NSCLC Mutated Isoforms of CCDC6 Affect the Intracellular Distribution of the Wild Type Protein Promoting Cisplatinum Resistance and PARP Inhibitors Sensitivity in Lung Cancer Cells
Abstract
1. Introduction
2. Results
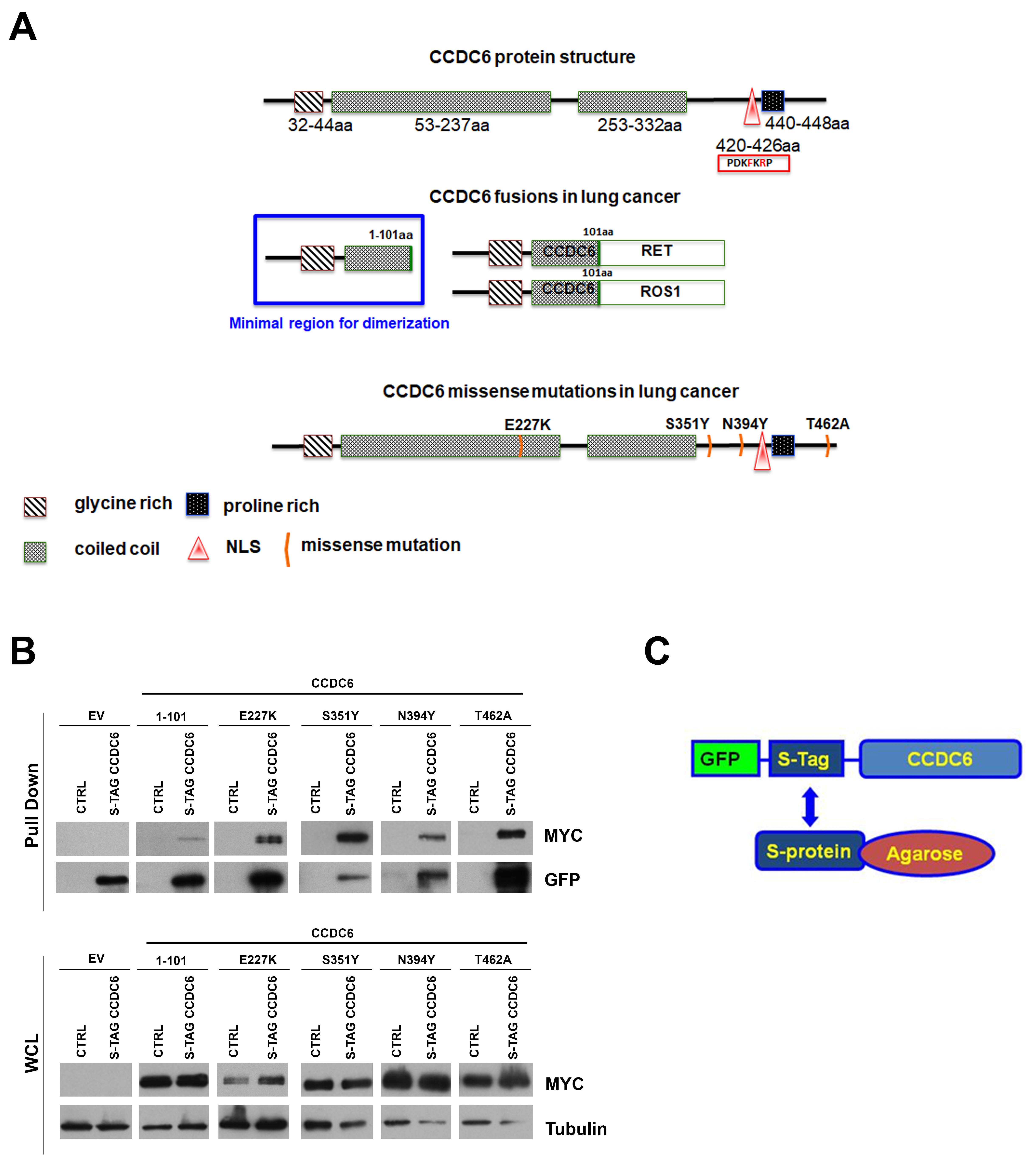
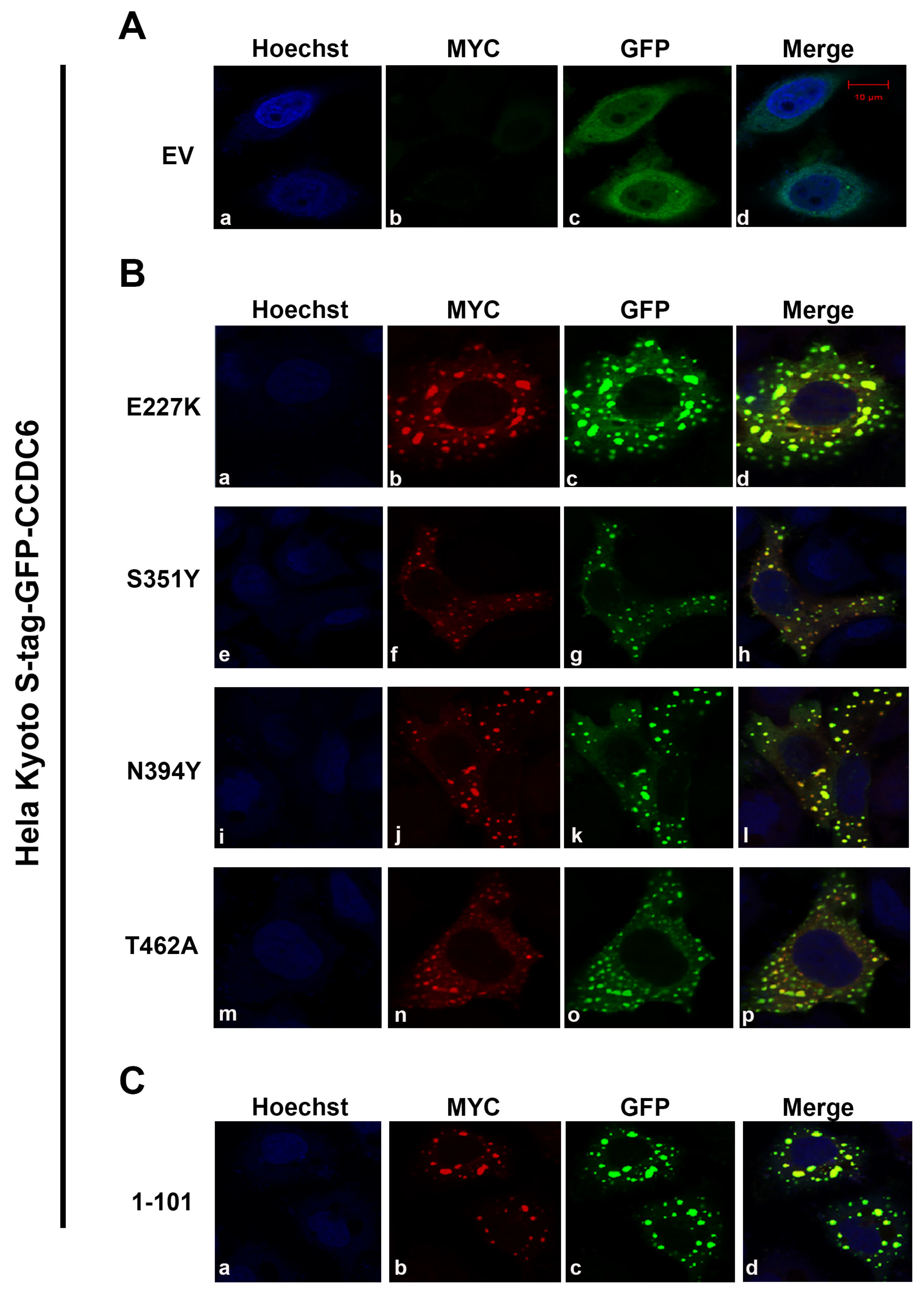
2.1. CCDC6 Lung-Mutants Form Heterodimers with the Wild Type Protein Shaping Its Intracellular Distribution
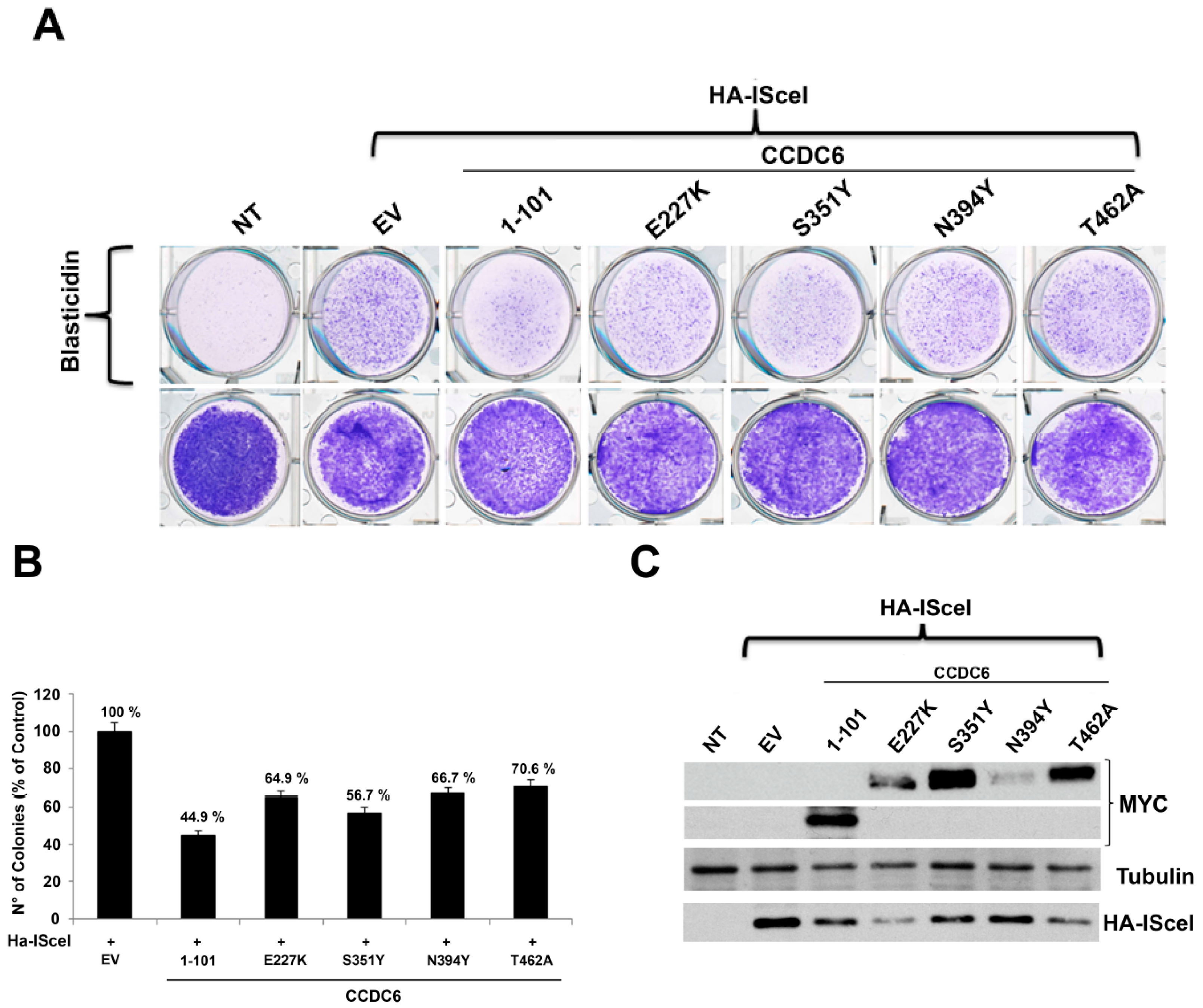
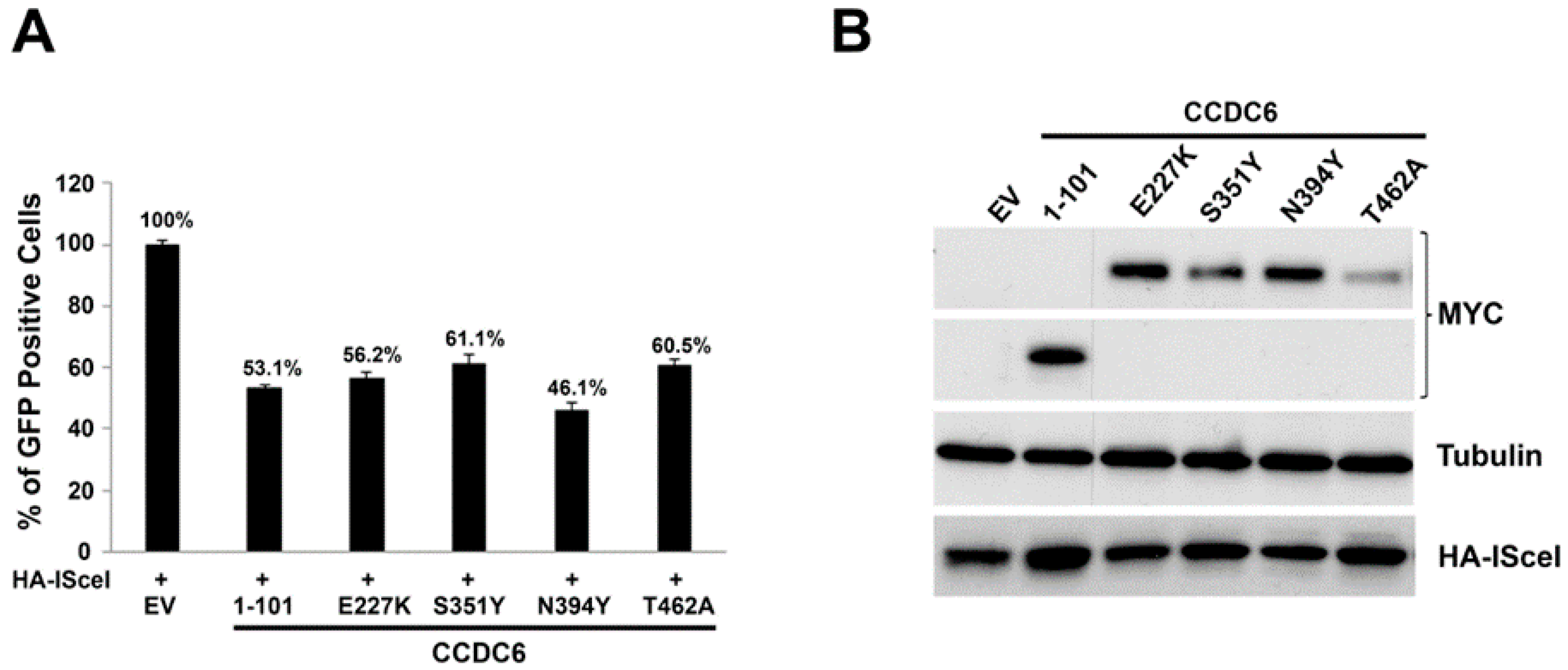
2.2. CCDC6 Lung-Mutants Reduce the DNA Repair Efficiency by Affecting the Homologous Recombination Process
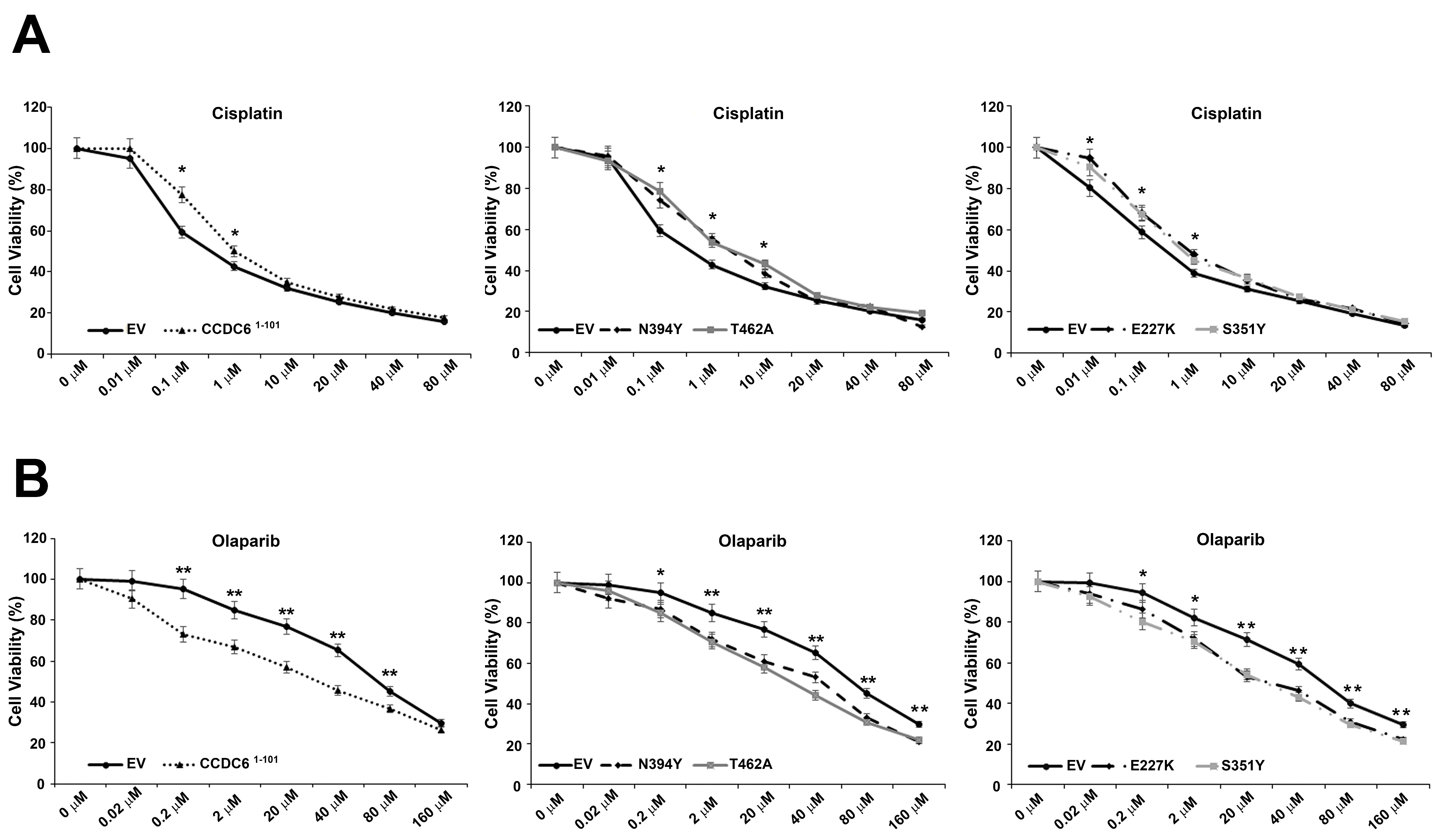
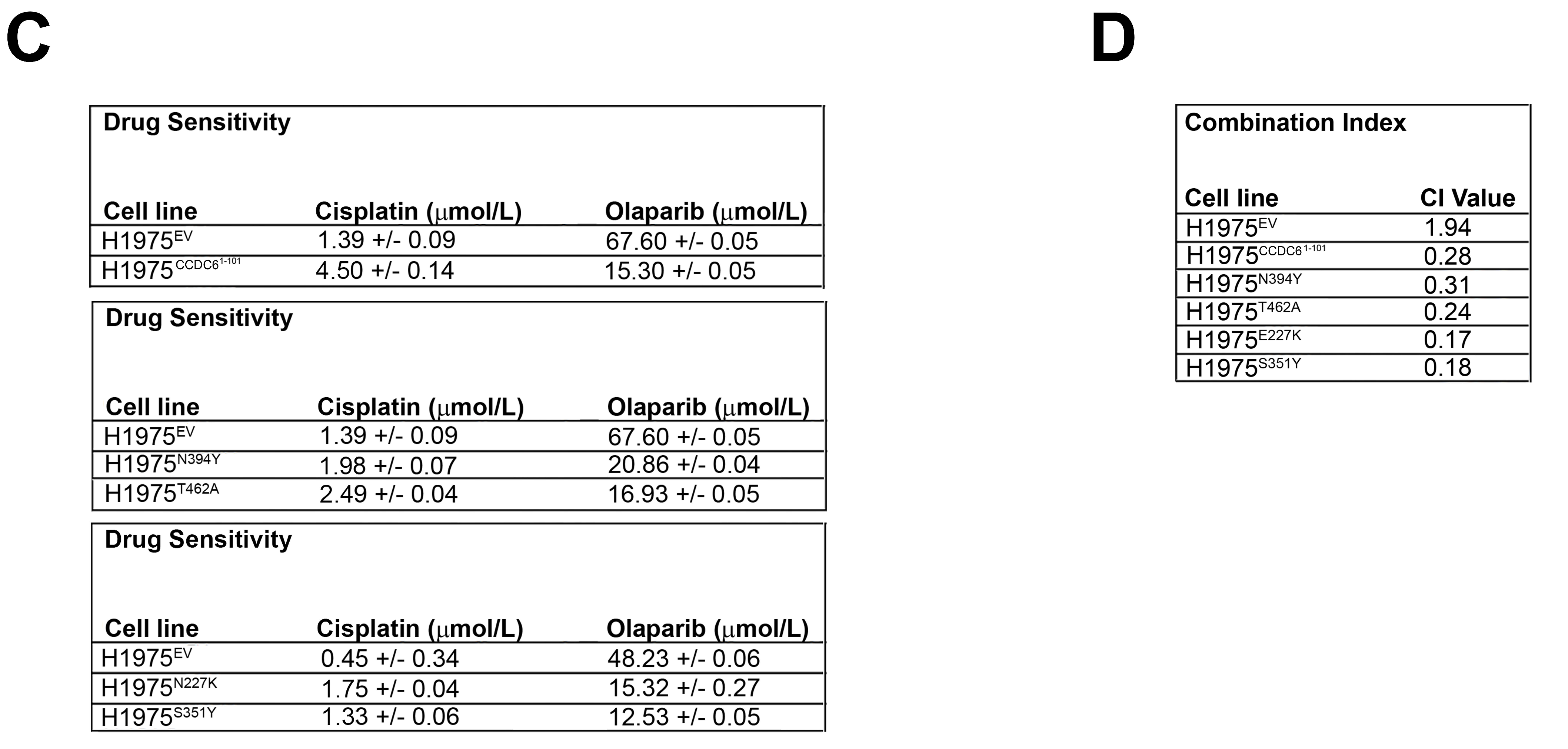
2.3. CCDC6 Lung-Mutants Induce Cisplatinum Resistance and Sensitivity to PARP-Inhibitors in NSCLC
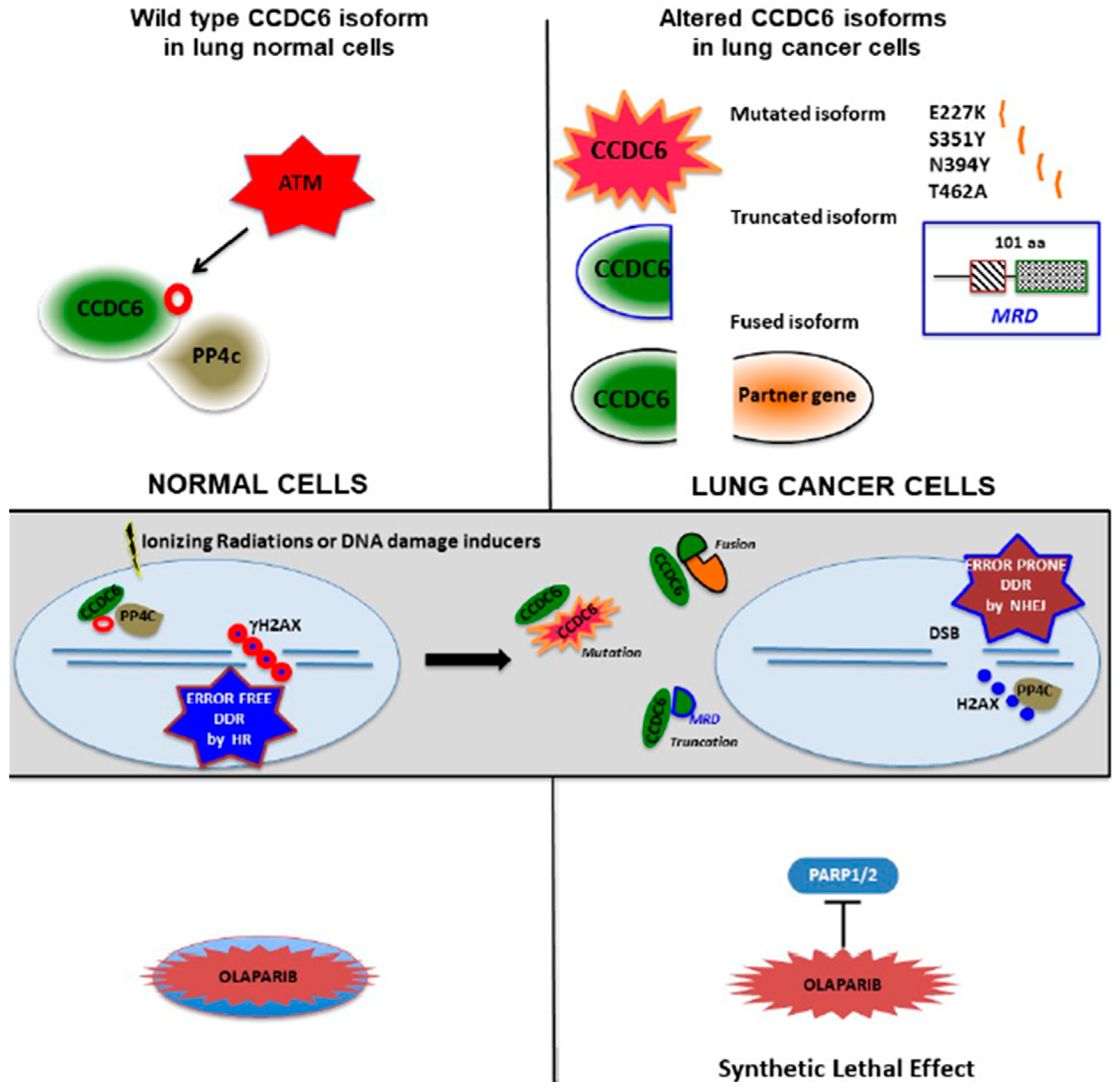
3. Discussion
4. Materials and Methods
4.1. Cell Lines, Drugs, and Chemicals
4.2. Plasmids and Transfection
4.3. Protein Extraction and Western Blot Analysis
4.4. Immunofluorescence Staining
4.5. HR Reporter Cell Assay
4.6. HR Transient Assay
4.7. Sensitivity Test and Design for Drug Combination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Celetti, A.; Cerrato, A.; Merolla, F.; Vitagliano, D.; Vecchio, G.; Grieco, M. H4(D10S170), a gene frequently rearranged with RET in papillary thyroid carcinomas: Functional characterization. Oncogene 2004, 23, 109–121. [Google Scholar] [CrossRef]
- Merolla, F.; Pentimalli, F.; Pacelli, R.; Vecchio, G.; Fusco, A.; Grieco, M.; Celetti, A. Involvement of H4(D10S170) protein in ATM-dependent response to DNA damage. Oncogene 2007, 26, 6167–6175. [Google Scholar] [CrossRef]
- Morra, F.; Miro, C.; Napolitano, V.; Merolla, F.; Celetti, A. CCDC6 (coiled-coil domain containing 6). Atlas Genet. Cytogenet. Oncol. Haematol. 2016, 20, 166–171. [Google Scholar] [CrossRef][Green Version]
- Cerrato, A.; Merolla, F.; Morra, F.; Celetti, A. CCDC6: The identity of a protein known to be partner in fusion. Int. J. Cancer 2018, 142, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Beausoleil, S.A.; Jedrychowski, M.; Schwartz, D.; Elias, J.E.; Villén, J.; Li, J.; Cohn, M.A.; Cantley, L.C.; Gygi, S.P. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc. Natl. Acad Sci. USA 2004, 101, 12130–12135. [Google Scholar] [CrossRef] [PubMed]
