google Scholar] [CrossRef]
- Fujiwara, T.; Stevenson, J.; Parry, M.; Tsuda, Y.; Tsoi, K.; Jeys, L. What is an adequate margin for infiltrative soft-tissue sarcomas? Eur. J. Surg. Oncol. 2019. [Google Scholar] [CrossRef]
- Kainhofer, V.; Smolle, M.A.; Szkandera, J.; Liegl-Atzwanger, B.; Maurer-Ertl, W.; Gerger, A.; Riedl, J.; Leithner, A. The width of resection margins influences local recurrence in soft tissue sarcoma patients. Eur. J. Surg. Oncol. 2016, 42, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Tunn, P.U.; Kettelhack, C.; Durr, H.R. Standardized approach to the treatment of adult soft tissue sarcoma of the extremities. Recent Results Cancer Res. 2009, 179, 211–228. [Google Scholar]
- Wittekind, C.; Compton, C.C.; Greene, F.L.; Sobin, L.H. TNM residual tumor classification revisited. Cancer 2002, 94, 2511–2516. [Google Scholar] [CrossRef]
- Trojani, M.; Contesso, G.; Coindre, J.M.; Rouesse, J.; Bui, N.B.; de Mascarel, A.; Goussot, J.F.; David, M.; Bonichon, F.; Lagarde, C. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int. J. Cancer 1984, 33, 37–42. [Google Scholar] [CrossRef]
- Mozumder, S.I.; Rutherford, M.J.; Lambert, P.C. stpm2cr: A flexible parametric competing risks model using a direct likelihood approach for the cause-specific cumulative incidence function. Stata J. 2017, 17, 462–489. [Google Scholar] [CrossRef]
- Durrleman, S.; Simon, R. Flexible regression models with cubic splines. Stat. Med. 1989, 8, 551–561. [Google Scholar] [CrossRef]
- Fine, J.P.; Gray, R.J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]



| Variables | Test Cohort (n = 1931) | Validation Cohort (n = 1085) | ||||
|---|---|---|---|---|---|---|
| N (%) | Missing (%) | N (%) | Missing (%) | p-Value * | ||
| Age (continuous; years; median + IQR) | 59 (44.7–70) | 45 (2.3) | 61 (47–74) | 0 (0.0) | <0.0001 | |
| Gender | Male | 1038 (53.8) | 0 (0.0) | 615 (56.7) | 0 (0.0) | 0.121 |
| Female | 893 (46.2) | 470 (43.3) | ||||
| Tumor Location | Upper Extremity | 520 (26.9) | 1 (0.05) | 312 (28.8) | 0 (0.0) | 0.285 |
| Lower Extremity | 1410 (73.1) | 773 (71.2) | ||||
| Depth | Epifascial | 518 (26.9) | 2 (0.1) | 291 (26.8) | 0 (0.0) | 0.984 |
| Subfascial | 1411 (73.1) | 794 (73.2) | ||||
| Tumor Size (continuous; cm; median + IQR) | 7 (4–11) | 30 (1.6) | 7.5 (5–12) | 5 (0.5) | 0.026 | |
| Histology | Myxoid liposarcoma | 222 (11.6) | 16 (0.8) | 111 (10.2) | 0 (0.0) | <0.0001 |
| MPNST | 83 (4.3) | 43 (4.0) | ||||
| Myxofibrosarcoma | 451(23.6) | 104 (9.6) | ||||
| Synovial Sarcoma | 174 (9.1) | 79 (7.3) | ||||
| UPS | 325 (17.0) | 375 (34.6) | ||||
| Angiosarcoma/Vascular Sarcoma | 22 (1.1) | 20 (1.8) | ||||
