Radiotherapy-Induced Changes in the Systemic Immune and Inflammation Parameters of Head and Neck Cancer Patients
Abstract
1. Introduction
2. Results
2.1. Clinical Parameters of HNSCC Patients
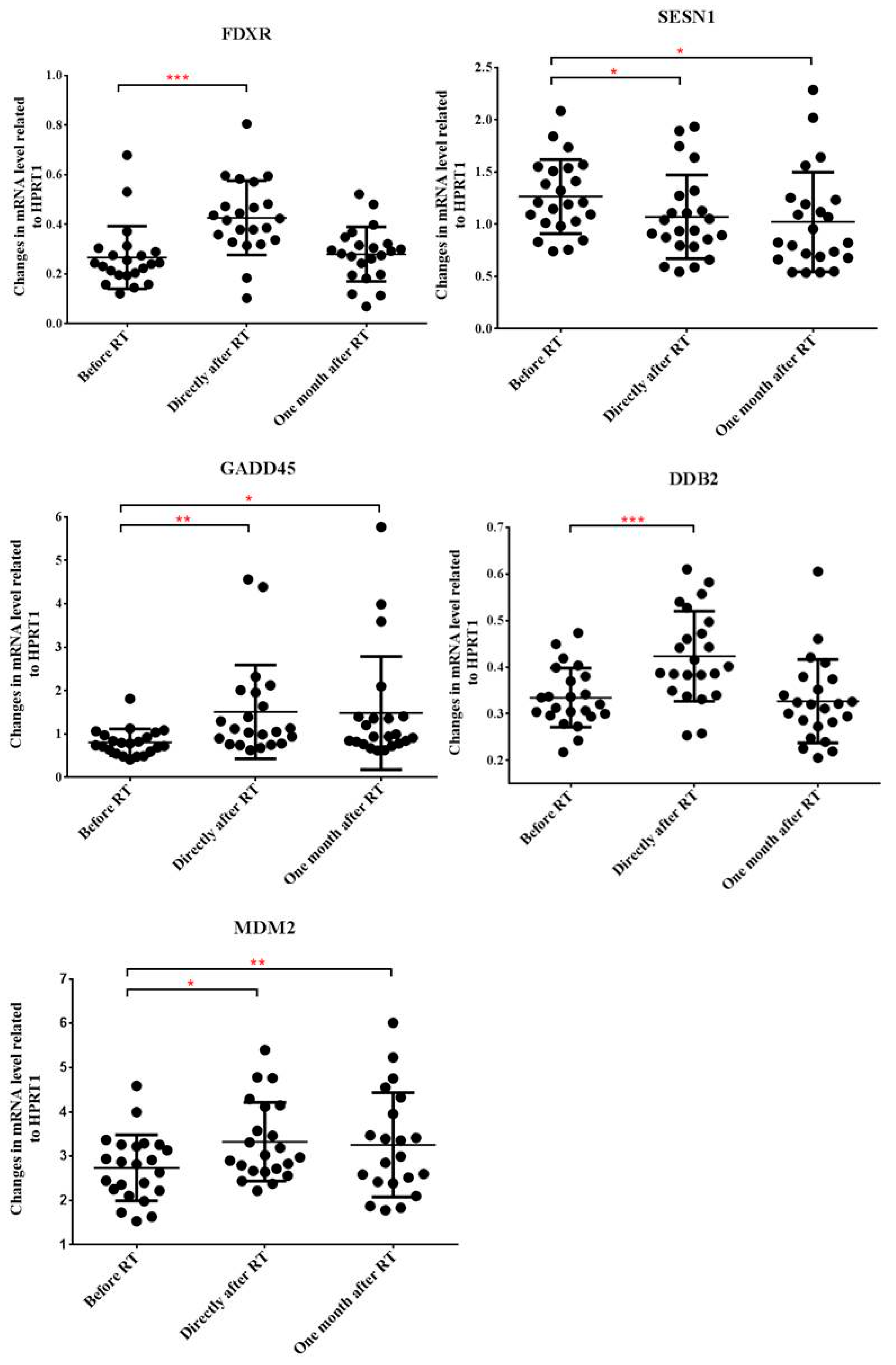
2.2. Radiotherapy Alters Gene Expression Profile of Peripheral Blood Cells
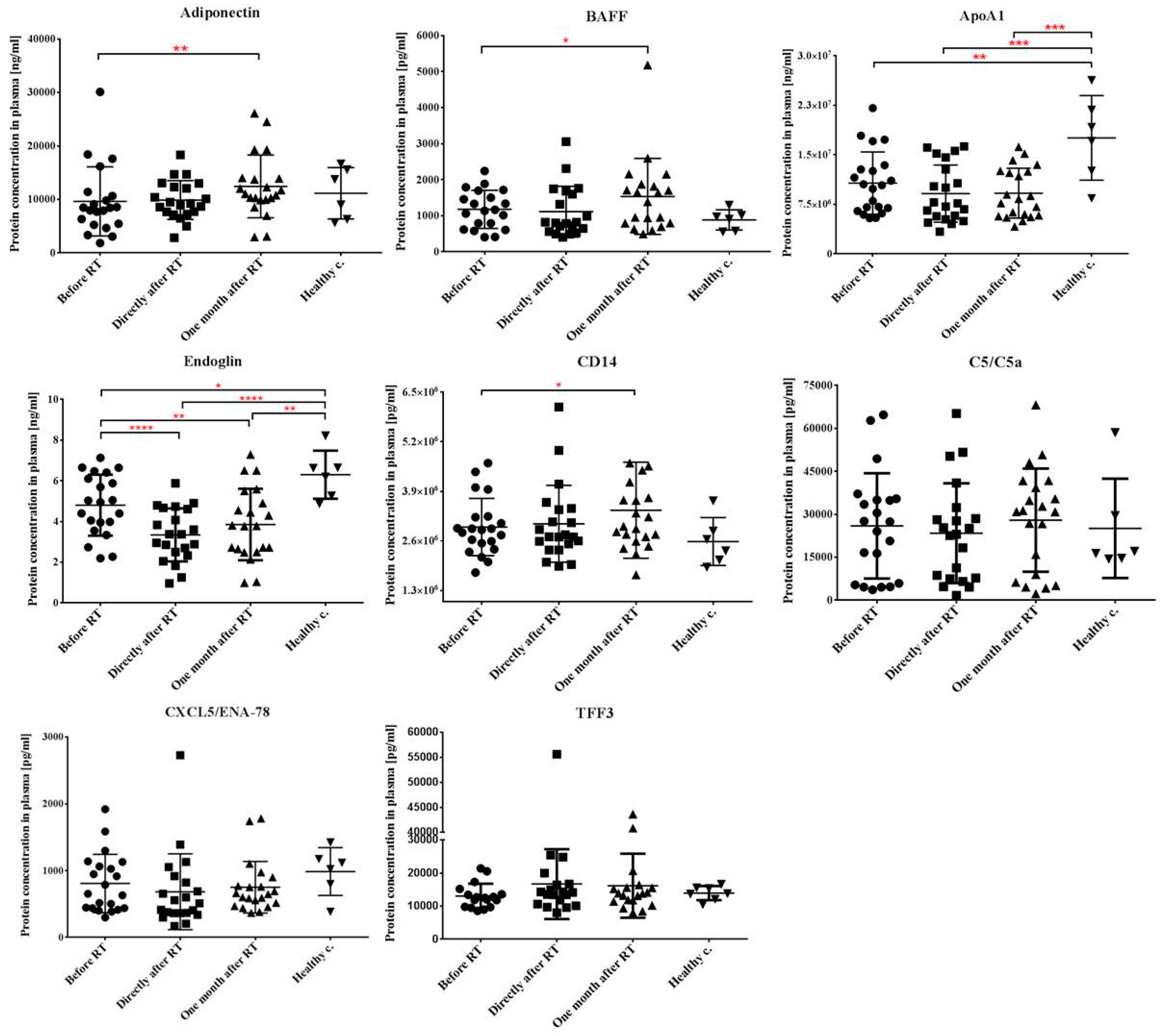
2.3. Local Tumor Irradiation Induces Systemic Changes in the Level of Immune and Inflammation-Related Plasma Proteins
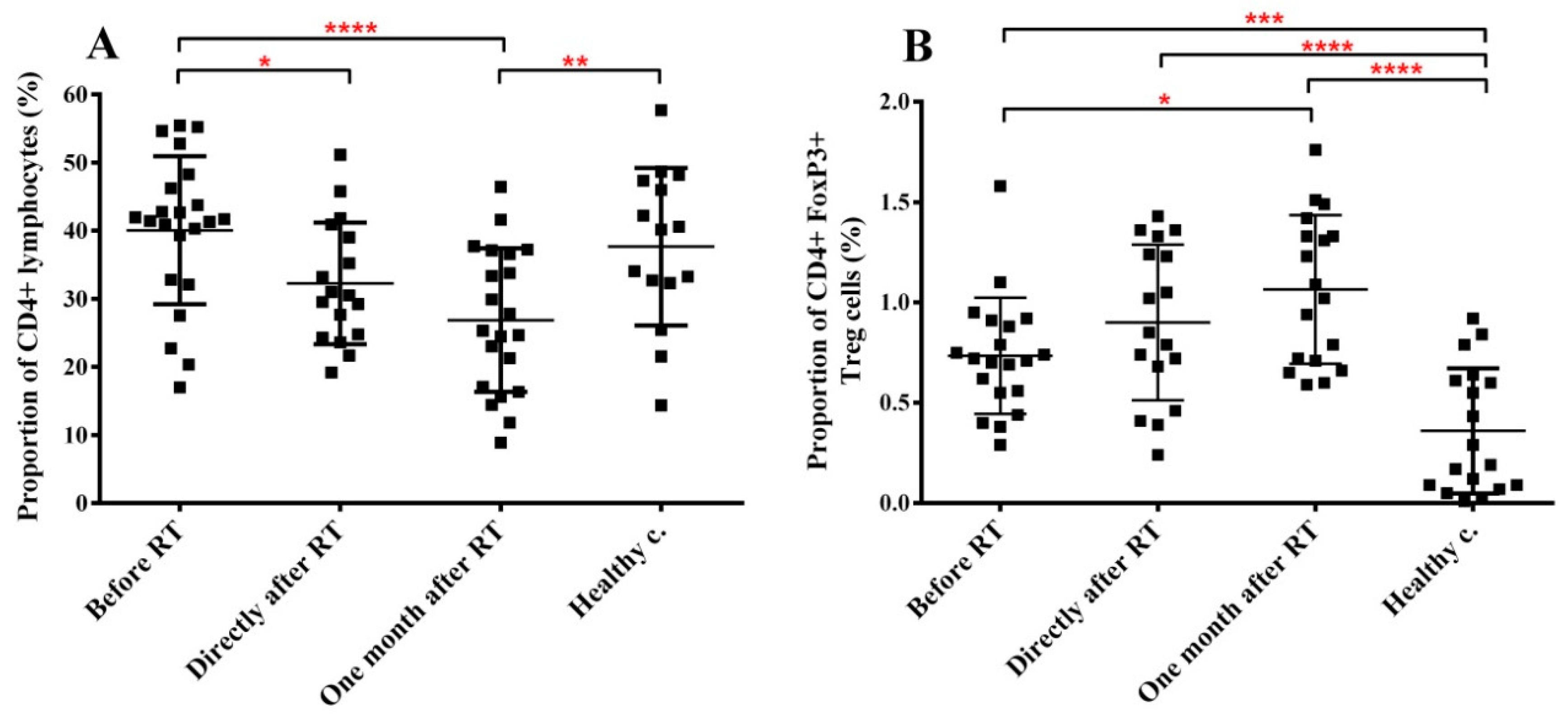
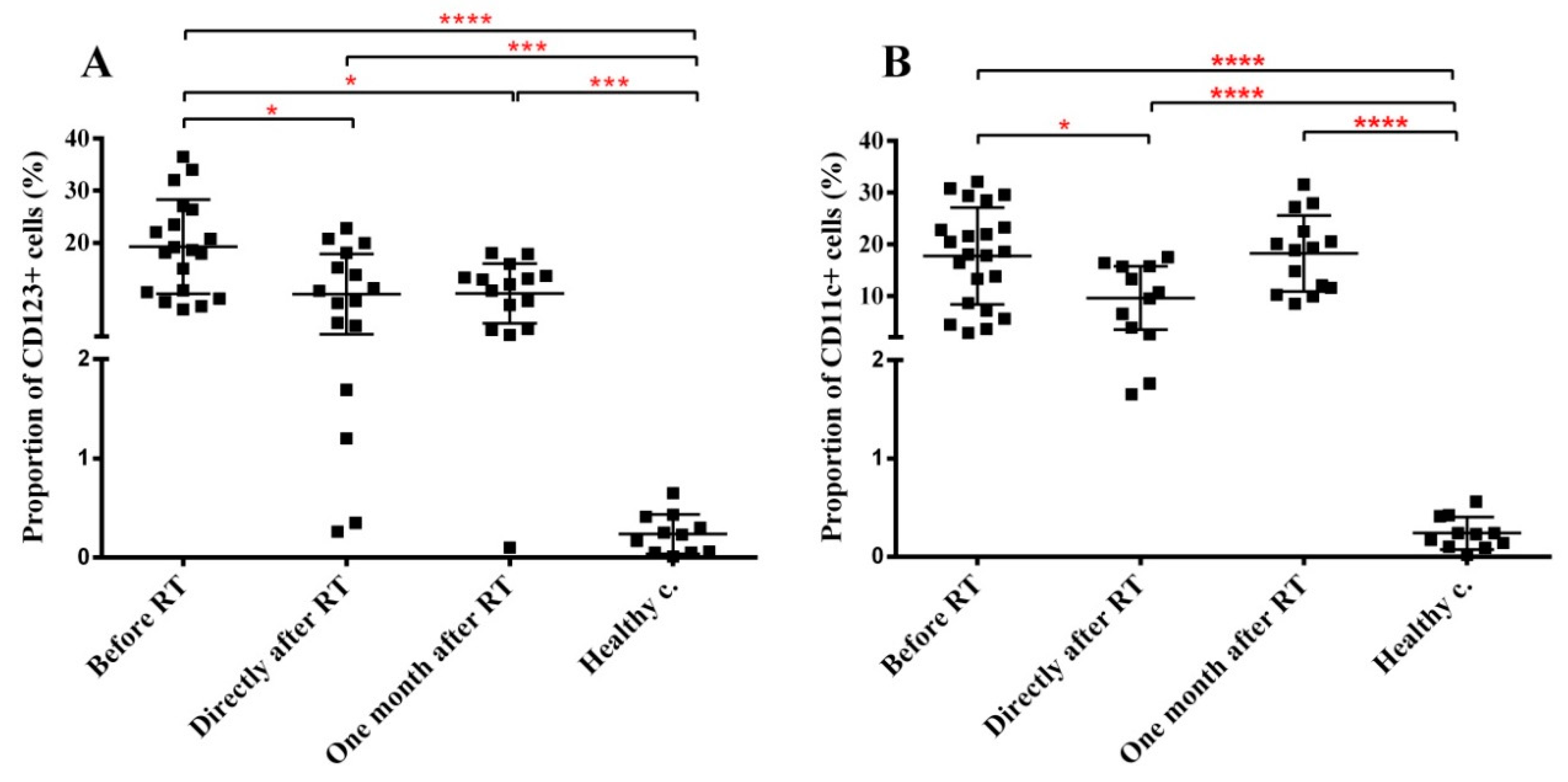
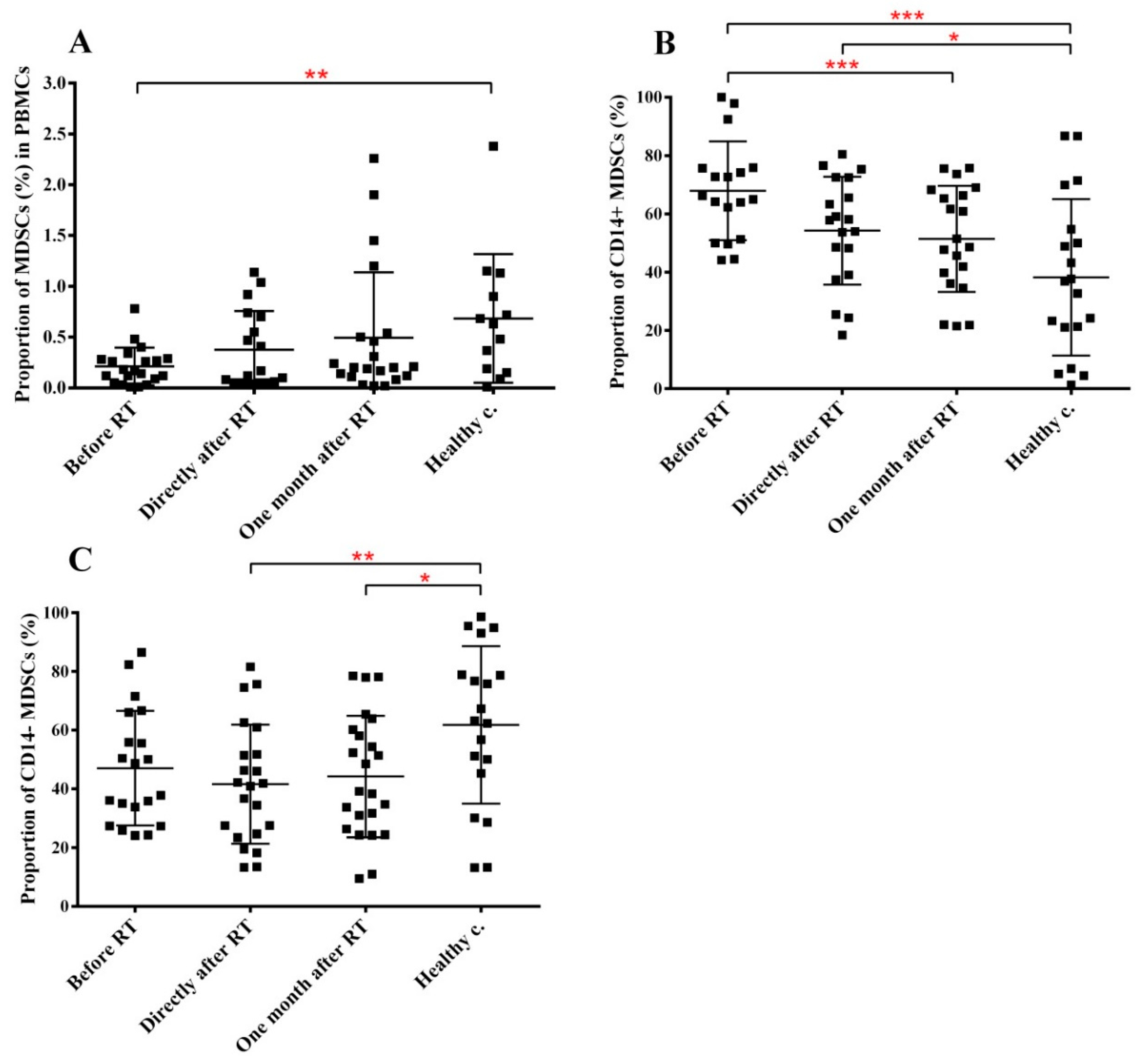
2.4. RT Induces Changes in the Immune Phenotype of PBMCs of HNSCC Patients
3. Discussion
4. Materials and Methods
4.1. Sample Collection from Radiotherapy Patients
4.2. Ethical Permissions
4.3. Isolation of PBMCs from Human Blood
4.4. Immune Phenotyping of PBMCs and Polymorphonuclear Cells
4.5. Hierarchical Gating Strategy
4.6. Analysis of Plasma Proteins in HNSCC Patients Treated with RT
4.7. RNA Isolation and Reverse Transcription
4.8. Quantitative Real-Time Polymerase Chain Reaction
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Yang, C.C.; Su, Y.C.; Lin, Y.W.; Huang, C.I.; Lee, C.C. Differential impact of age on survival in head and neck cancer according to classic Cox regression and decision tree analysis. Clin. Otolaryngol. 2019, 44, 244–253. [Google Scholar] [CrossRef]
- Bunbanjerdsuk, S.; Vorasan, N.; Saethang, T.; Pongrujikorn, T.; Pangpunyakulchai, D.; Mongkonsiri, N.; Arsa, L.; Thokanit, N.; Pongsapich, W.; Anekpuritanang, T.; et al. Oncoproteomic and gene expression analyses identify prognostic biomarkers for second primary malignancy in patients with head and neck squamous cell carcinoma. Mod. Pathol. 2019, 32, 943–956. [Google Scholar] [CrossRef]
- Barton, M.B.; Allen, S.; Delaney, G.P.; Hudson, H.M.; Hao, Z.; Allison, R.W.; van der Linden, Y.M. Patterns of retreatment by radiotherapy. Clin. Oncol. 2014, 26, 611–618. [Google Scholar] [CrossRef]
- Nikitaki, Z.; Mavragani, I.V.; Laskaratou, D.A.; Gika, V.; Moskvin, V.P.; Theofilatos, K.; Vougas, K.; Stewart, R.D.; Georgakilas, A.G. Systemic mechanisms and effects of ionizing radiation: A new ‘old’ paradigm of how the bystanders and distant can become the players. Semin. Cancer Biol. 2016, 37, 77–95. [Google Scholar] [CrossRef]
- Lumniczky, K.; Safrany, G. The impact of radiation therapy on the antitumor immunity: Local effects and systemic consequences. Cancer Lett. 2015, 356, 114–125. [Google Scholar] [CrossRef]
- Candeias, S.M.; Testard, I. The many interactions between the innate immune system and the response to radiation. Cancer Lett 2015, 368, 173–178. [Google Scholar] [CrossRef]
- De Ruiter, E.J.; Ooft, M.L.; Devriese, L.A.; Willems, S.M. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology 2017, 6, e1356148. [Google Scholar] [CrossRef]
- Ding, W.; Xu, X.; Qian, Y.; Xue, W.; Wang, Y.; Du, J.; Jin, L.; Tan, Y. Prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma: A meta-analysis. Medicine 2018, 97, e13301. [Google Scholar] [CrossRef]
