Preclinical Models of Craniospinal Irradiation for Medulloblastoma
Abstract
1. Introduction
2. Clinical Management of Medulloblastoma: The Evolving Role of Radiation Therapy
2.1. Background
2.2. Defining the Role of Radiotherapy across Clinical Risk Groups of Medulloblastoma
2.3. Towards Subgroup-Specific Radiotherapy Guidelines
2.4. Advances in Radiotherapy Techniques
3. Preclinical Modeling of CSI in Medulloblastoma
3.1. Current Models of Medulloblastoma
3.2. Preclinical Pipeline Limitations and Considerations
3.3. Existing Models of Craniospinal Irradiation
4. Outstanding Questions and Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vladoiu, M.C.; El-Hamamy, I.; Donovan, L.K.; Farooq, H.; Holgado, B.L.; Sundaravadanam, Y.; Ramaswamy, V.; Hendrikse, L.D.; Kumar, S.; Mack, S.C.; et al. Childhood cerebellar tumours mirror conserved fetal transcriptional programs. Nature 2019, 572, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Hovestadt, V.; Smith, K.S.; Bihannic, L.; Filbin, M.G.; Shaw, M.L.; Baumgartner, A.; DeWitt, J.C.; Groves, A.; Mayr, L.; Weisman, H.R.; et al. Resolving medulloblastoma cellular architecture by single-cell genomics. Nature 2019, 572, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.; Tong, Y.; Robinson, G.; Thompson, M.C.; Currle, D.S.; Eden, C.; Kranenburg, T.A.; Hogg, T.; Poppleton, H.; Martin, J.; et al. Subtypes of medulloblastoma have distinct developmental origins. Nature 2010, 468, 1095–1099. [Google Scholar] [CrossRef]
- McNeill, K.A. Epidemiology of Brain Tumors. Neurol. Clin. 2016, 34, 981–998. [Google Scholar] [CrossRef]
- Northcott, P.A.; Robinson, G.W.; Kratz, C.P.; Mabbott, D.J.; Pomeroy, S.L.; Clifford, S.C.; Rutkowski, S.; Ellison, D.W.; Malkin, D.; Taylor, M.D.; et al. Medulloblastoma. Nat. Rev. Dis. Primers 2019, 5, e11. [Google Scholar] [CrossRef]
- Juraschka, K.; Taylor, M.D. Medulloblastoma in the age of molecular subgroups: A review. J. Neurosurg. Pediatr. 2019, 24, 353–363. [Google Scholar] [CrossRef]
- Fouladi, M.; Gajjar, A.; Boyett, J.M.; Walter, A.W.; Thompson, S.J.; Merchant, T.E.; Jenkins, J.J.; Langston, J.W.; Liu, A.; Kun, L.E.; et al. Comparison of CSF cytology and spinal magnetic resonance imaging in the detection of leptomeningeal disease in pediatric medulloblastoma or primitive neuroectodermal tumor. J. Clin. Oncol. 1999, 17, 3234–3237. [Google Scholar] [CrossRef]
- Kortmann, R.D.; Kuhl, J.; Timmermann, B.; Mittler, U.; Urban, C.; Budach, V.; Richter, E.; Willich, N.; Flentje, M.; Berthold, F.; et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: Results of the German prospective randomized trial HIT ‘91. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 269–279. [Google Scholar] [CrossRef]
- Packer, R.J.; Goldwein, J.; Nicholson, H.S.; Vezina, L.G.; Allen, J.C.; Ris, M.D.; Muraszko, K.; Rorke, L.B.; Wara, W.M.; Cohen, B.H.; et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children’s Cancer Group Study. J. Clin. Oncol. 1999, 17, 2127–2136. [Google Scholar] [CrossRef]
- Packer, R.J.; Gajjar, A.; Vezina, G.; Rorke-Adams, L.; Burger, P.C.; Robertson, P.L.; Bayer, L.; LaFond, D.; Donahue, B.R.; Marymont, M.H.; et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J. Clin. Oncol. 2006, 24, 4202–4208. [Google Scholar] [CrossRef]
- Duffner, P.K.; Horowitz, M.E.; Krischer, J.P.; Burger, P.C.; Cohen, M.E.; Sanford, R.A.; Friedman, H.S.; Kun, L.E. The treatment of malignant brain tumors in infants and very young children: An update of the Pediatric Oncology Group experience. Neuro Oncol. 1999, 1, 152–161. [Google Scholar] [CrossRef]
- Grill, J.; Sainte-Rose, C.; Jouvet, A.; Gentet, J.C.; Lejars, O.; Frappaz, D.; Doz, F.; Rialland, X.; Pichon, F.; Bertozzi, A.I.; et al. Treatment of medulloblastoma with postoperative chemotherapy alone: An SFOP prospective trial in young children. Lancet Oncol. 2005, 6, 573–580. [Google Scholar] [CrossRef]
- Rutkowski, S.; Bode, U.; Deinlein, F.; Ottensmeier, H.; Warmuth-Metz, M.; Soerensen, N.; Graf, N.; Emser, A.; Pietsch, T.; Wolff, J.E.; et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N. Engl. J. Med. 2005, 352, 978–986. [Google Scholar] [CrossRef]
- Dhall, G.; Grodman, H.; Ji, L.; Sands, S.; Gardner, S.; Dunkel, I.J.; McCowage, G.B.; Diez, B.; Allen, J.C.; Gopalan, A.; et al. Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “Head Start” I and II protocols. Pediatr. Blood Cancer 2008, 50, 1169–1175. [Google Scholar] [CrossRef]