- Morra, F.; Luise, C.; Merolla, F.; Poser, I.; Visconti, R.; Ilardi, G.; Paladino, S.; Inuzuka, H.; Guggino, G.; Monaco, R.; et al. FBXW7 and USP7 regulate CCDC6 turnover during the cell cycle and affect cancer drugs susceptibility in NSCLC. Oncotarget 2015, 6, 12697–12709. [Google Scholar] [CrossRef] [PubMed]
- Luise, C.; Merolla, F.; Leone, V.; Paladino, S.; Sarnataro, D.; Fusco, A.; Celetti, A. Identification of sumoylation sites in CCDC6, the first identified RET partner gene in papillary thyroid carcinoma, uncovers a mode of regulating CCDC6 function on CREB1 transcriptional activity. PLoS ONE 2012, 7, e49298. [Google Scholar] [CrossRef] [PubMed]
- Nakada, S.; Chen, G.I.; Gingras, A.C.; Durocher, D. PP4 is a gamma H2AX phosphatase required for recovery from the DNA damage checkpoint. EMBO Rep. 2008, 9, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Merolla, F.; Luise, C.; Muller, M.T.; Pacelli, R.; Fusco, A.; Celetti, A. Loss of CCDC6, the first identified RET partner gene, affects pH2AX S139 levels and accelerates mitotic entry upon DNA damage. PLoS ONE 2012, 7, e36177. [Google Scholar] [CrossRef]
- Thanasopoulou, A.; Stravopodis, D.J.; Dimas, K.S.; Schwaller, J.; Anastasiadou, E. Loss of CCDC6 affects cell cycle through impaired intra-S-phase checkpoint control. PLoS ONE 2012, 7, e31007. [Google Scholar] [CrossRef]
- Cerrato, A.; Morra, F.; Celetti, A. Use of poly ADP-ribose polymerase [PARP] inhibitors in cancer cells bearing DDR defects: The rationale for their inclusion in the clinic. J. Exp. Clin. Cancer Res. 2016, 35, 179. [Google Scholar] [CrossRef] [PubMed]
- Malapelle, U.; Morra, F.; Ilardi, G.; Visconti, R.; Merolla, F.; Cerrato, A.; Napolitano, V.; Monaco, R.; Guggino, G.; Monaco, G.; et al. USP7 inhibitors, downregulating CCDC6, sensitize lung neuroendocrine cancer cells to PARP-inhibitor drugs. Lung Cancer 2017, 107, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Morra, F.; Merolla, F.; Napolitano, V.; Ilardi, G.; Miro, C.; Paladino, S.; Staibano, S.; Cerrato, A.; Celetti, A. The combined effect of USP7 inhibitors and PARP inhibitors in hormone-sensitive and castration-resistant prostate cancer cells. Oncotarget 2017, 8, 31815–31829. [Google Scholar] [CrossRef] [PubMed]
- Morra, F.; Merolla, F.; Criscuolo, D.; Insabato, L.; Giannella, R.; Ilardi, G.; Cerrato, A.; Visconti, R.; Staibano, S.; Celetti, A. CCDC6 and USP7 expression levels suggest novel treatment options in high-grade urothelial bladder cancer. J. Exp. Clin. Cancer Res. 2019, 38, 90. [Google Scholar] [CrossRef] [PubMed]