- Fu, Q.; Chen, N.; Ge, C.; Li, R.; Li, Z.; Zeng, B.; Li, C.; Wang, Y.; Xue, Y.; Song, X.; et al. Prognostic value of tumor-infiltrating lymphocytes in melanoma: A systematic review and meta-analysis. Oncoimmunology 2019, 8, 1593806. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Chen, R.; Bai, Y.; Lu, X. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget 2017, 8, 15621–15631. [Google Scholar] [CrossRef]
- Mao, Y.; Qu, Q.; Chen, X.; Huang, O.; Wu, J.; Shen, K. The Prognostic Value of Tumor-Infiltrating Lymphocytes in Breast Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0152500. [Google Scholar] [CrossRef]
- Nguyen, N.; Bellile, E.; Thomas, D.; McHugh, J.; Rozek, L.; Virani, S.; Peterson, L.; Carey, T.E.; Walline, H.; Moyer, J.; et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck 2016, 38, 1074–1084. [Google Scholar] [CrossRef]
- Zhao, Y.; Ge, X.; He, J.; Cheng, Y.; Wang, Z.; Wang, J.; Sun, L. The prognostic value of tumor-infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: A systematic review and meta-analysis. World J. Surg. Oncol. 2019, 17, 85. [Google Scholar] [CrossRef]
- Zheng, X.; Song, X.; Shao, Y.; Xu, B.; Chen, L.; Zhou, Q.; Hu, W.; Zhang, D.; Wu, C.; Tao, M.; et al. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: A meta-analysis. Oncotarget 2017, 8, 57386–57398. [Google Scholar] [CrossRef]
- Zheng, X.; Song, X.; Shao, Y.; Xu, B.; Hu, W.; Zhou, Q.; Chen, L.; Zhang, D.; Wu, C.; Jiang, J. Prognostic Role of Tumor-Infiltrating Lymphocytes in Esophagus Cancer: A Meta-Analysis. Cell. Phys. Biochem. Int. J. Exp. Cell. Phys. Biochem. Pharm. 2018, 45, 720–732. [Google Scholar] [CrossRef]
- Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef]
- Buffa, F.M.; Bentzen, S.M.; Daley, F.M.; Dische, S.; Saunders, M.I.; Richman, P.I.; Wilson, G.D. Molecular marker profiles predict locoregional control of head and neck squamous cell carcinoma in a randomized trial of continuous hyperfractionated accelerated radiotherapy. Clin. Cancer Res. 2004, 10, 3745–3754. [Google Scholar] [CrossRef]
- Chin, D.; Boyle, G.M.; Williams, R.M.; Ferguson, K.; Pandeya, N.; Pedley, J.; Campbell, C.M.; Theile, D.R.; Parsons, P.G.; Coman, W.B. Novel markers for poor prognosis in head and neck cancer. Int. J. Cancer 2005, 113, 789–797. [Google Scholar] [CrossRef]
- Homma, A.; Furuta, Y.; Oridate, N.; Nakano, Y.; Kohashi, G.; Yagi, K.; Nagahashi, T.; Yagi, K.; Nagahashi, T.; Fukuda, S.; et al. Prognostic significance of clinical parameters and biological markers in patients with squamous cell carcinoma of the head and neck treated with concurrent chemoradiotherapy. Clin. Cancer Res. 1999, 5, 801–806. [Google Scholar]
- Quon, H.; Liu, F.F.; Cummings, B.J. Potential molecular prognostic markers in head and neck squamous cell carcinomas. Head Neck 2001, 23, 147–159. [Google Scholar] [CrossRef]
- Bellairs, J.A.; Hasina, R.; Agrawal, N. Tumor DNA: An emerging biomarker in head and neck cancer. Cancer Metastasis Rev. 2017, 36, 515–523. [Google Scholar] [CrossRef]