- Gajjar, A.; Chintagumpala, M.; Ashley, D.; Kellie, S.; Kun, L.E.; Merchant, T.E.; Woo, S.; Wheeler, G.; Ahern, V.; Krasin, M.J.; et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): Long-term results from a prospective, multicentre trial. Lancet Oncol. 2006, 7, 813–820. [Google Scholar] [CrossRef]
- Merchant, T.E.; Kun, L.E.; Krasin, M.J.; Wallace, D.; Chintagumpala, M.M.; Woo, S.Y.; Ashley, D.M.; Sexton, M.; Kellie, S.J.; Ahern, V.; et al. Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 782–787. [Google Scholar] [CrossRef]
- Taylor, M.D.; Northcott, P.A.; Korshunov, A.; Remke, M.; Cho, Y.J.; Clifford, S.C.; Eberhart, C.G.; Parsons, D.W.; Rutkowski, S.; Gajjar, A.; et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol. 2012, 123, 465–472. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Remke, M.; Bouffet, E.; Bailey, S.; Clifford, S.C.; Doz, F.; Kool, M.; Dufour, C.; Vassal, G.; Milde, T.; et al. Risk stratification of childhood medulloblastoma in the molecular era: The current consensus. Acta Neuropathol. 2016, 131, 821–831. [Google Scholar] [CrossRef]
- Sharma, T.; Schwalbe, E.C.; Williamson, D.; Sill, M.; Hovestadt, V.; Mynarek, M.; Rutkowski, S.; Robinson, G.W.; Gajjar, A.; Cavalli, F.; et al. Second-generation molecular subgrouping of medulloblastoma: An international meta-analysis of Group 3 and Group 4 subtypes. Acta Neuropathol. 2019, 138, 309–326. [Google Scholar] [CrossRef]
- Jenkin, R.D. Medulloblastoma in childhood: Radiation therapy. Can. Med. Assoc. J. 1969, 100, 51–53. [Google Scholar]
- Evans, A.E.; Jenkin, R.D.; Sposto, R.; Ortega, J.A.; Wilson, C.B.; Wara, W.; Ertel, I.J.; Kramer, S.; Chang, C.H.; Leikin, S.L.; et al. The treatment of medulloblastoma. Results of a prospective randomized trial of radiation therapy with and without CCNU, vincristine, and prednisone. J. Neurosurg. 1990, 72, 572–582. [Google Scholar] [CrossRef]
- Chin, H.W.; Maruyama, Y. Age at treatment and long-term performance results in medulloblastoma. Cancer 1984, 53, 1952–1958. [Google Scholar] [CrossRef]
- Silverman, C.L.; Palkes, H.; Talent, B.; Kovnar, E.; Clouse, J.W.; Thomas, P.R. Late effects of radiotherapy on patients with cerebellar medulloblastoma. Cancer 1984, 54, 825–829. [Google Scholar] [CrossRef]
- Packer, R.J.; Sposto, R.; Atkins, T.E.; Sutton, L.N.; Bruce, D.A.; Siegel, K.R.; Rorke, L.B.; Littman, P.A.; Schut, L. Quality of life in children with primitive neuroectodermal tumors (medulloblastoma) of the posterior fossa. Pediatr. Neurosci. 1987, 13, 169–175. [Google Scholar] [CrossRef]
- Duffner, P.K.; Horowitz, M.E.; Krischer, J.P.; Friedman, H.S.; Burger, P.C.; Cohen, M.E.; Sanford, R.A.; Mulhern, R.K.; James, H.E.; Freeman, C.R.; et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N. Engl. J. Med. 1993, 328, 1725–1731. [Google Scholar] [CrossRef]
- Geyer, J.R.; Zeltzer, P.M.; Boyett, J.M.; Rorke, L.B.; Stanley, P.; Albright, A.L.; Wisoff, J.H.; Milstein, J.M.; Allen, J.C.; Finlay, J.L.; et al. Survival of infants with primitive neuroectodermal tumors or malignant ependymomas of the CNS treated with eight drugs in 1 day: A report from the Childrens Cancer Group. J. Clin. Oncol. 1994, 12, 1607–1615. [Google Scholar] [CrossRef]
- Chi, S.N.; Gardner, S.L.; Levy, A.S.; Knopp, E.A.; Miller, D.C.; Wisoff, J.H.; Weiner, H.L.; Finlay, J.L. Feasibility and response to induction chemotherapy intensified with high-dose methotrexate for young children with newly diagnosed high-risk disseminated medulloblastoma. J. Clin. Oncol. 2004, 22, 4881–4887. [Google Scholar] [CrossRef]
- Geyer, J.R.; Sposto, R.; Jennings, M.; Boyett, J.M.; Axtell, R.A.; Breiger, D.; Broxson, E.; Donahue, B.; Finlay, J.L.; Goldwein, J.W.; et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: A report from the Children’s Cancer Group. J. Clin. Oncol. 2005, 23, 7621–7631. [Google Scholar] [CrossRef]
- Rutkowski, S.; von Hoff, K.; Emser, A.; Zwiener, I.; Pietsch, T.; Figarella-Branger, D.; Giangaspero, F.; Ellison, D.W.; Garre, M.L.; Biassoni, V.; et al. Survival and prognostic factors of early childhood medulloblastoma: An international meta-analysis. J. Clin. Oncol. 2010, 28, 4961–4968. [Google Scholar] [CrossRef]
- Lafay-Cousin, L.; Bouffet, E.; Strother, D.; Rudneva, V.; Hawkins, C.; Eberhart, C.; Horbinski, C.; Heier, L.; Souweidane, M.; Williams-Hughes, C.; et al. Phase II Study of Nonmetastatic Desmoplastic Medulloblastoma in Children Younger Than 4 Years of Age: A Report of the Children’s Oncology Group (ACNS1221). J. Clin. Oncol. 2019. [Google Scholar] [CrossRef]