- Morra, F.; Merolla, F.; D’Abbiero, D.; Ilardi, G.; Campione, S.; Monaco, R.; Guggino, G.; Ambrosio, F.; Staibano, S.; Cerrato, A.; et al. Analysis of CCDC6 as a novel biomarker for the clinical use of PARP1 inhibitors in malignant pleural mesothelioma. Lung Cancer 2019, 135, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Morra, F.; Luise, C.; Visconti, R.; Staibano, S.; Merolla, F.; Ilardi, G.; Guggino, G.; Paladino, S.; Sarnataro, D.; Franco, R.; et al. New therapeutic perspectives in CCDC6 deficient lung cancer cells. Int. J. Cancer 2015, 136, 2146–2157. [Google Scholar] [CrossRef]
- Fusco, A.; Grieco, M.; Santoro, M.; Berlingieri, M.T.; Pilotti, S.; Pierotti, M.A.; Della Porta, G.; Vecchio, G. A new oncogene in human thyroid papillary carcinomas and their lymph-nodal metastases. Nature 1987, 328, 170–172. [Google Scholar] [CrossRef]
- Grieco, M.; Santoro, M.; Berlingieri, M.T.; Melillo, R.M.; Donghi, R.; Bongarzone, I.; Pierotti, M.A.; Della Porta, G.; Fusco, A.; Vecchio, G. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell 1990, 60, 557–563. [Google Scholar] [CrossRef]
- Drechsler, M.; Hildebrandt, B.; Kündgen, A.; Germing, U.; Royer-Pokora, B. Fusion of H4/D10S170 to PDGFRbeta in a patient with chronic myelomonocytic leukemia and long-term responsiveness to imatinib. Ann. Hematol. 2007, 86, 353–354. [Google Scholar] [CrossRef]
- Yamazaki, M.; Nakaseko, C.; Takeuchi, M.; Ozawa, S.; Ishizuka, Y.; Hatanaka, Y.; Oshima-Hasegawa, N.; Muto, T.; Tsukamoto, S.; Mitsukawa, S.; et al. Myeloid/Lymphoid Neoplasm with PDGFRB Rearrangement with t (5;10) (q33; q22) harboring a Novel Breakpoint of the CCDC6-PDGFRB Fusion Gene. Intern. Med. 2019, 58, 3449–3453. [Google Scholar] [CrossRef]
- Puxeddu, E.; Zhao, G.; Stringer, J.R.; Medvedovic, M.; Moretti, S.; Fagin, J.A. Characterization of novel non-clonal intrachromosomal rearrangements between the H4 and PTEN genes (H4/PTEN) in human thyroid cell lines and papillary thyroid cancer specimens. Mutat. Res. 2005, 570, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Su, F.; Kalyana-Sundaram, S.; Khazanov, N.; Ateeq, B.; Cao, X.; Lonigro, R.J.; Vats, P.; Wang, R.; Lin, S.F.; et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 2013, 3, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, X.; Wang, S.; Moser, C.D.; Shaleh, H.M.; Mohamed, E.A.; Chaiteerakij, R.; Allotey, L.K.; Chen, G.; Miyabe, K.; et al. Antitumor effect of FGFR inhibitors on a novel cholangiocarcinoma patient derived xenograft mouse model endogenously expressing an FGFR2-CCDC6 fusion protein. Cancer Lett. 2016, 380, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Earp, M.A.; Raghavan, R.; Li, Q.; Dai, J.; Winham, S.J.; Cunningham, J.M.; Natanzon, Y.; Kalli, K.R.; Hou, X.; Weroha, S.J.; et al. Characterization of fusion genes in common and rare epithelial ovarian cancer histologic subtypes. Oncotarget 2017, 8, 46891–46899. [Google Scholar] [CrossRef]
- Yoshihara, K.; Wang, Q.; Torres-Garcia, W.; Zheng, S.; Vegesna, R.; Kim, H.; Verhaak, R.G. The landscape and therapeutic relevance of cancer-associated transcript fusions. Oncogene 2015, 34, 4845–4854. [Google Scholar] [CrossRef]
- Laxmi, A.; Gupta, P.; Gupta, J. CCDC6, a gene product in fusion with different protoncogenes, as a potential chemotherapeutic target. Cancer Biomark. 2019, 24, 383–393. [Google Scholar] [CrossRef]
- Tong, Q.; Xing, S.; Jhiang, S.M. Leucine zipper-mediated dimerization is essential for the PTC1 oncogenic activity. J. Biol. Chem. 1997, 272, 9043–9047. [Google Scholar] [CrossRef]
- Jhiang, S.M. The RET proto-oncogene in human cancers. Oncogene 2000, 19, 5590–5597. [Google Scholar] [CrossRef]
- Jossart, G.H.; O’Brien, B.; Cheng, J.F.; Tong, Q.; Jhiang, S.M.; Duh, Q.; Clark, O.H.; Weier, H.U. A novel multicolor hybridization scheme applied to localization of a transcribed sequence (D10S170/H4) and deletion mapping in the thyroid cancer cell line TPC-1. Cytogenet. Cell Genet. 1996, 75, 254–257. [Google Scholar] [CrossRef]
- Takeuchi, K.; Soda, M.; Togashi, Y.; Suzuki, R.; Sakata, S.; Hatano, S.; Asaka, R.; Hamanaka, W.; Ninomiya, H.; Uehara, H.; et al. RET, ROS1 and ALK fusions in lung cancer. Nat. Med. 2012, 18, 378–381. [Google Scholar] [CrossRef]
- Matsubara, D.; Kanai, Y.; Ishikawa, S.; Ohara, S.; Yoshimoto, T.; Sakatani, T.; Oguni, S.; Tamura, T.; Kataoka, H.; Endo, S.; et al. Identification of CCDC6-RET fusion in the human lung adenocarcinoma cell line, LC-2/ad. J. Thorac. Oncol. 2012, 7, 1872–1876. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, H.; Pan, Y.; Li, Y.; Ye, T.; Li, C.; Luo, X.; Wang, L.; Li, H.; Zhang, Y.; et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J. Clin. Oncol. 2012, 30, 4352–4359. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Makinoshima, H.; Matsumoto, S.; Suzuki, A.; Mimaki, S.; Matsushima, K.; Yoh, K.; Goto, K.; Suzuki, Y.; Ishii, G.; et al. Identification of a lung adenocarcinoma cell line with CCDC6-RET fusion gene and the effect of RET inhibitors in vitro and in vivo. Cancer Sci. 2013, 104, 896–903. [Google Scholar] [CrossRef]
- Mizukami, T.; Shiraishi, K.; Shimada, Y.; Ogiwara, H.; Tsuta, K.; Ichikawa, H.; Sakamoto, H.; Kato, M.; Shibata, T.; Nakano, T.; et al. Molecular mechanisms underlying oncogenic RET fusion in lung adenocarcinoma. J. Thorac. Oncol. 2014, 9, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Imielinski, M.; Berger, A.H.; Hammerman, P.S.; Hernandez, B.; Pugh, T.J.; Hodis, E.; Cho, J.; Suh, J.; Capelletti, M.; Sivachenko, A.; et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 2012, 150, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.F.; Shihab, H.A.; Mort, M.; Cooper, D.N.; Gaunt, T.R.; Campbell, C. FATHMM-XF: Accurate prediction of pathogenic point mutations via extended features. Bioinformatics 2018, 34, 511–513. [Google Scholar] [CrossRef]
- Visconti, R.; Morra, F.; Guggino, G.; Celetti, A. The between now and then of lung cancer chemotherapy and immunotherapy. Int. J. Mol. Sci. 2017, 18, 1374. [Google Scholar] [CrossRef]
- Tutt, A.; Bertwistle, D.; Valentine, J.; Gabriel, A.; Swift, S.; Ross, G.; Griffin, C.; Thacker, J.; Ashworth, A. Mutation in Brca2 stimulates error-prone homology-directed repair of DNA double-strand breaks occurring between repeated sequences. EMBO J. 2001, 20, 4704–4716. [Google Scholar] [CrossRef]
- Hoeijmakers, J.H. Genome maintenance mechanisms for preventing cancer. Nature 2001, 411, 366–374. [Google Scholar] [CrossRef]
- Bartek, J.; Lukas, J. DNA damage checkpoints: From initiation to recovery or adaptation. Curr. Opin. Cell Biol. 2007, 19, 238–245. [Google Scholar] [CrossRef]
- Harper, J.W.; Elledge, S.J. The DNA damage response: Ten years after. Mol. Cell 2007, 28, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Shiloh, Y. ATM and related protein kinases: Safeguarding genome integrity. Nat. Rev. Cancer 2003, 3, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.B.; Elledge, S.J. The DNA damage response: Putting checkpoints in perspective. Nature 2000, 408, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Curtin, N.J. DNA repair dysregulation from cancer driver to therapeutic target. Nat. Rev. Cancer 2012, 12, 801–817. [Google Scholar] [CrossRef] [PubMed]