- Chera, B.S.; Kumar, S.; Beaty, B.T.; Marron, D.; Jefferys, S.; Green, R.; Goldman, E.C.; Amdur, R.; Sheets, N.; Dagan, R.; et al. Rapid Clearance Profile of Plasma Circulating Tumor HPV Type 16 DNA during Chemoradiotherapy Correlates with Disease Control in HPV-Associated Oropharyngeal Cancer. Clin. Cancer Res. 2019, 25, 4682–4690. [Google Scholar] [CrossRef]
- Jensen, K.K.; Gronhoj, C.; Jensen, D.H.; von Buchwald, C. Circulating human papillomavirus DNA as a surveillance tool in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Clin. Otolaryngol. 2018, 43, 1242–1249. [Google Scholar] [CrossRef]
- Amundson, S.A.; Grace, M.B.; McLeland, C.B.; Epperly, M.W.; Yeager, A.; Zhan, Q.; Greenberger, J.S.; Fornace, A.J., Jr. Human in vivo radiation-induced biomarkers: Gene expression changes in radiotherapy patients. Cancer Res. 2004, 64, 6368–6371. [Google Scholar] [CrossRef]
- Kis, E.; Szatmari, T.; Keszei, M.; Farkas, R.; Esik, O.; Lumniczky, K.; Falus, A.; Safrany, G. Microarray analysis of radiation response genes in primary human fibroblasts. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 1506–1514. [Google Scholar] [CrossRef]
- Tilton, S.C.; Markillie, L.M.; Hays, S.; Taylor, R.C.; Stenoien, D.L. Identification of Differential Gene Expression Patterns after Acute Exposure to High and Low Doses of Low-LET Ionizing Radiation in a Reconstituted Human Skin Tissue. Radiat. Res. 2016, 186, 531–538. [Google Scholar] [CrossRef]
- O’Brien, G.; Cruz-Garcia, L.; Majewski, M.; Grepl, J.; Abend, M.; Port, M.; Tichy, A.; Sirak, I.; Malkova, A.; Donovan, E.; et al. FDXR is a biomarker of radiation exposure in vivo. Sci. Rep. 2018, 8, 684. [Google Scholar]
- Kabacik, S.; Mackay, A.; Tamber, N.; Manning, G.; Finnon, P.; Paillier, F.; Ashworth, A.; Bouffler, S.; Badie, C. Gene expression following ionising radiation: Identification of biomarkers for dose estimation and prediction of individual response. Int. J. Radiat. Biol. 2011, 87, 115–129. [Google Scholar] [CrossRef]
- Brondum, L.; Eriksen, J.G.; Singers Sorensen, B.; Mortensen, L.S.; Toustrup, K.; Overgaard, J.; Alsner, J. Plasma proteins as prognostic biomarkers in radiotherapy treated head and neck cancer patients. Clin. Transl. Radiat. Oncol. 2017, 2, 46–52. [Google Scholar] [CrossRef][Green Version]
- Widlak, P.; Jelonek, K.; Wojakowska, A.; Pietrowska, M.; Polanska, J.; Marczak, L.; Miszczyk, L.; Skladowski, K. Serum Proteome Signature of Radiation Response: Up-regulation of Inflammation-Related Factors and Down-regulation of Apolipoproteins and Coagulation Factors in Cancer Patients Treated With Radiation Therapy–A Pilot Study. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 1108–1115. [Google Scholar] [CrossRef]
- Metcalfe, E.; Arik, D.; Oge, T.; Etiz, D.; Yalcin, O.T.; Kabukcuoglu, S.; Pasaoglu, O.; Ozalp, S.S. CD105 (endoglin) expression as a prognostic marker of angiogenesis in squamous cell cervical cancer treated with radical radiotherapy. J. Cancer Res. Ther. 2018, 14, 1373–1378. [Google Scholar] [CrossRef]