- Ashley, D.M.; Merchant, T.E.; Strother, D.; Zhou, T.; Duffner, P.; Burger, P.C.; Miller, D.C.; Lyon, N.; Bonner, M.J.; Msall, M.; et al. Induction chemotherapy and conformal radiation therapy for very young children with nonmetastatic medulloblastoma: Children’s Oncology Group study P9934. J. Clin. Oncol. 2012, 30, 3181–3186. [Google Scholar] [CrossRef]
- Blaney, S.M.; Kocak, M.; Gajjar, A.; Chintagumpala, M.; Merchant, T.; Kieran, M.; Pollack, I.F.; Gururangan, S.; Geyer, R.; Phillips, P.; et al. Pilot study of systemic and intrathecal mafosfamide followed by conformal radiation for infants with intracranial central nervous system tumors: A pediatric brain tumor consortium study (PBTC-001). J. Neurooncol. 2012, 109, 565–571. [Google Scholar] [CrossRef]
- Robinson, G.W.; Rudneva, V.A.; Buchhalter, I.; Billups, C.A.; Waszak, S.M.; Smith, K.S.; Bowers, D.C.; Bendel, A.; Fisher, P.G.; Partap, S.; et al. Risk-adapted therapy for young children with medulloblastoma (SJYC07): Therapeutic and molecular outcomes from a multicentre, phase 2 trial. Lancet Oncol. 2018, 19, 768–784. [Google Scholar] [CrossRef]
- Biroc, S.L.; Etzler, M.E. The effect of periodate oxidation and alpha-mannosidase treatment on Dolichos biflorus lectin. Biochim. Biophys. Acta 1978, 544, 85–92. [Google Scholar] [CrossRef]
- Zeltzer, P.M.; Boyett, J.M.; Finlay, J.L.; Albright, A.L.; Rorke, L.B.; Milstein, J.M.; Allen, J.C.; Stevens, K.R.; Stanley, P.; Li, H.; et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: Conclusions from the Children’s Cancer Group 921 randomized phase III study. J. Clin. Oncol. 1999, 17, 832–845. [Google Scholar] [CrossRef]
- Thomas, P.R.; Deutsch, M.; Kepner, J.L.; Boyett, J.M.; Krischer, J.; Aronin, P.; Albright, L.; Allen, J.C.; Packer, R.J.; Linggood, R.; et al. Low-stage medulloblastoma: Final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. J. Clin. Oncol. 2000, 18, 3004–3011. [Google Scholar] [CrossRef]
- Ris, M.D.; Packer, R.; Goldwein, J.; Jones-Wallace, D.; Boyett, J.M. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: A Children’s Cancer Group study. J. Clin. Oncol. 2001, 19, 3470–3476. [Google Scholar] [CrossRef]
- Bailey, C.C.; Gnekow, A.; Wellek, S.; Jones, M.; Round, C.; Brown, J.; Phillips, A.; Neidhardt, M.K. Prospective randomised trial of chemotherapy given before radiotherapy in childhood medulloblastoma. International Society of Paediatric Oncology (SIOP) and the (German) Society of Paediatric Oncology (GPO): SIOP II. Med. Pediatr. Oncol. 1995, 25, 166–178. [Google Scholar] [CrossRef]
- Tait, D.M.; Thornton-Jones, H.; Bloom, H.J.; Lemerle, J.; Morris-Jones, P. Adjuvant chemotherapy for medulloblastoma: The first multi-centre control trial of the International Society of Paediatric Oncology (SIOP I). Eur. J. Cancer 1990, 26, 464–469. [Google Scholar] [CrossRef]
- Bouffet, E.; Bernard, J.L.; Frappaz, D.; Gentet, J.C.; Roche, H.; Tron, P.; Carrie, C.; Raybaud, C.; Joannard, A.; Lapras, C.; et al. M4 protocol for cerebellar medulloblastoma: Supratentorial radiotherapy may not be avoided. Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 79–85. [Google Scholar] [CrossRef]
- Fukunaga-Johnson, N.; Lee, J.H.; Sandler, H.M.; Robertson, P.; McNeil, E.; Goldwein, J.W. Patterns of failure following treatment for medulloblastoma: Is it necessary to treat the entire posterior fossa? Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 143–146. [Google Scholar] [CrossRef]
- Merchant, T.E.; Happersett, L.; Finlay, J.L.; Leibel, S.A. Preliminary results of conformal radiation therapy for medulloblastoma. Neuro Oncol. 1999, 1, 177–187. [Google Scholar] [CrossRef]
- Wolden, S.L.; Dunkel, I.J.; Souweidane, M.M.; Happersett, L.; Khakoo, Y.; Schupak, K.; Lyden, D.; Leibel, S.A. Patterns of failure using a conformal radiation therapy tumor bed boost for medulloblastoma. J. Clin. Oncol. 2003, 21, 3079–3083. [Google Scholar] [CrossRef]
- Michalski, A.; Janss, A.; Vezina, G.; Gajjar, A.; Pollack, I.; Merchant, T.E.; FitzGerald, T.J.; Booth, T.; Tarbell, N.J.; Billups, C.A.; et al. Results of COG ACNS0331: A Phase III Trial of Involved-Field Radiotherapy (IFRT) and Low Dose Craniospinal Irradiation (LD-CSI) with Chemotherapy in Average-Risk Medulloblastoma: A Report from the Children’s Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 937–938. [Google Scholar] [CrossRef]
- Olson, J.M. Chemotherapy and Radiation Therapy in Treating Young Patients with Newly Diagnosed, Previously Untreated, High-Risk Medulloblastoma. ClinicalTrials.gov Identifier: NCT00392327; 2006. Available online: https://clinicaltrials.gov/ct2/show/NCT00392327?term=NCT00392327&draw=2&rank=1 (accessed on 12 December 2019).