- Zeng-Rong, N.; Paterson, J.; Alpert, L.; Tsao, M.S.; Viallet, J.; Alaoui-Jamali, M.A. Elevated DNA repair capacity is associated with intrinsic resistance of lung cancer to chemotherapy. Cancer Res. 1995, 55, 4760–4764. [Google Scholar] [PubMed]
- Bosken, C.H.; Wei, Q.; Amos, C.I.; Spitz, M.R. An analysis of DNA repair as a determinant of survival in patients with non-small-cell lung cancer. J. Natl. Cancer Inst. 2002, 94, 1091–1099. [Google Scholar] [CrossRef]
- Duchesne, G.M. Fundamental bases of combined therapy in lung cancer: Cell resistance to chemotherapy and radiotherapy. Lung Cancer 1994, 10, S67–S72. [Google Scholar] [CrossRef]
- Guo, W.F.; Lin, R.X.; Huang, J.; Zhou, Z.; Yang, J.; Guo, G.Z.; Wang, S.Q. Identification of differentially expressed genes contributing to radioresistance in lung cancer cells using microarray analysis. Radiat. Res. 2005, 164, 27–35. [Google Scholar] [CrossRef]
- Leone, V.; Langella, C.; Esposito, F.; Arra, C.; Palma, G.; Rea, D.; Paciello, O.; Merolla, F.; De Biase, D.; Papparella, S.; et al. Ccdc6 knock-in mice develop thyroid hyperplasia associated to an enhanced CREB1 activity. Oncotarget 2015, 6, 15628–15638. [Google Scholar] [CrossRef]
- Lord, C.J.; Tutt, A.N.; Ashworth, A. Synthetic lethality and cancer therapy: Lessons learned from the development of PARP inhibitors. Annu. Rev. Med. 2015, 66, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Oplustilova, L.; Wolanin, K.; Mistrik, M.; Korinkova, G.; Simkova, D.; Bouchal, J.; Lenobel, R.; Bartkova, J.; Lau, A.; O’Connor, M.J.; et al. Evaluation of candidate biomarkers to predict cancer cell sensitivity or resistance to PARP-1 inhibitor treatment. Cell Cycle 2012, 11, 3837–3850. [Google Scholar] [CrossRef] [PubMed]
- Postel-Vinay, S.; Bajrami, I.; Friboulet, L.; Elliott, R.; Fontebasso, Y.; Dorvault, N.; Olaussen, K.A.; André, F.; Soria, J.C.; Lord, C.J.; et al. A high-throughput screen identifies PARP1/2 inhibitors as a potential therapy for ERCC1-deficient non-small cell lung cancer. Oncogene 2013, 32, 5377–5387. [Google Scholar] [CrossRef]