- Marioni, G.; Ottaviano, G.; Marchese-Ragona, R.; Fasanaro, E.; Tealdo, G.; Zanotti, C.; Randon, B.; Giacomelli, L.; Stellini, E.; Blandamura, S. Nuclear survivin expression correlates with endoglin-assessed microvascularisation in laryngeal carcinoma. J. Clin. Pathol. 2017, 70, 1033–1037. [Google Scholar] [CrossRef]
- Marioni, G.; Staffieri, A.; Hagen, R.; Ottaviano, G.; Lionello, M.; Staffieri, C.; Giacomelli, L.; Blandamura, S. Prognostic value of hypoxia-inducible factors (angiogenin and endoglin) in open partial laryngectomies: Uni- and multivariate analyses. Am. J. Otolaryngol. 2013, 34, 3–9. [Google Scholar] [CrossRef]
- Demirci, U.; Yaman, M.; Buyukberber, S.; Coskun, U.; Baykara, M.; Uslu, K.; Ozet, A.; Benekli, M.; Bagriacik, E.U. Prognostic importance of markers for inflammation, angiogenesis and apoptosis in high grade glial tumors during temozolomide and radiotherapy. Int. Immunopharmacol. 2012, 14, 546–549. [Google Scholar] [CrossRef]
- Li, C.; Wilson, P.B.; Levine, E.; Barber, J.; Stewart, A.L.; Kumar, S. TGF-beta1 levels in pre-treatment plasma identify breast cancer patients at risk of developing post-radiotherapy fibrosis. Int. J. Cancer 1999, 84, 155–159. [Google Scholar] [CrossRef]
- Chang, H.; Wei, J.W.; Chen, K.; Zhang, S.; Han, F.; Lu, L.X.; Xiao, W.W.; Gao, Y.H. Apolipoprotein A-I Is a Prognosticator of Nasopharyngeal Carcinoma in the Era of Intensity-modulated Radiotherapy. J. Cancer 2018, 9, 702–710. [Google Scholar] [CrossRef]
- Luo, X.L.; Zhong, G.Z.; Hu, L.Y.; Chen, J.; Liang, Y.; Chen, Q.Y.; Liu, Q.; Rao, H.L.; Chen, K.L.; Cai, Q.Q. Serum apolipoprotein A-I is a novel prognostic indicator for non-metastatic nasopharyngeal carcinoma. Oncotarget 2015, 6, 44037–44048. [Google Scholar] [CrossRef]
- Pfannenstiel, L.W.; Diaz-Montero, C.M.; Tian, Y.F.; Scharpf, J.; Ko, J.S.; Gastman, B.R. Immune-Checkpoint Blockade Opposes CD8(+) T-cell Suppression in Human and Murine Cancer. Cancer Immunol. Res. 2019, 7, 510–525. [Google Scholar] [CrossRef]
- Matoba, T.; Imai, M.; Ohkura, N.; Kawakita, D.; Ijichi, K.; Toyama, T.; Morita, A.; Murakami, S.; Sakaguchi, S.; Yamazaki, S. Regulatory T cells expressing abundant CTLA-4 on the cell surface with a proliferative gene profile are key features of human head and neck cancer. Int. J. Cancer 2019, 144, 2811–2822. [Google Scholar] [CrossRef]
- Mann, J.E.; Smith, J.D.; Birkeland, A.C.; Bellile, E.; Swiecicki, P.; Mierzwa, M.; Chinn, S.B.; Shuman, A.G.; Malloy, K.M.; Casper, K.A.; et al. Analysis of tumor-infiltrating CD103 resident memory T-cell content in recurrent laryngeal squamous cell carcinoma. Cancer Immunol. Immunother. 2019, 68, 213–220. [Google Scholar] [CrossRef]
- Santegoets, S.J.; Duurland, C.L.; Jordanova, E.S.; van Ham, J.J.; Ehsan, I.; van Egmond, S.L.; Welters, M.J.P.; van der Burg, S.H. Tbet-positive regulatory T cells accumulate in oropharyngeal cancers with ongoing tumor-specific type 1 T cell responses. J. Immunother. Cancer 2019, 7, 14. [Google Scholar] [CrossRef]
- Aggarwal, S.; Sharma, S.C.; Das, N.S. Dynamics of regulatory T cells (Tregs) in patients with oral squamous cell carcinoma. J. Surg. Oncol. 2017, 116, 1103–1113. [Google Scholar] [CrossRef]
