The Future of Immunotherapy-Based Combination Therapy in Metastatic Renal Cell Carcinoma
Abstract
1. Introduction
2. The Rationale to Combine Immunotherapy with Angiogenesis Inhibitors
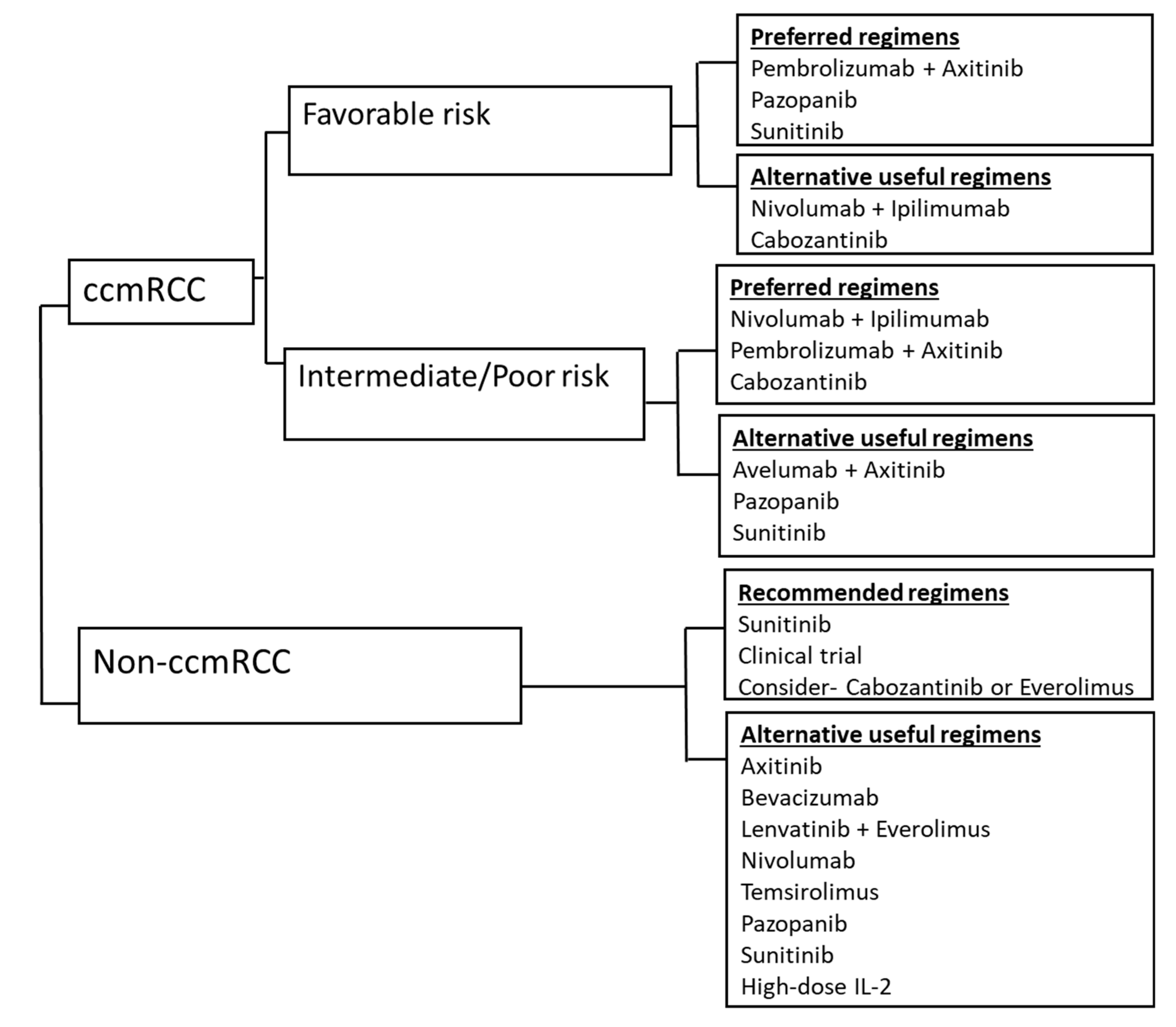
3. Nivolumab in Combination with Ipilimumab versus Sunitinib Monotherapy
4. Pembrolizumab in Combination with Axitinib in Metastatic ccRCC
5. Avelumab in Combination with Axitinib in Metastatic ccRCC
6. Atezolizumab in Combination with Bevacizumab versus Sunitinib Monotherapy in Metastatic ccRCC
7. Nivolumab in Combination with Tivozanib in mRCC
8. Nivolumab in Combination with Sunitinib or Pazopanib in mRCC
9. Pembrolizumab in Combination with Bevacizumab in mRCC
10. Pembrolizumab in Combination with Lenvatinib in mRCC
11. Pembrolizumab and Pazopanib in Patients with Advanced or mRCC
12. Cabozantinib in Combination with Atezolizumab in Advance Renal Cell Carcinoma
13. Discussion
14. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Belldegrun, A.S.; Klatte, T.; Shuch, B.; LaRochelle, J.C.; Miller, D.C.; Said, J.W.; Riggs, S.B.; Zomorodian, N.; Kabbinavar, F.F.; Dekernion, J.B.; et al. Cancer-specific survival outcomes among patients treated during the cytokine era of kidney cancer (1989–2005): A benchmark for emerging targeted cancer therapies. Cancer 2008, 113, 2457–2463. [Google Scholar] [CrossRef]
- Fyfe, G.; Fisher, R.I.; Rosenberg, S.A.; Sznol, M.; Parkinson, D.R.; Louie, A.C. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. 1995, 13, 688–696. [Google Scholar] [CrossRef]
- Voron, T.; Marcheteau, E.; Pernot, S.; Colussi, O.; Tartour, E.; Taieb, J.; Terme, M. Control of the immune response by pro-angiogenic factors. Front. Oncol. 2014, 4, 70. [Google Scholar] [CrossRef]
- Yasuda, S.; Sho, M.; Yamato, I.; Yoshiji, H.; Wakatsuki, K.; Nishiwada, S.; Yagita, H.; Nakajima, Y. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin. Exp. Immunol. 2013, 172, 500–506. [Google Scholar] [CrossRef]
- Brauer, M.J.; Zhuang, G.; Schmidt, M.; Yao, J.; Wu, X.; Kaminker, J.S.; Jurinka, S.S.; Kolumam, G.; Chung, A.S.; Jubb, A.; et al. Identification and Analysis of In Vivo VEGF Downstream Markers Link VEGF Pathway Activity with Efficacy of Anti-VEGF Therapies. Clin. Cancer Res. 2013, 19, 3681–3692. [Google Scholar] [CrossRef]
- Powles, T.; Nickles, D.; Van Allen, E.; Chappey, C.; Zou, W.; Kowanetz, M.; Kadel, E.; Denker, M.; Boyd, Z.; Vogelzang, N.; et al. Immune biomarkers associated with clinical benefit from atezolizumab (MPDL3280a; anti-PD-L1) in advanced urothelial bladder cancer (UBC). J. Immunother. Cancer 2015, 3, P83. [Google Scholar] [CrossRef]