- Fong, P.C.; Boss, D.S.; Yap, T.A.; Tutt, A.; Wu, P.; Mergui-Roelvink, M.; Mortimer, P.; Swaisland, H.; Lau, A.; O’Connor, M.J.; et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009, 361, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.; Robson, M.; Garber, J.E.; Domchek, S.M.; Audeh, M.W.; Weitzel, J.N.; Friedlander, M.; Arun, B.; Loman, N.; Schmutzler, R.K.; et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet 2010, 376, 235–244. [Google Scholar] [CrossRef]
- Chang, A. Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer 2011, 71, 3–10. [Google Scholar] [CrossRef]
- Birkelbach, M.; Ferraiolo, N.; Gheorghiu, L.; Pfäffle, H.N.; Daly, B.; Ebright, M.I.; Spencer, C.; O’Hara, C.; Whetstine, J.R.; Benes, C.H.; et al. Detection of impaired homologous recombination repair in NSCLC cells and tissues. J. Thorac. Oncol. 2013, 8, 279–286. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, Z.; Borczuk, A.; Powell, C.A.; Balajee, A.S.; Lieberman, H.B.; Halmos, B. PARP inhibition selectively increases sensitivity to cisplatin in ERCC1-low non-small cell lung cancer cells. Carcinogenesis 2013, 34, 739–749. [Google Scholar] [CrossRef]
- Gridelli, C.; Rossi, A.; Di Maio, M.; Leo, S.; Filipazzi, V.; Favaretto, A.G.; Burgio, M.A.; Cinieri, S.; Bianco, R.; Ciardiello, F.; et al. Rationale and design of MILES-3 and MILES-4 studies: Two randomized phase 3 trials comparing single-agent chemotherapy versus cisplatin-based doublets in elderly patients with advanced Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2014, 15, 166–170. [Google Scholar] [CrossRef]
- Bowden, N.A. Nucleotide excision repair: Why is it not used to predict response to platinum-based chemotherapy? Cancer Lett. 2014, 346, 163–171. [Google Scholar] [CrossRef]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Bang, Y.J.; Kwak, E.L.; Iafrate, A.J.; Varella-Garcia, M.; Fox, S.B.; Riely, G.J.; Solomon, B.; Ou, S.H.; Kim, D.W.; et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: Up dated results from a phase 1 study. Lancet Oncol. 2012, 13, 1011–1019. [Google Scholar] [CrossRef]
- Okamoto, K.; Kodama, K.; Takase, K.; Sugi, N.H.; Yamamoto, Y.; Iwata, M.; Tsuruoka, A. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013, 340, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, P.; Bandlamudi, C.; Cheng, M.L.; Srinivasan, P.; Chavan, S.S.; Friedman, N.D.; Rosen, E.Y.; Richards, A.L.; Bouvier, N.; Selcuklu, S.D.; et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature 2019, 571, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Curtin, N.J.; Drew, Y.; Sharma-Saha, S. Why BRCA mutations are not tumour-agnostic biomarkers for PARP inhibitor therapy. Nat. Rev. Clin. Oncol. 