- Drennan, S.; Stafford, N.D.; Greenman, J.; Green, V.L. Increased frequency and suppressive activity of CD127(low/-) regulatory T cells in the peripheral circulation of patients with head and neck squamous cell carcinoma are associated with advanced stage and nodal involvement. Immunology 2013, 140, 335–343. [Google Scholar]
- Strauss, L.; Bergmann, C.; Gooding, W.; Johnson, J.T.; Whiteside, T.L. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin. Cancer Res. 2007, 13, 6301–6311. [Google Scholar] [CrossRef]
- Gaur, P.; Shukla, N.K.; Das, S.N. Phenotypic and Functional Characteristics of Th17 (CD4(+)IL17A(+)) Cells in Human Oral Squamous Cell Carcinoma and Its Clinical Relevance. Immunol. Investig. 2017, 46, 689–702. [Google Scholar] [CrossRef]
- Balermpas, P.; Rodel, F.; Rodel, C.; Krause, M.; Linge, A.; Lohaus, F.; Baumann, M.; Tinhofer, I.; Budach, V.; Gkika, E.; et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG). Int. J. Cancer 2016, 138, 171–181. [Google Scholar] [CrossRef]
- Schuler, P.J.; Harasymczuk, M.; Schilling, B.; Saze, Z.; Strauss, L.; Lang, S.; Johnson, J.T.; Whiteside, T.L. Effects of adjuvant chemoradiotherapy on the frequency and function of regulatory T cells in patients with head and neck cancer. Clin. Cancer Res. 2013, 19, 6585–6596. [Google Scholar] [CrossRef]
- Young, M.R.; Wright, M.A.; Lozano, Y.; Matthews, J.P.; Benefield, J.; Prechel, M.M. Mechanisms of immune suppression in patients with head and neck cancer: Influence on the immune infiltrate of the cancer. Int. J. Cancer 1996, 67, 333–338. [Google Scholar] [CrossRef]
- Tsai, M.S.; Chen, W.C.; Lu, C.H.; Chen, M.F. The prognosis of head and neck squamous cell carcinoma related to immunosuppressive tumor microenvironment regulated by IL-6 signaling. Oral Oncol. 2019, 91, 47–55. [Google Scholar] [CrossRef]
- Balogh, A.; Persa, E.; Bogdandi, E.N.; Benedek, A.; Hegyesi, H.; Safrany, G.; Lumniczky, K. The effect of ionizing radiation on the homeostasis and functional integrity of murine splenic regulatory T cells. Inflamm. Res. 2013, 62, 201–212. [Google Scholar] [CrossRef]
- Muroyama, Y.; Nirschl, T.R.; Kochel, C.M.; Lopez-Bujanda, Z.; Theodros, D.; Mao, W.; Carrera-Haro, M.A.; Ghasemzadeh, A.; Marciscano, A.E.; Velarde, E.; et al. Stereotactic Radiotherapy Increases Functionally Suppressive Regulatory T Cells in the Tumor Microenvironment. Cancer Immunol. Res. 2017, 5, 992–1004. [Google Scholar] [CrossRef]
- Parikh, F.; Duluc, D.; Imai, N.; Clark, A.; Misiukiewicz, K.; Bonomi, M.; Gupta, V.; Patsias, A.; Parides, M.; Demicco, E.G.; et al. Chemoradiotherapy-induced up-regulation of PD-1 antagonizes immunity to HPV-related oropharyngeal cancer. Cancer Res. 2014, 74, 7205–7216. [Google Scholar] [CrossRef]
- Sridharan, V.; Margalit, D.N.; Lynch, S.A.; Severgnini, M.; Zhou, J.; Chau, N.G.; Rabinowits, G.; Lorch, J.H.; Hammerman, P.S.; Hodi, F.S.; et al. Definitive chemoradiation alters the immunologic landscape and immune checkpoints in head and neck cancer. Br. J. Cancer 2016, 115, 252–260. [Google Scholar] [CrossRef]
