Neurocognitive Decline Following Radiotherapy: Mechanisms and Therapeutic Implications
Abstract
1. Introduction
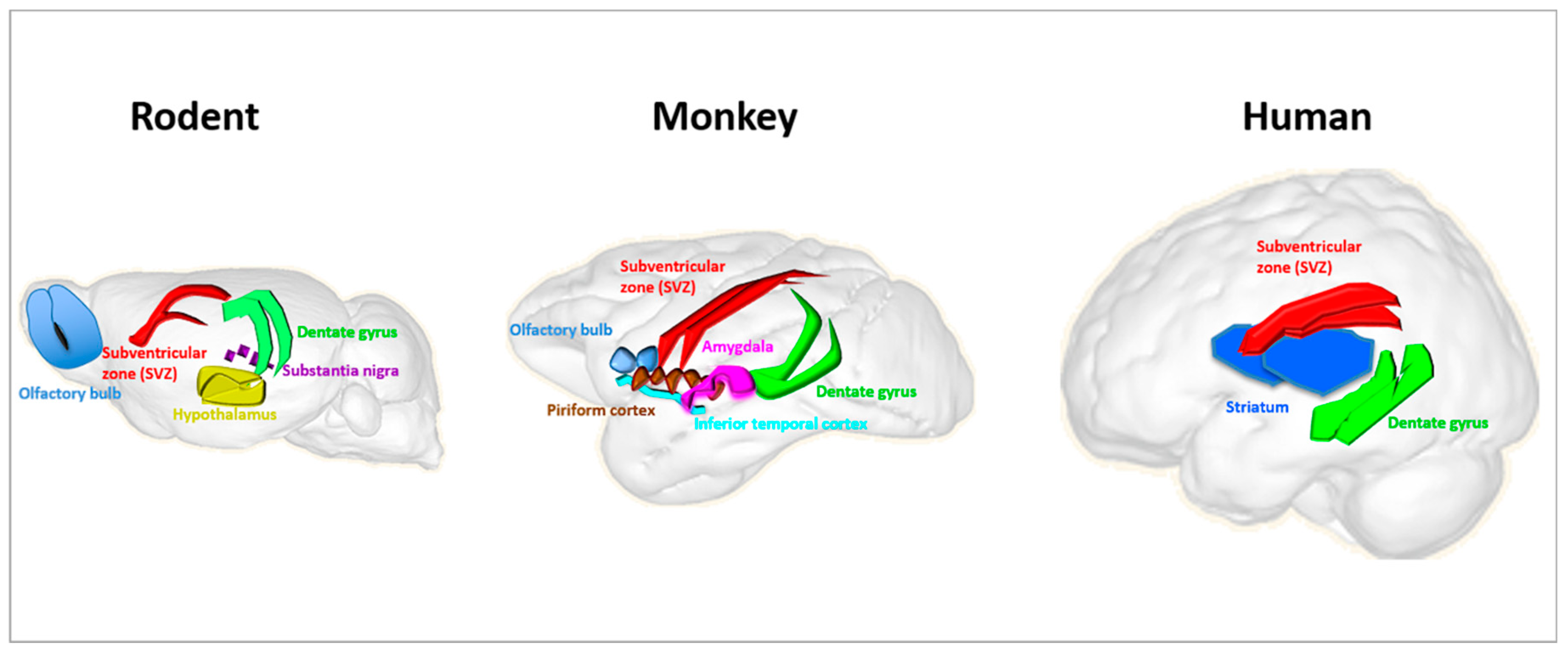
2. Neural Stem Cells
2.1. Human Adult Neurogenesis
2.2. Radiation Effects on Cognitive Function
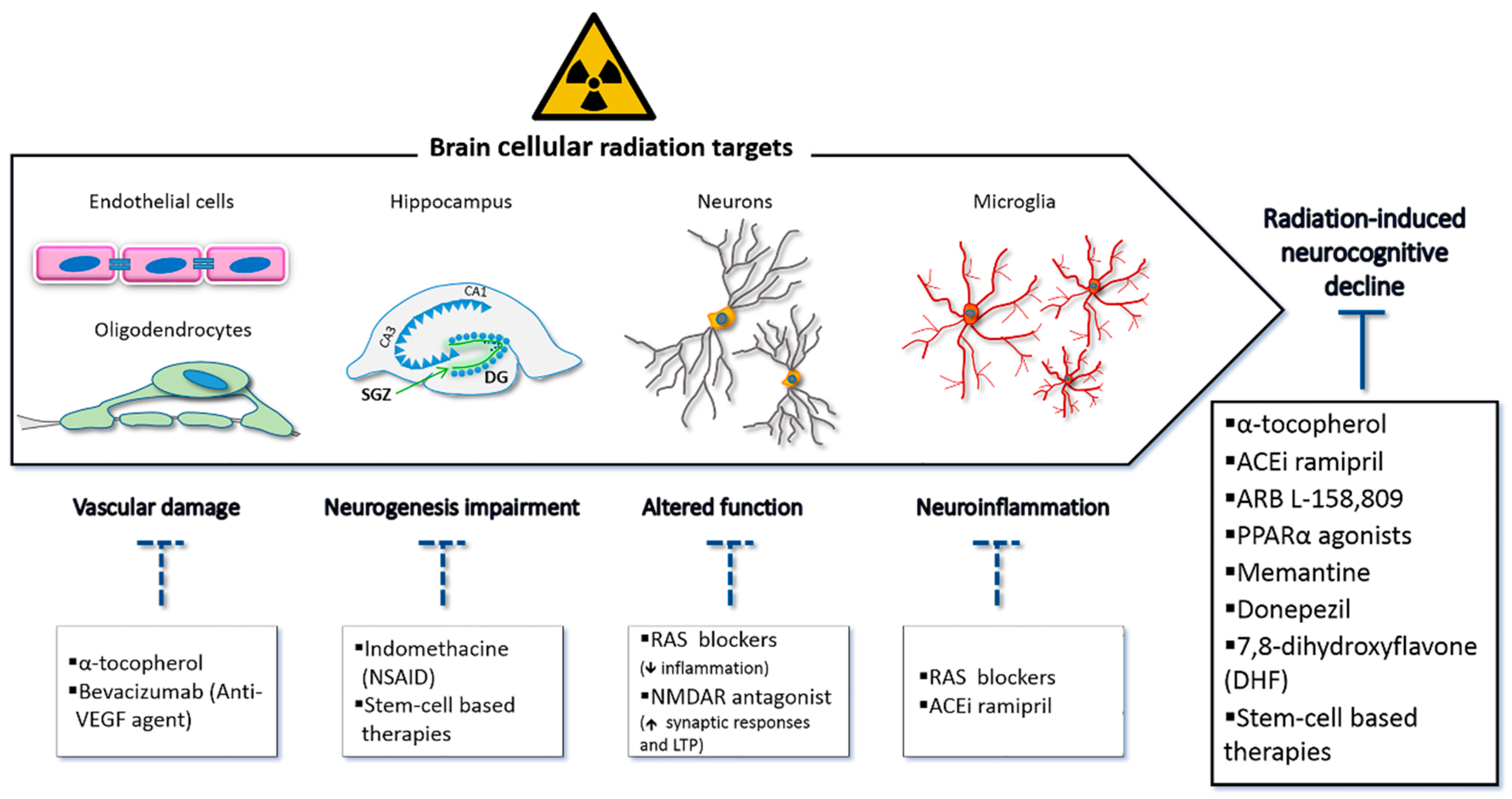
3. Strategies for Preventive and Therapeutic Measures: Current Knowledge and Perspectives
3.1. Improvement of Techniques in Radiation Therapy
3.2. Pharmacologic Interventions
3.3. Stem-Cell Transplantation Approaches
4. Summary
5. Conclusions
Funding
Conflicts of Interest
References
- Thomas, G.A.; Symonds, P. Radiation exposure and health effects—is it time to reassess the real consequences? Clin. Oncol. (R. Coll. Radiol.) 2016, 28, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Michaelidesová, A.; Konířová, J.; Bartůněk, P.; Zíková, M. Effects of radiation therapy on neural stem cells. Genes 2019, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, H.; Yakisich, J.S. Overcoming the blood-brain barrier for chemotherapy: Limitations, challenges and rising problems. Anticancer Agents Med. Chem. 2014, 14, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.; Komaki, R. Treatment of brain metastasis from lung cancer. Cancers 2010, 2, 2100–2137. [Google Scholar] [CrossRef]
- Askins, M.A.; Moore, B.D., 3rd. Preventing neurocognitive late effects in childhood cancer survivors. J. Child Neurol. 2008, 23, 1160–1171. [Google Scholar] [CrossRef]
- Monje, M.; Dietrich, J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav. Brain Res. 2012, 227, 376–379. [Google Scholar] [CrossRef]
- Makale, M.T.; McDonald, C.R.; Hattangadi-Gluth, J.A.; Kesari, S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat. Rev. Neurol. 2017, 13, 52–64. [Google Scholar] [CrossRef]
- Russo, I.; Barlati, S.; Bosetti, F. Effects of neuroinflammation on the regenerative capacity of brain stem cells. J. Neurochem. 2011, 116, 947–956. [Google Scholar] [CrossRef]
- Jenrow, K.A.; Brown, S.L.; Lapanowski, K.; Naei, H.; Kolozsvary, A.; Kim, J.H. Selective inhibition of microglia-mediated neuroinflammation mitigates radiation-induced cognitive impairment. Radiat. Res. 2013, 179, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Prise, K.M.; Saran, A. Concise review: Stem cell effects in radiation risk. Stem Cells 2011, 29, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Tomé, W.A.; Mehta, M.P. Why avoid the hippocampus? A comprehensive review. Radiother. Oncol. 2010, 97, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, J.; Li, G.; Li, Y.; Wu, R.; Cheng, J.; Tang, Y. Pathophysiological responses in rat and mouse models of radiation-induced brain injury. Mol. Neurobiol. 2017, 54, 1022–1032. [Google Scholar] [CrossRef]