- Northcott, P.A.; Pfister, S.M.; Jones, D.T. Next-generation (epi)genetic drivers of childhood brain tumours and the outlook for targeted therapies. Lancet Oncol. 2015, 16, 293–302. [Google Scholar] [CrossRef]
- Wang, X.; Dubuc, A.M.; Ramaswamy, V.; Mack, S.; Gendoo, D.M.; Remke, M.; Wu, X.; Garzia, L.; Luu, B.; Cavalli, F.; et al. Medulloblastoma subgroups remain stable across primary and metastatic compartments. Acta Neuropathol. 2015, 129, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.; Remke, M.; Bouffet, E.; Faria, C.C.; Perreault, S.; Cho, Y.J.; Shih, D.J.; Luu, B.; Dubuc, A.M.; Northcott, P.A.; et al. Recurrence patterns across medulloblastoma subgroups: An integrated clinical and molecular analysis. Lancet Oncol. 2013, 14, 1200–1207. [Google Scholar] [CrossRef]
- Northcott, P.A.; Buchhalter, I.; Morrissy, A.S.; Hovestadt, V.; Weischenfeldt, J.; Ehrenberger, T.; Grobner, S.; Segura-Wang, M.; Zichner, T.; Rudneva, V.A.; et al. The whole-genome landscape of medulloblastoma subtypes. Nature 2017, 547, 311–317. [Google Scholar] [CrossRef]
- Schwalbe, E.C.; Lindsey, J.C.; Nakjang, S.; Crosier, S.; Smith, A.J.; Hicks, D.; Rafiee, G.; Hill, R.M.; Iliasova, A.; Stone, T.; et al. Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: A cohort study. Lancet Oncol. 2017, 18, 958–971. [Google Scholar] [CrossRef]
- Cavalli, F.M.G.; Remke, M.; Rampasek, L.; Peacock, J.; Shih, D.J.H.; Luu, B.; Garzia, L.; Torchia, J.; Nor, C.; Morrissy, A.S.; et al. Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell 2017, 31, 737–754. [Google Scholar] [CrossRef]
- Morfouace, M.; Shelat, A.; Jacus, M.; Freeman, B.B., 3rd; Turner, D.; Robinson, S.; Zindy, F.; Wang, Y.D.; Finkelstein, D.; Ayrault, O.; et al. Pemetrexed and gemcitabine as combination therapy for the treatment of Group3 medulloblastoma. Cancer Cell 2014, 25, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, A.; Robinson, G. A Clinical and Molecular Risk-Directed Therapy for Newly Diagnosed Medulloblastoma. ClinicalTrials.gov Identifier: NCT01878617; 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT01878617?term=nct01878617&rank=1 (accessed on 27 November 2019).
- Cohen, K.; Bandopadhayay, P.; Chi, S.; London, W.; Rodriguez, F.; Hawkins, C.; Yang, E.; Aguilera, D.; Castellino, R.; MacDonald, T.; et al. MEDU-34. Pilot of Study of a Surgery and Chemotherapy-only approach in the upfront therapy of children with WNT-Positive Standard Risk Medulloblastoma. Neuro Oncol. 2019, 21, ii110. [Google Scholar] [CrossRef]
- Gottardo, N. Reduced Craniospinal Radiation Therapy and Chemotherapy in Treating Younger Patients with Newly Diagnosed WNT-Driven Medulloblastoma. Clinical Trials.gov Identifier: NCT02724579; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02724579 (accessed on 28 November 2019).