- Gabitass, R.F.; Annels, N.E.; Stocken, D.D.; Pandha, H.A.; Middleton, G.W. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol. Immunother. 2011, 60, 1419–1430. [Google Scholar] [CrossRef]
- Ohki, S.; Shibata, M.; Gonda, K.; Machida, T.; Shimura, T.; Nakamura, I.; Ohtake, T.; Koyama, Y.; Suzuki, S.; Ohto, H.; et al. Circulating myeloid-derived suppressor cells are increased and correlate with immune suppression, inflammation and hypoproteinemia in patients with cancer. Oncol. Rep. 2012, 28, 453–458. [Google Scholar] [CrossRef]
- Wang, D.; An, G.; Xie, S.; Yao, Y.; Feng, G. The clinical and prognostic significance of CD14(+)HLA-DR(-/low) myeloid-derived suppressor cells in hepatocellular carcinoma patients receiving radiotherapy. Tumour Biol. 2016, 37, 10427–10433. [Google Scholar] [CrossRef]
- Yuan, X.K.; Zhao, X.K.; Xia, Y.C.; Zhu, X.; Xiao, P. Increased circulating immunosuppressive CD14(+)HLA-DR(-/low) cells correlate with clinical cancer stage and pathological grade in patients with bladder carcinoma. J. Int. Med. Res. 2011, 39, 1381–1391. [Google Scholar] [CrossRef]
- Van Meir, H.; Nout, R.A.; Welters, M.J.; Loof, N.M.; de Kam, M.L.; van Ham, J.J.; Samuels, S.; Kenter, G.G.; Cohen, A.F.; Melief, C.J.; et al. Impact of (chemo)radiotherapy on immune cell composition and function in cervical cancer patients. Oncoimmunology 2017, 6, e1267095. [Google Scholar] [CrossRef]
- Melioli, G.; Semino, C.; Margarino, G.; Mereu, P.; Scala, M.; Cangemi, G.; Crocetti, E.; Machi, A.M.; Ferlazzo, G. Expansion of natural killer cells in patients with head and neck cancer: Detection of “noninhibitory” (activating) killer Ig-like receptors on circulating natural killer cells. Head Neck 2003, 25, 297–305. [Google Scholar] [CrossRef]
- Accomando, W.P.; Wiencke, J.K.; Houseman, E.A.; Butler, R.A.; Zheng, S.; Nelson, H.H.; Kelsey, K.T. Decreased NK cells in patients with head and neck cancer determined in archival DNA. Clin. Cancer Res. 2012, 18, 6147–6154. [Google Scholar] [CrossRef]
- Wulff, S.; Pries, R.; Borngen, K.; Trenkle, T.; Wollenberg, B. Decreased levels of circulating regulatory NK cells in patients with head and neck cancer throughout all tumor stages. Anticancer Res. 2009, 29, 3053–3057. [Google Scholar]
- Schmidt, M.A.; Fortsch, C.; Schmidt, M.; Rau, T.T.; Fietkau, R.; Distel, L.V. Circulating regulatory T cells of cancer patients receiving radiochemotherapy may be useful to individualize cancer treatment. Radiother. Oncol. 2012, 104, 131–138. [Google Scholar] [CrossRef]
- Sakakura, K.; Chikamatsu, K.; Takahashi, K.; Whiteside, T.L.; Furuya, N. Maturation of circulating dendritic cells and imbalance of T-cell subsets in patients with squamous cell carcinoma of the head and neck. Cancer Immunol. Immunother. 2006, 55, 151–159. [Google Scholar] [CrossRef]
- Hoffmann, T.K.; Muller-Berghaus, J.; Ferris, R.L.; Johnson, J.T.; Storkus, W.J.; Whiteside, T.L. Alterations in the frequency of dendritic cell subsets in the peripheral circulation of patients with squamous cell carcinomas of the head and neck. Clin. Cancer Res. 2002, 8, 1787–1793. [Google Scholar]