- Tada, E.; Yang, C.; Gobbel, G.T.; Lamborn, K.R.; Fike, J.R. Long-term impairment of subependymal repopulation following damage by ionizing irradiation. Exp. Neurol. 1999, 160, 66–77. [Google Scholar] [CrossRef]
- Mizumatsu, S.; Monje, M.L.; Morhardt, D.R.; Rola, R.; Palmer, T.D.; Fike, J.R. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003, 63, 4021–4027. [Google Scholar]
- Casciati, A.; Dobos, K.; Antonelli, F.; Benedek, A.; Kempf, S.J.; Bellés, M.; Balogh, A.; Tanori, M.; Heredia, L.; Atkinson, M.J.; et al. Age-related effects of X-ray irradiation on mouse hippocampus. Oncotarget 2016, 7, 28040–28058. [Google Scholar] [CrossRef]
- Kempf, S.J.; Casciati, A.; Buratovic, S.; Janik, D.; von Toerne, C.; Ueffing, M.; Neff, F.; Moertl, S.; Stenerlöw, B.; Saran, A.; et al. The cognitive defects of neonatally irradiated mice are accompanied by changed synaptic plasticity, adult neurogenesis and neuroinflammation. Mol. Neurodegener. 2014, 9, 57. [Google Scholar] [CrossRef]
- Lumniczky, K.; Szatmári, T.; Sáfrány, G. Ionizing radiation-induced immune and inflammatory reactions in the brain. Front. Immunol. 2017, 8, 517. [Google Scholar] [CrossRef]
- Chesnokova, V.; Pechnick, R.N.; Wawrowsky, K. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav. Immun. 2016, 58, 1–8. [Google Scholar] [CrossRef]
- Lee, W.H.; Sonntag, W.E.; Mitschelen, M.; Yan, H.; Lee, Y.W. Irradiation induces regionally specific alterations in pro-inflammatory environments in rat brain. Int. J. Radiat. Biol. 2010, 86, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Hladik, D.; Tapio, S. Effects of ionizing radiation on the mammalian brain. Mutat. Res. 2016, 770 Pt B, 219–230. [Google Scholar] [CrossRef]
- Benatti, C.; Blom, J.M.; Rigillo, G.; Alboni, S.; Zizzi, F.; Torta, R.; Brunello, N.; Tascedda, F. Disease-induced neuroinflammation and depression. CNS Neurol. Disord. Drug Targets 2016, 15, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Danzer, S.C. Adult neurogenesis in the human brain: Paradise lost? Epilepsy Curr. 2018, 18, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Nguyen, T.; Ihrie, R.A.; Mirzadeh, Z.; Tsai, H.H.; Wong, M.; Gupta, N.; Berger, M.S.; Huang, E.; Garcia-Verdugo, J.M.; et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 2011, 478, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Dennis, C.V.; Suh, L.S.; Rodriguez, M.L.; Kril, J.J.; Sutherland, G.T. Human adult neurogenesis across the ages: An immunohistochemical study. Neuropathol. Appl. Neurobiol. 2016, 42, 621–638. [Google Scholar] [CrossRef]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 2018, 22, 589–599. [Google Scholar] [CrossRef]