- Thompson, E.M.; Hielscher, T.; Bouffet, E.; Remke, M.; Luu, B.; Gururangan, S.; McLendon, R.E.; Bigner, D.D.; Lipp, E.S.; Perreault, S.; et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: A retrospective integrated clinical and molecular analysis. Lancet Oncol. 2016, 17, 484–495. [Google Scholar] [CrossRef]
- Moxon-Emre, I.; Taylor, M.D.; Bouffet, E.; Hardy, K.; Campen, C.J.; Malkin, D.; Hawkins, C.; Laperriere, N.; Ramaswamy, V.; Bartels, U.; et al. Intellectual Outcome in Molecular Subgroups of Medulloblastoma. J. Clin. Oncol. 2016, 34, 4161–4170. [Google Scholar] [CrossRef]
- Carrie, C.; Alapetite, C.; Mere, P.; Aimard, L.; Pons, A.; Kolodie, H.; Seng, S.; Lagrange, J.L.; Pontvert, D.; Pignon, T.; et al. Quality control of radiotherapeutic treatment of medulloblastoma in a multicentric study: The contribution of radiotherapy technique to tumour relapse. The French Medulloblastoma Group. Radiother. Oncol. 1992, 24, 77–81. [Google Scholar] [CrossRef]
- Carrie, C.; Hoffstetter, S.; Gomez, F.; Moncho, V.; Doz, F.; Alapetite, C.; Murraciole, X.; Maire, J.P.; Benhassel, M.; Chapet, S.; et al. Impact of targeting deviations on outcome in medulloblastoma: Study of the French Society of Pediatric Oncology (SFOP). Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 435–439. [Google Scholar] [CrossRef]
- Oyharcabal-Bourden, V.; Kalifa, C.; Gentet, J.C.; Frappaz, D.; Edan, C.; Chastagner, P.; Sariban, E.; Pagnier, A.; Babin, A.; Pichon, F.; et al. Standard-risk medulloblastoma treated by adjuvant chemotherapy followed by reduced-dose craniospinal radiation therapy: A French Society of Pediatric Oncology Study. J. Clin. Oncol. 2005, 23, 4726–4734. [Google Scholar] [CrossRef]
- Paulino, A.C.; Lobo, M.; Teh, B.S.; Okcu, M.F.; South, M.; Butler, E.B.; Su, J.; Chintagumpala, M. Ototoxicity after intensity-modulated radiation therapy and cisplatin-based chemotherapy in children with medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1445–1450. [Google Scholar] [CrossRef]
- Huang, E.; Teh, B.S.; Strother, D.R.; Davis, Q.G.; Chiu, J.K.; Lu, H.H.; Carpenter, L.S.; Mai, W.Y.; Chintagumpala, M.M.; South, M.; et al. Intensity-modulated radiation therapy for pediatric medulloblastoma: Early report on the reduction of ototoxicity. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 599–605. [Google Scholar] [CrossRef]
- Wong, K.K.; Ragab, O.; Tran, H.N.; Pham, A.; All, S.; Waxer, J.; Olch, A.J. Acute toxicity of craniospinal irradiation with volumetric-modulated arc therapy in children with solid tumors. Pediatr. Blood Cancer 2018, 65, e27050. [Google Scholar] [CrossRef]
- Barra, S.; Gusinu, M.; Timon, G.; Giannelli, F.; Vidano, G.; Garre, M.L.; Corvo, R. Pediatric craniospinal irradiation with conventional technique or helical tomotherapy: Impact of age and body volume on integral dose. Tumori 2016, 102, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.S.Q.; Barrett, S.A.; Mullaney, L.M. A review of dosimetric and toxicity modeling of proton versus photon craniospinal irradiation for pediatrics medulloblastoma. Acta Oncol. 2017, 56, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Yuh, G.E.; Loredo, L.N.; Yonemoto, L.T.; Bush, D.A.; Shahnazi, K.; Preston, W.; Slater, J.M.; Slater, J.D. Reducing toxicity from craniospinal irradiation: Using proton beams to treat medulloblastoma in young children. Cancer J. 2004, 10, 386–390. [Google Scholar] [CrossRef]