- Gottfried, E.; Kreutz, M.; Mackensen, A. Tumor-induced modulation of dendritic cell function. Cytokine Growth Factor Rev. 2008, 19, 65–77. [Google Scholar] [CrossRef]
- Wang, E.; Uccellini, L.; Marincola, F.M. A genetic inference on cancer immune responsiveness. Oncoimmunology 2012, 1, 520–525. [Google Scholar] [CrossRef]
- Pilones, K.A.; Vanpouille-Box, C.; Demaria, S. Combination of radiotherapy and immune checkpoint inhibitors. Semin. Radiat. Oncol. 2015, 25, 28–33. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Pilones, K.A.; Wennerberg, E.; Formenti, S.C.; Demaria, S. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine 2015, 33, 7415–7422. [Google Scholar] [CrossRef]
- Young, K.H.; Baird, J.R.; Savage, T.; Cottam, B.; Friedman, D.; Bambina, S.; Messenheimer, D.J.; Fox, B.; Newell, P.; Bahjat, K.S.; et al. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PLoS ONE 2016, 11, e0157164. [Google Scholar] [CrossRef]
- Donaubauer, A.J.; Ruhle, P.F.; Becker, I.; Fietkau, R.; Gaipl, U.S.; Frey, B. One-Tube Multicolor Flow Cytometry Assay (OTMA) for Comprehensive Immunophenotyping of Peripheral Blood. Methods Mol. Biol. 2019, 1904, 189–212. [Google Scholar]

| Patient Code | Pathology | Localization | TNM Classification | Gender | Age | Total Dose (Gy) | Number of Fractions | Dose/ Fraction (Gy) | Overall Treatment Time (days) | AMR (Acute Mucosal Reaction) | Response to Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1_HNC | Carcinoma planoepitheliale G2 | larynx (glottis) | T1N0M0 | man | 62 | 51 | 17 | 3 | 25 | 3 | 1 |
| 2_HNC | Carcinoma planoepitheliale akeratodes | larynx | T2N0M0 | man | 72 | 64.8 | 37 | 1.8 | 38 | 2 | 1 |
| 3_HNC | Carcinoma planoepitheliale keratodes G2 | oral cavity | T2N0M0 | man | 44 | 52.8 | 33 | 1.6 | 61 | 3 | 4 |
| 4_HNC | Carcinoma planoepitheliale | larynx | T1N0M0 | man | 66 | 51 | 17 | 3 | 26 | 3 | 1 |
| 10_HNC | Squamous cell carcinoma | oral cavity | T1N0M0 | man | 43 | 52.8 | 33 | 1.6 | 48 | 2 | 1 |
| 12_HNC | Carcinoma planoepiheliale keratodes | larynx | T1N0M0 | man | 76 | 51 | 17 | 3 | 25 | 3 | 1 |
| 14_HNC | Carcinoma planoepitheliale keratodes | oral cavity | T4N0M0 | man | 63 | 57.6 | 36 | 1.6 | 50 | 1 | 6 |
| 17_HNC | Carcinoma planoepitheliale keratodes G1 | larynx | T2N0M0 | man | 64 | 72 | 40 | 1.8 | 38 | 3 | 1 |
| 18_HNC | Carcinoma planoepitheliale invasium G1 keratodes | larynx | T1N0M0 | man | 70 | 51 | 17 | 3 | 23 | 2 | 1 |
| 19_HNC | Carcinoma planoepitheliale | larynx (epiglottis) | T2N0M0 | woman | 67 | 72 | 40 | 1.8 | 41 | 3 | 1 |
| 20_HNC | Carcinoma planoepitheliale G3 | oral cavity | T4N0M0 | man | 59 | 57.6 | 36 | 1.6 | 50 | 0 | 1 |
| 21_HNC | Carcinoma planoepitheliale keratodes G1 | larynx (epiglottis) | T3N2M0 | woman | 52 | 74 | 40 | 1.8 | 41 | 3 | 1 |
| 23_HNC | Carcinoma planoepitheliale non keratodes G2 | larynx | T2N0M0 | woman | 57 | 66 | 30 | 2.2 | 48 | 3 | 3 |
| 24_HNC | Carcinoma planoepitheliale akeratodes | larynx (glottis) | T1N0M0 | man | 55 | 51 | 17 | 3 | 24 | 1 | 5 |