2019, 16, 725–726. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Merolla, F.; Mascolo, M.; Ilardi, G.; Romano, S.; Varricchio, S.; Napolitano, V.; Celetti, A.; Postiglione, L.; Di Lorenzo, P.P.; et al. FKBP51 Immunohistochemical Expression: A New Prognostic Biomarker for OSCC? Int. J. Mol. Sci. 2017, 18, 443. [Google Scholar] [CrossRef]
- Poser, I.; Sarov, M.; Hutchins, J.R.; Hériché, J.K.; Toyoda, Y.; Pozniakovsky, A.; Weigl, D.; Nitzsche, A.; Hegemann, B.; Bird, A.W.; et al. BAC TransgeneOmics: A high-throughput method for exploration of protein function in mammals. Nat. Methods 2008, 5, 409–415. [Google Scholar] [CrossRef]
- Jasin, M. Genetic manipulation of genomes with rare-cutting endonucleases. Trends Genet. 1996, 12, 224–228. [Google Scholar] [CrossRef]
- Chou, T.C.; Talaly, P. A simple generalized equation for the analysis of multiple inhibitions of Michaelis-Menten kinetic systems. J. Biol. Chem. 1977, 252, 6438–6442. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerrato, A.; Morra, F.; Di Domenico, I.; Celetti, A. NSCLC Mutated Isoforms of CCDC6 Affect the Intracellular Distribution of the Wild Type Protein Promoting Cisplatinum Resistance and PARP Inhibitors Sensitivity in Lung Cancer Cells. Cancers 2020, 12, 44. https://doi.org/10.3390/cancers12010044
Cerrato A, Morra F, Di Domenico I, Celetti A. NSCLC Mutated Isoforms of CCDC6 Affect the Intracellular Distribution of the Wild Type Protein Promoting Cisplatinum Resistance and PARP Inhibitors Sensitivity in Lung Cancer Cells. Cancers. 2020; 12(1):44. https://doi.org/10.3390/cancers12010044
Chicago/Turabian StyleCerrato, Aniello, Francesco Morra, Imma Di Domenico, and Angela Celetti. 2020. "NSCLC Mutated Isoforms of CCDC6 Affect the Intracellular Distribution of the Wild Type Protein Promoting Cisplatinum Resistance and PARP Inhibitors Sensitivity in Lung Cancer Cells" Cancers 12, no. 1: 44. https://doi.org/10.3390/cancers12010044
APA StyleCerrato, A., Morra, F., Di Domenico, I., & Celetti, A. (2020). NSCLC Mutated Isoforms of CCDC6 Affect the Intracellular Distribution of the Wild Type Protein Promoting Cisplatinum Resistance and PARP Inhibitors Sensitivity in Lung Cancer Cells. Cancers, 12(1), 44. https://doi.org/10.3390/cancers12010044