- McDermott, D.F.; Huseni, M.A.; Atkins, M.B.; Motzer, R.J.; Rini, B.I.; Escudier, B.; Fong, L.; Joseph, R.W.; Pal, S.K.; Reeves, J.A.; et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 2018, 24, 749–757. [Google Scholar] [CrossRef]
- Wallin, J.J.; Bendell, J.C.; Funke, R.; Sznol, M.; Korski, K.; Jones, S.; Hernandez, G.; Mier, J.; He, X.; Hodi, F.S.; et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat. Commun. 2016, 7, 12624. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Ko, J.J.; Xie, W.; Kroeger, N.; Lee, J.L.; Rini, B.I.; Knox, J.J.; Bjarnason, G.A.; Srinivas, S.; Pal, S.K.; Yuasa, T.; et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: A population-based study. Lancet Oncol. 2015, 16, 293–300. [Google Scholar] [CrossRef]
- Grünwald, V.; Choueiri, T.K.; Rini, B.I.; Powles, T.; George, S.; Grimm, M.-O.; McHenry, M.B.; Maurer, M.; Motzer, R.J.; Hammers, H.J.; et al. 950PAssociation between depth of response and overall survival: Exploratory analysis in patients with previously untreated advanced renal cell carcinoma (aRCC) in CheckMate 214. Ann. Oncol. 2019, 30, mdz249-046. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulieres, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Rini, B.I.; Powles, T.; Atkins, M.B.; Escudier, B.; McDermott, D.F.; Suarez, C.; Bracarda, S.; Stadler, W.M.; Donskov, F.; Lee, J.L.; et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019, 393, 2404–2415. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Soulieres, D.; Melichar, B.; Vynnychenko, I.; et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for metastatic renal cell carcinoma (mRCC): Outcomes in the combined IMDC intermediate/poor risk and sarcomatoid subgroups of the phase 3 KEYNOTE-426 study. J. Clin. Oncol. 2019, 37, 4500. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Larkin, J.; Oya, M.; Thistlethwaite, F.; Martignoni, M.; Nathan, P.; Powles, T.; McDermott, D.; Robbins, P.B.; Chism, D.D.; et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): An open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol. 2018, 19, 451–460. [Google Scholar] [CrossRef]
- Albiges, L.; Rini, B.I.; Haanen, J.B.A.G.; Motzer, R.J.; Kollmannsberger, C.K.; Negrier, S.; Nole, F.; Bedke, J.; Bilen, M.A.; Nathan, P.; et al. 908PDPrimary renal tumour shrinkage in patients (pts) who did not undergo upfront cytoreductive nephrectomy (uCN): Subgroup analysis from the phase III JAVELIN Renal 101 trial of first-line avelumab + axitinib (A + Ax) vs sunitinib (S) for advanced renal cell carcinoma (aRCC). Ann. Oncol. 2019, 30, mdz249-007. [Google Scholar] [CrossRef]