- Eaton, B.R.; Esiashvili, N.; Kim, S.; Patterson, B.; Weyman, E.A.; Thornton, L.T.; Mazewski, C.; MacDonald, T.J.; Ebb, D.; MacDonald, S.M.; et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro Oncol. 2016, 18, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Yock, T.I.; Yeap, B.Y.; Ebb, D.H.; Weyman, E.; Eaton, B.R.; Sherry, N.A.; Jones, R.M.; MacDonald, S.M.; Pulsifer, M.B.; Lavally, B.; et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: A phase 2 single-arm study. Lancet Oncol. 2016, 17, 287–298. [Google Scholar] [CrossRef]
- Eaton, B.R.; Esiashvili, N.; Kim, S.; Weyman, E.A.; Thornton, L.T.; Mazewski, C.; MacDonald, T.; Ebb, D.; MacDonald, S.M.; Tarbell, N.J.; et al. Clinical Outcomes Among Children with Standard-Risk Medulloblastoma Treated with Proton and Photon Radiation Therapy: A Comparison of Disease Control and Overall Survival. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 133–138. [Google Scholar] [CrossRef]
- Kahalley, L.S.; Peterson, R.; Ris, M.D.; Janzen, L.; Okcu, M.F.; Grosshans, D.R.; Ramaswamy, V.; Paulino, A.C.; Hodgson, D.; Mahajan, A.; et al. Superior Intellectual Outcomes After Proton Radiotherapy Compared with Photon Radiotherapy for Pediatric Medulloblastoma. J. Clin. Oncol. 2019. [Google Scholar] [CrossRef]
- Roussel, M.F.; Stripay, J.L. Modeling Pediatric Medulloblastoma. Brain Pathol. 2019. [Google Scholar] [CrossRef]
- Brabetz, S.; Leary, S.E.S.; Grobner, S.N.; Nakamoto, M.W.; Seker-Cin, H.; Girard, E.J.; Cole, B.; Strand, A.D.; Bloom, K.L.; Hovestadt, V.; et al. A biobank of patient-derived pediatric brain tumor models. Nat. Med. 2018, 24, 1752–1761. [Google Scholar] [CrossRef]
- Sanden, E.; Dyberg, C.; Krona, C.; Gallo-Oller, G.; Olsen, T.K.; Enriquez Perez, J.; Wickstrom, M.; Estekizadeh, A.; Kool, M.; Visse, E.; et al. Establishment and characterization of an orthotopic patient-derived Group 3 medulloblastoma model for preclinical drug evaluation. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Morrissy, A.S.; Garzia, L.; Shih, D.J.; Zuyderduyn, S.; Huang, X.; Skowron, P.; Remke, M.; Cavalli, F.M.; Ramaswamy, V.; Lindsay, P.E.; et al. Divergent clonal selection dominates medulloblastoma at recurrence. Nature 2016, 529, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.N.; Higgins, G.S.; Brown, J.M.; Baumann, M.; Kirsch, D.G.; Willers, H.; Prasanna, P.G.; Dewhirst, M.W.; Bernhard, E.J.; Ahmed, M.M. Improving the Predictive Value of Preclinical Studies in Support of Radiotherapy Clinical Trials. Clin. Cancer Res. 2016, 22, 3138–3147. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, R.; Lindsay, P.E.; Ansell, S.; Wilson, G.; Jelveh, S.; Hill, R.P.; Jaffray, D.A. Characterization of image quality and image-guidance performance of a preclinical microirradiator. Med. Phys. 2011, 38, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Armour, E.; Kazanzides, P.; Iordachita, I.; Tryggestad, E.; Deng, H.; Matinfar, M.; Kennedy, C.; Liu, Z.; Chan, T.; et al. High-resolution, small animal radiation research platform with x-ray tomographic guidance capabilities. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1591–1599. [Google Scholar] [CrossRef]