| Dedifferentiated/Pleomorphic Liposarcoma | 141 (7.4) | 85 (7.8) | ||||
| Leiomyosarcoma | 221 (11.5) | 118 (10.9) | ||||
| Others | 276 (14.4) | 150 (13.8) | ||||
| Grading | G2 | 719 (37.8) | 30 (1.6) | 382 (35.8) | 19 (1.8) | 0.282 |
| G3 | 1182 (62.2) | 684 (64.2) | ||||
| Margins | R0 | 1494 (77.4) | 0 (0.0) | 768 (70.8) | 0 (0.0) | <0.0001 |
| R1/2 | 437 (22.6) | 317 (29.2) | ||||
| CTX | No | 1408 (73.0) | 1 (0. 05) | 1039 (95.8) | 0 (0.0) | <0.0001 |
| Neoadjuvant | 262 (13.6) | 40 (3.7) | ||||
| Adjuvant | 209 (10.8) | 6 (0.6) | ||||
| Neoadjuvant + Adjuvant | 51 (2.6) | 0 (0.0) | ||||
| RTX | No | 619 (32.9) | 50 (2.6) | 335 (30.9) | 0 (0.0) | <0.0001 |
| Neoadjuvant | 303 (16.1) | 460 (42.4) | ||||
| Adjuvant | 956 (50.8) | 275 (25.4) | ||||
| Neoadjuvant + Adjuvant | 3 (0.2) | 15 (1.4) | ||||
| Follow-up (continuous; months; median + IQR) | 50 (23.3–95) | 11 (0.6) | 56 (21–91) | 0 (0.0) | 0.254 | |
| Variables | Coefficient | 95%-CI | p-value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Local Recurrence | |||||
| Gender | Male | 1 | 0.011 | ||
| Female | 0.698 | 0.529 | 0.921 | ||
| Grading | G2 | 1 | 0.199 | ||
| G3 | 0.816 | 0.598 | 1.113 | ||
| Tumor size | 1.026 | 1.004 | 1.049 | 0.019 | |
| Margins | R0 | 1 | <0.001 | ||
| R1/R2 | 2.761 | 2.021 | 3.774 | ||
| Histology | Myxoid Liposarcoma | 1 | |||
| MPNST | 4.227 | 1.837 | 9.729 | 0.001 | |
| Myxofibrosarcoma | 4.156 | 2.056 | 8.400 | <0.001 | |
| Synovial Sarcoma | 3.116 | 1.429 | 7.014 | 0.005 | |
| UPS | 3.373 | 1.620 | 7.025 | 0.001 | |
| Angiosarcoma/Vascular Sarcoma | 3.316 | 0.981 | 12.341 | 0.074 | |
| Dedifferentiated/Pleomorphic Liposarcoma | 1.727 | 0.719 | 4.143 | 0.221 | |
| Leiomyosarcoma | 2.779 | 1.294 | 5.966 | 0.009 | |
| Others | 2.385 | 1.123 | 5.065 | 0.024 | |
| Neoadjuvant RTX | No | 1 | <0.001 | ||
| Yes | 0.298 | 0.178 | 0.494 | ||
| Adjuvant RTX | No | 1 | 0.001 | ||
| Yes | 0.603 | 0.447 | 0.814 | ||
| Adjuvant CTX | No | 1 | 0.008 | ||
| Yes | 1.711 | 1.154 | 2.538 | ||
| Restricted cubic spline 1 | 2.104 | 1.851 | 2.392 | <0.001 | |
| Restricted cubic spline 2 | 1.332 | 1.230 | 1.442 | <0.001 | |
| Restricted cubic spline 3 | 0.980 | 0.937 | 1.026 | 0.391 | |
| Restricted cubic spline for time-dependent effect of grading | 0.944 | 0.813 | 1.096 | 0.449 | |
| Constant | 0.048 | 0.024 | 0.097 | <0.001 | |
| Death | |||||
| Gender | Male | 1 | 0.005 | ||
| Female | 0.736 | 0.595 | 0.910 | ||
| Grading | G2 | 1 | <0.001 | ||
| G3 | 2.215 | 1.655 | 2.964 | ||
| Tumor size | 1.065 | 1.048 | 1.081 | <0.001 | |
| Margins | R0 | 1 | 0.296 | ||
| R1/R2 | 1.153 | 0.883 | 1.057 | ||
| Histology | Myxoid Liposarcoma | 1 | |||