- Motzer, R.J.; Powles, T.; Atkins, M.B.; Escudier, B.; McDermott, D.F.; Suarez, C.; Bracarda, S.; Stadler, W.M.; Donskov, F.; Lee, J.-L.; et al. IMmotion151: A Randomized Phase III Study of Atezolizumab Plus Bevacizumab vs Sunitinib in Untreated Metastatic Renal Cell Carcinoma (mRCC). J. Clin. Oncol. 2018, 36, 578. [Google Scholar] [CrossRef]
- Escudier, B.; Barthelemy, P.; Ravaud, A.; Negrier, S.; Needle, M.N.; Albiges, L. Tivozanib combined with nivolumab: Phase Ib/II study in metastatic renal cell carcinoma (mRCC). J. Clin. Oncol. 2018, 36, 618. [Google Scholar] [CrossRef]
- Barthelemy, P.; Escudier, B.; Ravaud, A.; Negrier, S.; Needle, M.N.; Albiges, L. 878PTiNivo—Tivozanib combined with nivolumab: Safety and efficacy in patients with metastatic renal cell carcinoma (mRCC). Ann. Oncol. 2018, 29, mdy283-087. [Google Scholar] [CrossRef]
- Barthelemy, P.; Escudier, B.; Negrier, S.; Ravaud, A.; Needle, M.N.; Albiges, L. 947PTiNivo: Tivozanib combined with nivolumab results in prolonged progression free survival in patients with metastatic renal cell carcinoma (mRCC): Final results. Ann. Oncol. 2019, 30, mdz249-043. [Google Scholar] [CrossRef]
- Amin, A.; Plimack, E.R.; Ernstoff, M.S.; Lewis, L.D.; Bauer, T.M.; McDermott, D.F.; Carducci, M.; Kollmannsberger, C.; Rini, B.I.; Heng, D.Y.C.; et al. Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: The CheckMate 016 study. J. Immunother. Cancer 2018, 6, 109. [Google Scholar] [CrossRef]
- Dudek, A.Z.; Sica, R.A.; Sidani, A.; Jha, G.G.; Xie, H.; Alva, A.S.; Stein, M.N.; Singer, E.A. Phase Ib study of pembrolizumab in combination with bevacizumab for the treatment of metastatic renal cell carcinoma: Big Ten Cancer Research Consortium BTCRC-GU14-003. J. Clin. Oncol. 2016, 34, 559. [Google Scholar] [CrossRef]
- Matsui, J.; Yamamoto, Y.; Funahashi, Y.; Tsuruoka, A.; Watanabe, T.; Wakabayashi, T.; Uenaka, T.; Asada, M. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int. J. Cancer 2008, 122, 664–671. [Google Scholar] [CrossRef]
- Lee, C.-H.; Makker, V.; Rasco, D.W.; Taylor, M.H.; Stepan, D.E.; Shumaker, R.C.; Schmidt, E.V.; Guo, M.; Dutcus, C.E.; Motzer, R.J. Lenvatinib + pembrolizumab in patients with renal cell carcinoma: Updated results. J. Clin. Oncol. 2018, 36, 4560. [Google Scholar] [CrossRef]