- Smith, S.M.C.; Bianski, B.M.; Orr, B.A.; Harknett, G.; Onar-Thomas, A.; Gilbertson, R.J.; Merchant, T.E.; Roussel, M.F.; Tinkle, C.L. Preclinical Modeling of Image-Guided Craniospinal Irradiation for Very-High-Risk Medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 728–737. [Google Scholar] [CrossRef]
- Huang, L.; Garrett Injac, S.; Cui, K.; Braun, F.; Lin, Q.; Du, Y.; Zhang, H.; Kogiso, M.; Lindsay, H.; Zhao, S.; et al. Systems biology-based drug repositioning identifies digoxin as a potential therapy for groups 3 and 4 medulloblastoma. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Stone, H.B.; Bernhard, E.J.; Coleman, C.N.; Deye, J.; Capala, J.; Mitchell, J.B.; Brown, J.M. Preclinical Data on Efficacy of 10 Drug-Radiation Combinations: Evaluations, Concerns, and Recommendations. Transl. Oncol. 2016, 9, 46–56. [Google Scholar] [CrossRef]
- Tuli, R.; Surmak, A.; Reyes, J.; Hacker-Prietz, A.; Armour, M.; Leubner, A.; Blackford, A.; Tryggestad, E.; Jaffee, E.M.; Wong, J.; et al. Development of a novel preclinical pancreatic cancer research model: Bioluminescence image-guided focal irradiation and tumor monitoring of orthotopic xenografts. Transl. Oncol. 2012, 5, 77–84. [Google Scholar] [CrossRef]
- Hirata, E.; Sahai, E. Tumor Microenvironment and Differential Responses to Therapy. Cold Spring Harb. Perspect. Med. 2017, 7. [Google Scholar] [CrossRef]
- Ford, E.; Emery, R.; Huff, D.; Narayanan, M.; Schwartz, J.; Cao, N.; Meyer, J.; Rengan, R.; Zeng, J.; Sandison, G.; et al. An image-guided precision proton radiation platform for preclinical in vivo research. Phys. Med. Biol. 2017, 62, 43–58. [Google Scholar] [CrossRef]
- Kim, M.M.; Irmen, P.; Shoniyozov, K.; Verginadis, I.I.; Cengel, K.A.; Koumenis, C.; Metz, J.M.; Dong, L.; Diffenderfer, E.S. Design and commissioning of an image-guided small animal radiation platform and quality assurance protocol for integrated proton and x-ray radiobiology research. Phys. Med. Biol. 2019, 64, e135013. [Google Scholar] [CrossRef] [PubMed]
- Fertil, B.; Malaise, E.P. Intrinsic radiosensitivity of human cell lines is correlated with radioresponsiveness of human tumors: Analysis of 101 published survival curves. Int. J. Radiat. Oncol. Biol. Phys. 1985, 11, 1699–1707. [Google Scholar] [CrossRef]
- Deacon, J.; Peckham, M.J.; Steel, G.G. The radioresponsiveness of human tumours and the initial slope of the cell survival curve. Radiother. Oncol. 1984, 2, 317–323. [Google Scholar] [CrossRef]
- Zhukova, N.; Ramaswamy, V.; Remke, M.; Martin, D.C.; Castelo-Branco, P.; Zhang, C.H.; Fraser, M.; Tse, K.; Poon, R.; Shih, D.J.; et al. WNT activation by lithium abrogates TP53 mutation associated radiation resistance in medulloblastoma. Acta Neuropathol. Commun. 2014, 2, e174. [Google Scholar] [CrossRef] [PubMed]