- Tobin, M.K.; Musaraca, K.; Disouky, A.; Shetti, A.; Bheri, A.; Honer, W.G.; Kim, N.; Dawe, R.J.; Bennett, D.A.; Arfanakis, K.; et al. Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer’s Disease Patients. Cell Stem Cell 2019, 24, 974–982. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Nogueira, A.B.; Nogueira, A.B.; Veiga, J.C.E.; Teixeira, M.J. Letter: Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Neurosurgery 2018, 83, E133–E137. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human adult neurogenesis: Evidence and remaining questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H. Adult neurogenesis in mammals. Science 2019, 364, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Mulhern, R.K.; Merchant, T.E.; Gajjar, A.; Reddick, W.E.; Kun, L.E. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004, 5, 399–408. [Google Scholar] [CrossRef]
- Duffner, P.K. Risk factors for cognitive decline in children treated for brain tumors. Eur. J. Paediatr. Neurol. 2010, 14, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.H.; Pagnier, A.; Guichardet, K.; Dubois-Teklali, F.; Schiff, I.; Lyard, G.; Cousin, E.; Krainik, A. Cognitive disorders in pediatric medulloblastoma: What neuroimaging has to offer. J. Neurosurg. Pediatr. 2014, 14, 136–144. [Google Scholar] [CrossRef]
- Crossen, J.R.; Garwood, D.; Glatstein, E.; Neuwelt, E.A. Neurobehavioral sequelae of cranial irradiation in adults: A review of radiation-induced encephalopathy. J. Clin. Oncol. 1994, 12, 627–642. [Google Scholar] [CrossRef]
- Surma-aho, O.; Niemelä, M.; Vilkki, J.; Kouri, M.; Brander, A.; Salonen, O.; Paetau, A.; Kallio, M.; Pyykkönen, J.; Jääskeläinen, J. Adverse long-term effects of brain radiotherapy in adult low-grade glioma patients. Neurology 2001, 56, 1285–1290. [Google Scholar] [CrossRef]
- Meyers, C.A.; Brown, P.D. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J. Clin. Oncol. 2006, 24, 1305–1309. [Google Scholar] [CrossRef]
- Robbins, M.E.; Bourland, J.D.; Cline, J.M.; Wheeler, K.T.; Deadwyler, S.A. A model for assessing cognitive impairment after fractionated whole-brain irradiation in nonhuman primates. Radiat. Res. 2011, 175, 519–525. [Google Scholar] [CrossRef]