- Lee, C.-H.; Shah, A.Y.; Makker, V.; Taylor, M.H.; Shaffer, D.; Hsieh, J.J.; Cohn, A.L.; DiSimone, C.; Marin, A.P.; Rasco, D.W.; et al. 1187PDPhase II study of lenvatinib plus pembrolizumab for disease progression after PD-1/PD-L1 immune checkpoint inhibitor in metastatic clear cell renal cell carcinoma (mccRCC): Results of an interim analysis. Ann. Oncol. 2019, 30, mdz253-013. [Google Scholar] [CrossRef]
- Chowdhury, S.; McDermott, D.F.; Voss, M.H.; Hawkins, R.E.; Aimone, P.; Voi, M.; Isabelle, N.; Wu, Y.; Infante, J.R. A phase I/II study to assess the safety and efficacy of pazopanib (PAZ) and pembrolizumab (PEM) in patients (pts) with advanced renal cell carcinoma (aRCC). J. Clin. Oncol. 2017, 35, 4506. [Google Scholar] [CrossRef]
- Agarwal, N.; Vaishampayan, U.; Green, M.; di Nucci, F.; Chang, P.-Y.; Scheffold, C.; Pal, S. 872PPhase Ib study (COSMIC-021) of cabozantinib in combination with atezolizumab: Results of the dose escalation stage in patients (pts) with treatment-naïve advanced renal cell carcinoma (RCC). Ann. Oncol. 2018, 29, mdy283-081. [Google Scholar] [CrossRef]
- Kidney Cancer NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf (accessed on 15 December 2019).
| Study | N | Compounds | Median OS, mo (95% CI) | Median PFS, mo (95% CI) | CRR | ORR (95% CI) | Grade 3 and 4 TRAEs | Treatment-Related Deaths | Treatment Discontinuation Rate | IRAE Needing ≥40 mg Total Daily Dose of Prednisone or Equivalent |
|---|---|---|---|---|---|---|---|---|---|---|
| Checkmate 214 [11] | 1096 | Intermediate and poor risk: Nivolumab + ipilimumab vs. sunitinib | NR vs. 26.0 HR = 0.63; p < 0.001. | 11.6 vs. 8.4 (HR = 0.82; p = 0.0331. | 9% vs. 1% | 42% vs. 27% | 46% vs. 63% | 1.5% vs. 0.74% | 22% vs. 12% | 35% |
| KEYNOTE-426 [14] | 861 | Pembrolizumab + axitinib vs. sunitinib | NR, HR 0.53; p < 0.0001 12-mo OS: 90% vs. 78% | 15.1 vs. 11.1 HR 0.69; 0.57–0.84; p = 0.0001) | 5.8% vs. 1.9% | 59.3% vs. 35.7%; p < 0.0001 | 62.9% vs. 58.1% | 0.9% vs. 1.6% | both drugs: 30.5%, sunitinib: 13.9% | N/a |
| JAVELIN Renal 101 [15] | 886 | Avelumab plus axitinib vs. sunitinib | NR; 12-mo: 86% vs. 83% (HR 0.78; 0.55 to 1.08; p = 0.14) | 13.8 vs 8.4 (HR 0.69; 0.56 to 0.84; p < 0.0001) | 3.4% vs 1.8% | 51.4% vs. 25.7 % | 71.2% vs. 71.5% | 0.7% vs. 0.2% | 7.6 vs.13.4 | 11.1% |
| IMmotion151 [16] | 915; PDL1+: 362 | Atezolizumab + bevacizumab vs. sunitinib | NR, 24-mo: 63% vs. 60% (HR 0.93; 0.76 to 1.14; p = 0.4751) | ITT: 11.2 vs. 8.4 (HR 0.83; 0.70–0.97; p = 0.0219) PDL1+: 11.2 vs. 7.7 | ITT: 5% vs. 2%; PD-L1+: 9% vs. 4% | ITT: 37% vs. 33% PD-L1+: 43% vs. 35% | 40% vs. 54% | 1.1% vs. 0.22% | 5% vs. 8% | 9% |