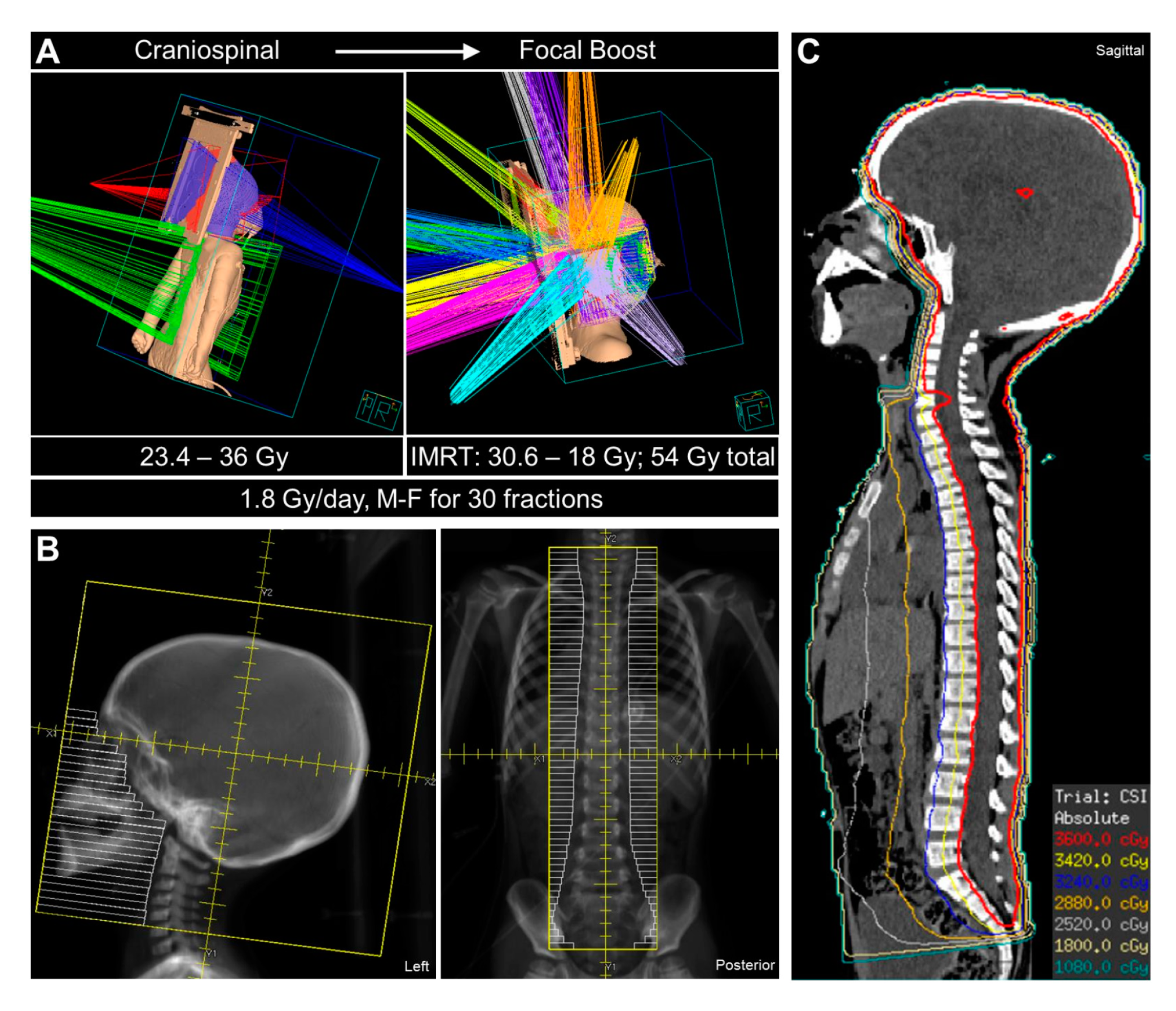
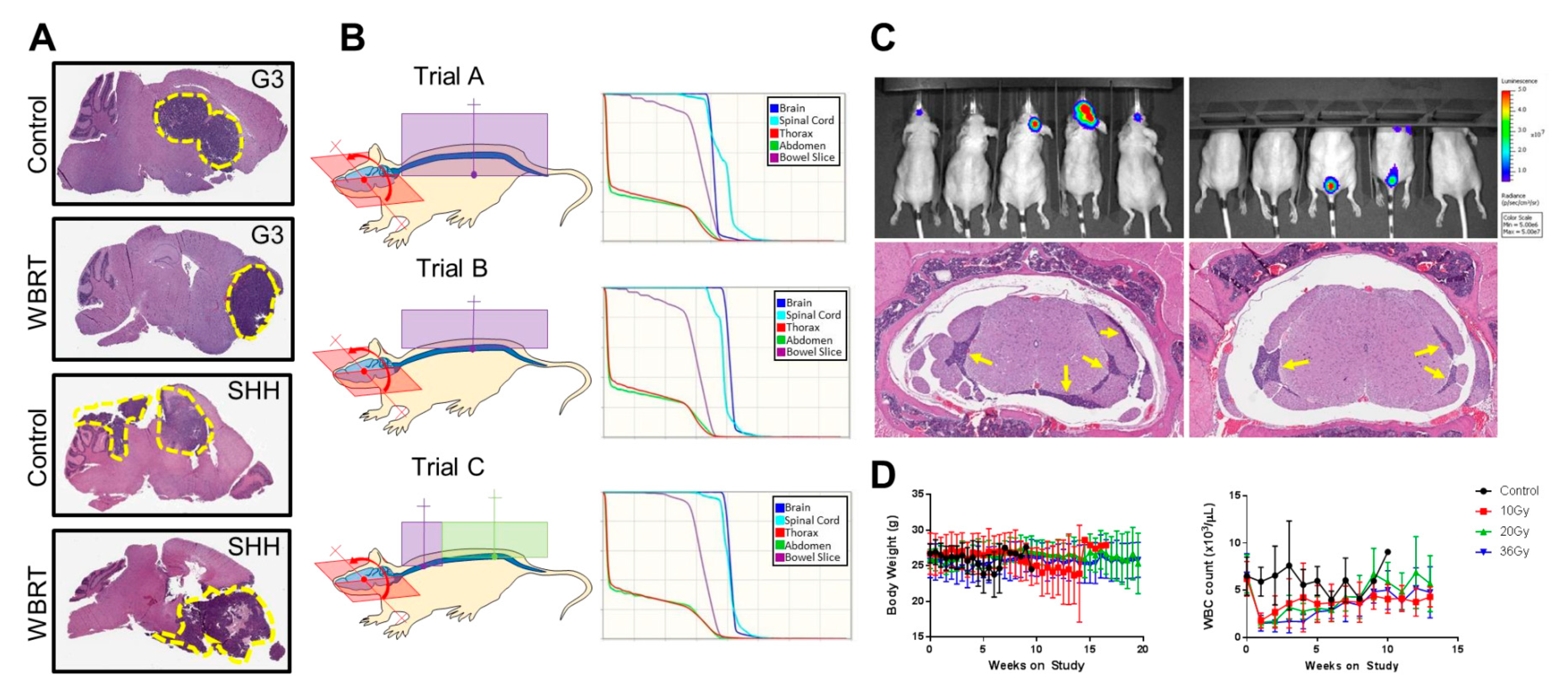
| Characteristic | SARRP-Based Irradiation (Xstrahl Ltd., UK) [74,75] | X-RAD 225Cx Irradiation (Precision X-ray Inc., USA) [71,73] | Custom Irradiation [76] |
|---|---|---|---|
| Photon energy range | 5–225 keV | 5–225 keV | 5–225 keV |
| Image guidance | Volumetric cone-beam CT | 2D fluoroscopic images Volumetric cone-beam CT | Clinical set-up |
| Beam collimation | Motorized variable collimator | Fixed collimators | Custom CSI lead aperture |
| Phase of radiotherapy | Primary | Post-operative | Primary |
| CSI beam arrangement | Brain: arc (−90–+90°) Spine: 2 field PA | Brain: opposed laterals Spine: single or multiple PA | Planar |
| Boost treatment | No | No | Yes |
| Dose per fraction | 2 Gy | Brain: 2 Gy Spine: 4.76 Gy | 2 Gy |
| Cumulative dose | 10–36 Gy | Brain: 36 Gy Spine: 28.56 Gy | 20 Gy |
| Medulloblastoma model system | PDOX (Group 3, SHH) | GEMM (SHH) | PDOX (Group 3) |
| Tumor burden assessment | Bioluminescence Histologic examination | Clinical symptoms Histologic examination | Clinical symptoms Histologic examination |
| Oustanding Questions | Considerations and Current Approaches |
|---|---|
| Clinically relevant CSI dosing regimens | Fully fractionated “human” dosing regimens vs. non-curative empiric murine regimens vs. feasible dosing regimens |
| Optimal enrollment threshold | Minimal tumor burden to mimic post-surgical conditions vs. moribund conditions to mimic recurrent setting; impact on RT efficacy |
| Tumor burden assessment | Bioluminescence imaging vs. MRI/CT vs. histopathology |
| Primary site tumor boost | Targeting of primary site: IGRT vs. biologic RT targeting vs. historic histopathology; integration into non-curative CSI regimens |
| Integration of other treatment modalities | Value of surgical resection; integration of adjuvant systemic therapy and preclinical modeling of clinically relevant systemic therapy exposure |
| Optimal endpoints | Overall survival vs. tumor-specific survival; tumor growth delay assays vs. tumor control dose 50% (TCD50) |
| Optimal MB models | GEMM vs. PDOX vs. organoids |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stripay, J.L.; Merchant, T.E.; Roussel, M.F.; Tinkle, C.L. Preclinical Models of Craniospinal Irradiation for Medulloblastoma. Cancers 2020, 12, 133. https://doi.org/10.3390/cancers12010133
Stripay JL, Merchant TE, Roussel MF, Tinkle CL. Preclinical Models of Craniospinal Irradiation for Medulloblastoma. Cancers. 2020; 12(1):133. https://doi.org/10.3390/cancers12010133
Chicago/Turabian StyleStripay, Jennifer L., Thomas E. Merchant, Martine F. Roussel, and Christopher L. Tinkle. 2020. "Preclinical Models of Craniospinal Irradiation for Medulloblastoma" Cancers 12, no. 1: 133. https://doi.org/10.3390/cancers12010133
APA StyleStripay, J. L., Merchant, T. E., Roussel, M. F., & Tinkle, C. L. (2020). Preclinical Models of Craniospinal Irradiation for Medulloblastoma. Cancers, 12(1), 133. https://doi.org/10.3390/cancers12010133