- Hanbury, D.B.; Robbins, M.E.; Bourland, J.D.; Wheeler, K.T.; Peiffer, A.M.; Mitchell, E.L.; Daunais, J.B.; Deadwyler, S.A.; Cline, J.M. Pathology of fractionated whole-brain irradiation in rhesus monkeys (Macaca mulatta). Radiat. Res. 2015, 183, 367–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Augusto-Oliveira, M.; Arrifano, G.P.F.; Malva, J.O.; Crespo-Lopez, M.E. Adult hippocampal neurogenesis in different taxonomic groups: Possible functional similarities and striking controversies. Cells 2019, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Li, W.; Ge, L.; Chen, G. Non-engineered and engineered adult neurogenesis in mammalian brains. Front. Neurosci. 2019, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Leibel, S.A.; Sheline, G.E. Radiation therapy for neoplasms of the brain. J. Neurosurg. 1987, 66, 1–22. [Google Scholar] [CrossRef]
- Robin, T.P.; Rusthoven, C.G. Strategies to preserve cognition in patients with brain metastases: A review. Front. Oncol. 2018, 8, 415. [Google Scholar] [CrossRef]
- Mehta, M.P. The controversy surrounding the use of whole-brain radiotherapy in brain metastases patients. Neuro Oncol. 2015, 17, 919–923. [Google Scholar] [CrossRef][Green Version]
- Scaringi, C.; Agolli, L.; Minniti, G. Technical advances in radiation therapy for brain tumors. Anticancer Res. 2018, 38, 6041–6045. [Google Scholar] [CrossRef]
- Gondi, V.; Pugh, S.L.; Tome, W.A.; Caine, C.; Corn, B.; Kanner, A.; Rowley, H.; Kundapur, V.; DeNittis, A.; Greenspoon, J.N.; et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase II multi-institutional trial. J. Clin. Oncol. 2014, 32, 3810–3816. [Google Scholar] [CrossRef]
- Oskan, F.; Ganswindt, U.; Schwarz, S.B.; Manapov, F.; Belka, C.; Niyazi, M. Hippocampus sparing in whole-brain radiotherapy. A review. Strahlenther. Onkol. 2014, 190, 337–341. [Google Scholar] [CrossRef]
- Kim, K.S.; Wee, C.W.; Seok, J.Y.; Hong, J.W.; Chung, J.B.; Eom, K.Y.; Kim, J.S.; Kim, C.Y.; Park, Y.H.; Kim, Y.J.; et al. Hippocampus-sparing radiotherapy using volumetric modulated arc therapy (VMAT) to the primary brain tumor: The result of dosimetric study and neurocognitive function assessment. Radiat. Oncol. 2018, 13, 29. [Google Scholar] [CrossRef]
- Atkins, K.M.; Pashtan, I.M.; Bussière, M.R.; Kang, K.H.; Niemierko, A.; Daly, J.E.; Botticello, T.M.; Hurd, M.C.; Chapman, P.H.; Oh, K.; et al. Proton stereotactic radiosurgery for brain metastases: A single-institution analysis of 370 patients. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Kazda, T.; Jancalek, R.; Pospisil, P.; Sevela, O.; Prochazka, T.; Vrzal, M.; Burkon, P.; Slavik, M.; Hynkova, L.; Slampa, P.; et al. Why and how to spare the hippocampus during brain radiotherapy: The developing role of hippocampal avoidance in cranial radiotherapy. Radiat. Oncol. 2014, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.J.; Kummerlowe, M.N.; Redmond, K.J.; Rigamonti, D.; Lim, M.K.; Kleinberg, L.R. Stereotactic radiosurgery: Treatment of brain metastasis without interruption of systemic therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, E.J.; Peterson, J.; Brown, P.D.; Sheehan, J.P.; Quiñones-Hinojosa, A.; Zaorsky, N.G.; Trifiletti, D.M. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: An international meta-analysis of individual patient data. Radiother. Oncol. 2019, 130, 104–112. [Google Scholar] [CrossRef]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef]
- Chan, A.S.; Cheung, M.C.; Law, S.C.; Chan, J.H. Phase II study of alpha-tocopherol in improving the cognitive function of patients with temporal lobe radionecrosis. Cancer 2004, 100, 398–404. [Google Scholar] [CrossRef]
- Raber, J.; Villasana, L.; Rosenberg, J.; Zou, Y.; Huang, T.T.; Fike, J.R. Irradiation enhances hippocampus-dependent cognition in mice deficient in extracellular superoxide dismutase. Hippocampus 2011, 21, 72–80. [Google Scholar] [CrossRef]
- Zou, Y.; Corniola, R.; Leu, D.; Khan, A.; Sahbaie, P.; Chakraborti, A.; Clark, D.J.; Fike, J.R.; Huang, T.T. Extracellular superoxide dismutase is important for hippocampal neurogenesis and preservation of cognitive functions after irradiation. Proc. Natl. Acad. Sci. USA 2012, 109, 21522–21527. [Google Scholar] [CrossRef]
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef]
- Wright, J.W.; Harding, J.W. The brain angiotensin system and extracellular matrix molecules in neural plasticity, learning, and memory. Prog. Neurobiol. 2004, 72, 263–293. [Google Scholar] [CrossRef]
- Jenrow, K.A.; Brown, S.L.; Liu, J.; Kolozsvary, A.; Lapanowski, K.; Kim, J.H. Ramipril mitigates radiation-induced impairment of neurogenesis in the rat dentate gyrus. Radiat. Oncol. 2010, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Greene-Schloesser, D.; Payne, V.; Diz, D.I.; Hsu, F.C.; Kooshki, M.; Mustafa, R.; Riddle, D.R.; Zhao, W.; Chan, M.D.; et al. Chronic administration of the angiotensin-converting enzyme inhibitor, ramipril, prevents fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment. Radiat. Res. 2012, 178, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.E.; Payne, V.; Tommasi, E.; Diz, D.I.; Hsu, F.C.; Brown, W.R.; Wheeler, K.T.; Olson, J.; Zhao, W. The AT1 receptor antagonist, L-158,809, prevents or ameliorates fractionated whole-brain irradiation-induced cognitive impairment. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Conner, K.R.; Forbes, M.E.; Lee, W.H.; Lee, Y.W.; Riddle, D.R. AT1 receptor antagonism does not influence early radiation-induced changes in microglial activation or neurogenesis in the normal rat brain. Radiat. Res. 2011, 176, 71–83. [Google Scholar] [CrossRef][Green Version]
- Parihar, V.K.; Limoli, C.L. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc. Natl. Acad. Sci. USA 2013, 110, 12822–12827. [Google Scholar] [CrossRef]
- Fidaleo, M.; Fanelli, F.; Ceru, M.P.; Moreno, S. Neuroprotective properties of peroxisome proliferator-activated receptor alpha (PPARα) and its lipid ligands. Curr. Med. Chem. 2014, 21, 2803–2821. [Google Scholar] [CrossRef]
- Ramanan, S.; Kooshki, M.; Zhao, W.; Hsu, F.C.; Riddle, D.R.; Robbins, M.E. The PPAR alpha agonist fenofibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole-brain irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 870–877. [Google Scholar] [CrossRef]
- Greene-Schloesser, D.; Payne, V.; Peiffer, A.M.; Hsu, F.C.; Riddle, D.R.; Zhao, W.; Chan, M.D.; Metheny-Barlow, L.; Robbins, M.E. The peroxisomal proliferator-activated receptor (PPAR) α agonist, fenofibrate, prevents fractionated whole-brain irradiation-induced cognitive impairment. Radiat. Res. 2014, 181, 33–44. [Google Scholar] [CrossRef]
- Zhao, W.; Payne, V.; Tommasi, E.; Diz, D.I.; Hsu, F.C.; Robbins, M.E. Administration of the peroxisomal proliferator-activated receptor gamma agonist pioglitazone during fractionated brain irradiation prevents radiation-induced cognitive impairment. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 6–9. [Google Scholar] [CrossRef]
- Reis, D.J.; Casteen, E.J.; Ilardi, S.S. The antidepressant impact of minocycline in rodents: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 261. [Google Scholar] [CrossRef]
- Zhang, L.; Li, K.; Sun, R.; Zhang, Y.; Ji, J.; Huang, P.; Yang, H.; Tian, Y. Minocycline ameliorates cognitive impairment induced by whole-brain irradiation: An animal study. Radiat. Oncol. 2014, 9, 281. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, P.; Chen, H.; Tan, W.; Lu, J.; Liu, W.; Wang, J.; Zhang, S.; Zhu, W.; Cao, J.; et al. The inhibitory effect of minocycline on radiation-induced neuronal apoptosis via AMPKα1 signaling-mediated autophagy. Sci. Rep. 2017, 7, 16373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, K.; Li, T.; Xu, Y.; Xie, C.; Sun, Y.; Zhang, Y.; Rodriguez, J.; Blomgren, K.; Zhu, C. Inhibition of autophagy prevents irradiation-induced neural stem and progenitor cell death in the juvenile mouse brain. Cell Death Dis. 2017, 8, e2694. [Google Scholar] [CrossRef] [PubMed]