| National Clinical Trial ID Number (Study) | Treatment | Phase | Clinical Trial Status | Treatment Line | Patients | Primary Outcome Measures |
|---|---|---|---|---|---|---|
| Lenvatinib | ||||||
| NCT02811861 (CLEAR) | Lenvatinib with everolimus or pembrolizumab compared to SOC sunitinib | III, randomized 1:1:1, open label | active, not-recruiting | First-line treatment of subjects with advanced renal cell carcinoma | 1069 | PFS by independent review |
| Cabozantinib | ||||||
| NCT03141177 (CheckMate 9ER) | Nivolumab + Cabozantinib vs. Sunitinib | Phase III, randomized, open-label study | active, not recruiting | First line, metastatic RCC | 638 | PFS per blinded independent central review (BICR) |
| NCT03937219 (COSMIC-313) | Cabozantinib + nivolumab + ipilimumab vs. Nivolumab + ipilimumab | III, randomized, 1:1, double-blind | recruiting | First line, intermediate- or poor-risk metastatic RCC | 676 | PFS by blinded independent radiology committee (BIRC) |
| NCT03793166 (PDIGREE study) | Nivolumab and Ipilimumab followed by Nivolumab vs. Cabozantinib with Nivolumab | III, randomized, open label | recruiting | First line | 1046 | OS |
| NCT03149822 | Pembrolizumab plus Cabozantinib | I/II, open label, single arm | recruiting | First or second line | 55 | ORR (CR + PR) |
| NCT03200587 | Avelumab and Cabozantinib | Ib, open label | recruiting | First line | 20 | DLTs, AEs, RP2D |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garje, R.; An, J.; Greco, A.; Vaddepally, R.K.; Zakharia, Y. The Future of Immunotherapy-Based Combination Therapy in Metastatic Renal Cell Carcinoma. Cancers 2020, 12, 143. https://doi.org/10.3390/cancers12010143
Garje R, An J, Greco A, Vaddepally RK, Zakharia Y. The Future of Immunotherapy-Based Combination Therapy in Metastatic Renal Cell Carcinoma. Cancers. 2020; 12(1):143. https://doi.org/10.3390/cancers12010143
Chicago/Turabian StyleGarje, Rohan, Josiah An, Austin Greco, Raju Kumar Vaddepally, and Yousef Zakharia. 2020. "The Future of Immunotherapy-Based Combination Therapy in Metastatic Renal Cell Carcinoma" Cancers 12, no. 1: 143. https://doi.org/10.3390/cancers12010143
APA StyleGarje, R., An, J., Greco, A., Vaddepally, R. K., & Zakharia, Y. (2020). The Future of Immunotherapy-Based Combination Therapy in Metastatic Renal Cell Carcinoma. Cancers, 12(1), 143. https://doi.org/10.3390/cancers12010143