| MPNST | 1.205 | 0.664 | 2.187 | 0.540 | |
| Myxofibrosarcoma | 1.208 | 0.795 | 1.836 | 0.375 | |
| Synovial Sarcoma | 1.461 | 0.888 | 2.404 | 0.136 | |
| UPS | 1.150 | 0.753 | 1.758 | 0.517 | |
| Angiosarcoma/Vascular Sarcoma | 4.729 | 2.335 | 9.580 | <0.001 | |
| Dedifferentiated/Pleomorphic Liposarcoma | 1.420 | 0.863 | 2.338 | 0.167 | |
| Leiomyosarcoma | 2.154 | 1.402 | 3.309 | <0.001 | |
| Others | 1.516 | 0.975 | 2.356 | 0.065 | |
| Neoadjuvant RTX | No | 1 | 0.007 | ||
| Yes | 1.543 | 1.127 | 2.111 | ||
| Adjuvant RTX | No | 1 | 0.296 | ||
| Yes | 1.145 | 0.888 | 1.476 | ||
| Adjuvant CTX | No | 1 | 0.022 | ||
| Yes | 0.679 | 0.488 | 0.946 | ||
| Restricted cubic spline 1 | 4.220 | 3.393 | 5.250 | <0.001 | |
| Restricted cubic spline 2 | 1.487 | 1.329 | 1.663 | <0.001 | |
| Restricted cubic spline 3 | 0.965 | 0.921 | 1.011 | 0.139 | |
| Restricted cubic spline for time-dependent effect of grading | 0.714 | 0.580 | 0.889 | 0.002 | |
| Constant | 0.050 | 0.032 | 0.078 | <0.001 | |
| Coefficient | 95%-CI | p-Value | |||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Distant Metastasis | |||||
| Gender | Male | 1 | <0.001 | ||
| Female | 0.720 | 0.605 | 0.857 | ||
| Grading | G2 | 1 | <0.001 | ||
| G3 | 1.737 | 1.412 | 2.136 | ||
| Tumor size | 1.069 | 1.056 | 1.083 | <0.001 | |
| Margins | R0 | 1 | 0.006 | ||
| R1/R2 | 1.347 | 1.087 | 1.669 | ||
| Histology | Myxoid Liposarcoma | 1 | |||
| MPNST | 1.825 | 1.158 | 2.875 | 0.009 | |
| Myxofibrosarcoma | 1.064 | 0.750 | 1.508 | 0.729 | |
| Synovial Sarcoma | 1.986 | 1.343 | 3.976 | 0.001 | |
| UPS | 1.445 | 1.033 | 2.022 | 0.032 | |
| Angiosarcoma/Vascular Sarcoma | 2.016 | 1.022 | 3.797 | 0.043 | |
| Dedifferentiated/Pleomorphic Liposarcoma | 1.209 | 0.786 | 1.861 | 0.387 | |
| Leiomyosarcoma | 2.689 | 1.900 | 3.797 | <0.001 | |
| Other | 1.835 | 1.293 | 2.604 | 0.001 | |
| Neoadjuvant RTX | No | 1 | 0.005 | ||
| Yes | 1.351 | 1.097 | 1.663 | ||
| Restricted cubic spline 1 | 2.928 | 2.591 | 3.308 | <0.001 | |
| Restricted cubic spline 2 | 1.458 | 1.374 | 1.547 | <0.001 | |
| Restricted cubic spline 3 | 0.965 | 0.926 | 1.006 | 0.096 | |
| Restricted cubic spline 4 | 1.040 | 1.020 | 1.062 | <0.001 | |
| Restricted cubic spline 5 | 0.995 | 0.982 | 1.008 | 0.427 | |
| Restricted cubic spline for time-dependent effect of grading | 0.723 | 0.640 | 0.817 | <0.001 | |
| Constant | 0.108 | 0.078 | 0.149 | <0.001 | |
| Death | |||||
| Gender | Male | 1 | 0.864 | ||
| Female | 0.968 | 0.666 | 1.407 | ||
| Grading | G2 | 1 | 0.018 | ||
| G3 | 1.873 | 1.116 | 3.145 | ||
| Tumor size | 1.023 | 0.997 | 1.050 | 0.087 | |
| Margins | R0 | 1 | 0.198 | ||