- Plangár, I.; Szabó, E.R.; Tőkés, T.; Mán, I.; Brinyiczki, K.; Fekete, G.; Németh, I.; Ghyczy, M.; Boros, M.; Hideghéty, K. Radio-neuroprotective effect of L-alpha-glycerylphosphorylcholine (GPC) in an experimental rat model. J. Neurooncol. 2014, 119, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gao, X.; Zhao, S.; Hu, W.; Chen, J. The Small-Molecule TrkB Agonist 7, 8-Dihydroxyflavone Decreases Hippocampal Newborn Neuron Death After Traumatic Brain Injury. J. Neuropathol. Exp. Neurol. 2015, 74, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Leu, D.; Ye, K.; Srinivasan, C.; Fike, J.R.; Huang, T.T. Cognitive impairments following cranial irradiation can be mitigated by treatment with a tropomyosin receptor kinase B agonist. Exp. Neurol. 2016, 279, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Cosman, K.M.; Boyle, L.L.; Porsteinsson, A.P. Memantine in the treatment of mild-to-moderate Alzheimer’s disease. Expert Opin. Pharmacother. 2007, 8, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Pugh, S.; Laack, N.N.; Wefel, J.S.; Khuntia, D.; Meyers, C.; Choucair, A.; Fox, S.; Suh, J.H.; Roberge, D.; et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013, 15, 1429–1437. [Google Scholar] [CrossRef]
- Acharya, M.M.; Christie, L.A.; Lan, M.L.; Giedzinski, E.; Fike, J.R.; Rosi, S.; Limoli, C.L. Human neural stem cell transplantation ameliorates radiation-induced cognitive dysfunction. Cancer Res. 2011, 71, 4834–4845. [Google Scholar] [CrossRef]
- Acharya, M.M.; Roa, D.E.; Bosch, O.; Lan, M.L.; Limoli, C.L. Stem cell transplantation strategies for the restoration of cognitive dysfunction caused by cranial radiotherapy. J. Vis. Exp. 2011, 56, 3107. [Google Scholar] [CrossRef]
- Joo, K.M.; Jin, J.; Kang, B.G.; Lee, S.J.; Kim, K.H.; Yang, H.; Lee, Y.A.; Cho, Y.J.; Im, Y.S.; Lee, D.S.; et al. Trans-differentiation of neural stem cells: A therapeutic mechanism against the radiation induced brain damage. PLoS ONE 2012, 7, e25936. [Google Scholar] [CrossRef] [PubMed]
- Baulch, J.E.; Acharya, M.M.; Allen, B.D.; Ru, N.; Chmielewski, N.N.; Martirosian, V.; Giedzinski, E.; Syage, A.; Park, A.L.; Benke, S.N.; et al. Cranial grafting of stem cell-derived microvesicles improves cognition and reduces neuropathology in the irradiated brain. Proc. Natl. Acad. Sci. USA 2016, 113, 4836–4841. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; Major, T.; Auyeung, G.; Policarpio, E.; Menon, J.; Droms, L.; Gutin, P.; Uryu, K.; Tchieu, J.; Soulet, D.; et al. Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell 2015, 16, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Soria, B.; Martin-Montalvo, A.; Aguilera, Y.; Mellado-Damas, N.; López-Beas, J.; Herrera-Herrera, I.; López, E.; Barcia, J.A.; Alvarez-Dolado, M.; Hmadcha, A.; et al. Human mesenchymal stem cells prevent neurological complications of radiotherapy. Front. Cell. Neurosci. 2019, 13, 204. [Google Scholar] [CrossRef]
- Duncan, T.; Valenzuela, M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res. Ther. 2017, 8, 111. [Google Scholar] [CrossRef]
- Bergami, M.; Rimondini, R.; Santi, S.; Blum, R.; Götz, M.; Canossa, M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc. Natl. Acad. Sci. USA 2008, 105, 15570–15575. [Google Scholar] [CrossRef]
- Groves, J.O.; Leslie, I.; Huang, G.J.; McHugh, S.B.; Taylor, A.; Mott, R.; Munafò, M.; Bannerman, D.M.; Flint, J. Ablating adult neurogenesis in the rat has no effect on spatial processing: Evidence from a novel pharmacogenetic model. PLoS Genet. 2013, 9, e1003718. [Google Scholar] [CrossRef]
- Tofilon, P.J.; Fike, J.R. The radioresponse of the central nervous system: A dynamic process. Radiat. Res. 2000, 153, 357–370. [Google Scholar] [CrossRef]
- Wu, P.H.; Coultrap, S.; Pinnix, C.; Davies, K.D.; Tailor, R.; Ang, K.K.; Browning, M.D.; Grosshans, D.R. Radiation induces acute alterations in neuronal function. PLoS ONE 2012, 7, e37677. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pazzaglia, S.; Briganti, G.; Mancuso, M.; Saran, A. Neurocognitive Decline Following Radiotherapy: Mechanisms and Therapeutic Implications. Cancers 2020, 12, 146. https://doi.org/10.3390/cancers12010146
Pazzaglia S, Briganti G, Mancuso M, Saran A. Neurocognitive Decline Following Radiotherapy: Mechanisms and Therapeutic Implications. Cancers. 2020; 12(1):146. https://doi.org/10.3390/cancers12010146
Chicago/Turabian StylePazzaglia, Simonetta, Giovanni Briganti, Mariateresa Mancuso, and Anna Saran. 2020. "Neurocognitive Decline Following Radiotherapy: Mechanisms and Therapeutic Implications" Cancers 12, no. 1: 146. https://doi.org/10.3390/cancers12010146
APA StylePazzaglia, S., Briganti, G., Mancuso, M., & Saran, A. (2020). Neurocognitive Decline Following Radiotherapy: Mechanisms and Therapeutic Implications. Cancers, 12(1), 146. https://doi.org/10.3390/cancers12010146