| R1/R2 | 1.378 | 0.846 | 2.244 | ||
| Histology | Myxoid Liposarcoma | 1 | |||
| MPNST | 2.506 | 0.844 | 7.442 | 0.098 | |
| Myxofibrosarcoma | 3.136 | 1.325 | 7.418 | 0.009 | |
| Synovial Sarcoma | 0.600 | 0.150 | 2.416 | 0.472 | |
| UPS | 1.781 | 0.714 | 4.443 | 0.216 | |
| Angiosarcoma/Vascular Sarcoma | 11.165 * | 3.507 * | 35.542 * | <0.001 * | |
| Dedifferentiated/Pleomorphic Liposarcoma | 3.331 | 1.259 | 8.812 | 0.015 | |
| Leiomyosarcoma | 1.798 | 0.675 | 4.782 | 0.241 | |
| Other | 2.408 | 0.963 | 4.782 | 0.060 | |
| Neoadjuvant RTX | No | 1 | 0.048 | ||
| Yes | 0.541 | 0.295 | 0.993 | ||
| Restricted cubic spline 1 | 3.604 | 2.494 | 5.211 | <0.001 | |
| Restricted cubic spline 2 | 1.270 | 1.060 | 1.523 | 0.010 | |
| Restricted cubic spline 3 | 0.952 | 0.863 | 1.049 | 0.316 | |
| Restricted cubic spline 4 | 0.953 | 0.908 | 1.001 | 0.057 | |
| Restricted cubic spline 5 | 0.974 | 0.569 | 1.199 | 0.097 | |
| Restricted cubic spline for time-dependent effect of grading | 0.826 | 0.569 | 1.199 | 0.314 | |
| Constant | 0.010 | 0.004 | 0.025 | <0.001 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolle, M.A.; van de Sande, M.; Callegaro, D.; Wunder, J.; Hayes, A.; Leitner, L.; Bergovec, M.; Tunn, P.-U.; van Praag, V.; Fiocco, M.; et al. Individualizing Follow-Up Strategies in High-Grade Soft Tissue Sarcoma with Flexible Parametric Competing Risk Regression Models. Cancers 2020, 12, 47. https://doi.org/10.3390/cancers12010047
Smolle MA, van de Sande M, Callegaro D, Wunder J, Hayes A, Leitner L, Bergovec M, Tunn P-U, van Praag V, Fiocco M, et al. Individualizing Follow-Up Strategies in High-Grade Soft Tissue Sarcoma with Flexible Parametric Competing Risk Regression Models. Cancers. 2020; 12(1):47. https://doi.org/10.3390/cancers12010047
Chicago/Turabian StyleSmolle, Maria Anna, Michiel van de Sande, Dario Callegaro, Jay Wunder, Andrew Hayes, Lukas Leitner, Marko Bergovec, Per-Ulf Tunn, Veroniek van Praag, Marta Fiocco, and et al. 2020. "Individualizing Follow-Up Strategies in High-Grade Soft Tissue Sarcoma with Flexible Parametric Competing Risk Regression Models" Cancers 12, no. 1: 47. https://doi.org/10.3390/cancers12010047
APA StyleSmolle, M. A., van de Sande, M., Callegaro, D., Wunder, J., Hayes, A., Leitner, L., Bergovec, M., Tunn, P.-U., van Praag, V., Fiocco, M., Panotopoulos, J., Willegger, M., Windhager, R., Dijkstra, S. P. D., van Houdt, W. J., Riedl, J. M., Stotz, M., Gerger, A., Pichler, M., ... Szkandera, J. (2020). Individualizing Follow-Up Strategies in High-Grade Soft Tissue Sarcoma with Flexible Parametric Competing Risk Regression Models. Cancers, 12(1), 47. https://doi.org/10.3390/cancers12010047