| 25_HNC | Carcinoma planoepitheliale keratodes G1 | oral cavity (tonque) | T2N0M0 | man | 66 | 57.6 | 36 | 1.6 | 55 | 2 | 1 |
| 26_HNC | Carcinoma planoepitheliale keratodes | oropharynx (tonsil) | T3N0M0 | man | 73 | 70 | 35 | 2 | 51 | 2 | 1 |
| 27_HNC | Carcinoma planoepitheliale G1 | oral cavity | T4N0M0 | woman | 49 | 57.6 | 36 | 1.6 | 50 | 2 | 1 |
| 28_HNC | Carcinoma planoepitheliale in situ | larynx | T2N0M0 | woman | 65 | 70.2 | 39 | 1.8 | 40 | 3 | 1 |
| 30_HNC | Carcinoma planoepitheliale akeratodes G2 | larynx | T3N0M0 | woman | 68 | 72 | 40 | 1.8 | 41 | 3 | 1 |
| 31_HNC | Carcinoma planoepitheliale keratodes G1 | oral cavity | T4N0M0 | man | 67 | 57.6 | 36 | 1.6 | 50 | 2 | 4 |
| 32_HNC | Carcinoma planoepitheliale keratodes G1 | oropharynx | T2N0M0 | woman | 79 | 57.6 | 36 | 1.6 | 52 | 2 | 1 |
| 33_HNC | Low Grade Mucoepidermoid Carcinoma | parotid | T1N0M0 | woman | 57 | 66 | 33 | 2 | 46 | 3 | 1 |
| 34_HNC | Squamous cell carcinoma | oral cavity (tonque) | T2N2M0 | woman | 60 | 60 | 30 | 2 | 44 | 2 | 1 |
| Cell Population | Before the Radiotherapy | Directly after Radiotherapy | 1 Month after Radiotherapy |
|---|---|---|---|
| CD4+ lymphocytes | n.s. | n.s. | ↓ ** |
| CD4+ FoxP3+ Treg | ↑ *** | ↑ **** | ↑ **** |
| CTLA-4+ in CD4+ lymphocytes | ↑ ** | ↑ **** | ↑ **** |
| PD-1+ in CD4+ FoxP3- lymphocytes | ↑ * | ↑ *** | ↑ ** |
| CD39+ in CD4+ FoxP3+ lymphocytes | ↓ **** | ↓ ** | ↓ ** |
| Ki-67+ in CD4+ lymphocytes | n.s. | ↑ ** | ↑ *** |
| MDSCs in PBMCs | ↓ ** | n.s. | n.s. |
| CD14+ in MDSCs | ↑ *** | ↑ * | ↑ |
| CD14− in MDSCs | ↓ p = 0.0569 | ↓ ** | ↓ * |
| CD123+ DCs | ↑ **** | ↑ *** | ↑ *** |
| DC11c+ DCs | ↑ **** | ↑ **** | ↑ **** |
| Gene Name | Abbreviation of Gene Name | Primer Sequences |
|---|---|---|
| Hypoxanthine Phosphoribosyl-transferase 1 | HPRT1 | F: 5′ TCAGGCAGTATAATCCAAAGATGGT 3′ |
| R: 5′ AGTCTGGCTTATATCCAACACTTCG 3′ | ||
| P: 5′ CGCAAGCTTGCTGGTGAAAAGGACCC 3′ | ||
| Damage Specific DNA Binding Protein 2 | DDB2 | F: 5′ GTCACTTCCAGCACCTCACA 3′ |
| R: 5′ ACGTCGATCGTCCTCAATTC 3′ | ||
| P: 5′ AGCCTGGCATCCTCGCTACAACC 3′ | ||
| Growth Arrest and DNA Damage Inducible Alpha | GADD45 | F: 5′ CTGCGAGAACGACATCAAC 3′ |
| R: 5′ AGCGTCGGTCTCCAAGAG 3′ | ||
| P: 5′ ATCCTGCGCGTCAGCAACCCG 3′ | ||
| Sestrin 1 | SESN1 | F: 5′ GCTGTCTTGTGCATTACTTGTG 3′ |
| R: 5′ CTGCGCAGCAGTCTACAG 3′ | ||
| P: 5′ ACATGTCCCACAACTTTGGTGCTGG 3′ | ||
| Ferredoxin reductase | FDXR | F: 5′ GTACAACGGGCTTCCTGAGA3′ |
| R: 5′ CTCAGGTGGGGTCAGTAGGA 3′ | ||
| P: 5′ CGGGCCACGTCCAGAGCCA 3′ | ||
| Murine double minus-2 proto-oncogene | MDM2 | F: 5′ CCATGATCTACAGGAACTTGGTAGTA 3′ |
| R: 5′ ACACCTGTTCTCACTCACAGATG 3′ | ||
| P: 5′ CAATCAGCAGGAATCATCGGACTCAG 3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balázs, K.; Kis, E.; Badie, C.; Bogdándi, E.N.; Candéias, S.; Cruz Garcia, L.; Dominczyk, I.; Frey, B.; Gaipl, U.; Jurányi, Z.; et al. Radiotherapy-Induced Changes in the Systemic Immune and Inflammation Parameters of Head and Neck Cancer Patients. Cancers 2019, 11, 1324. https://doi.org/10.3390/cancers11091324
Balázs K, Kis E, Badie C, Bogdándi EN, Candéias S, Cruz Garcia L, Dominczyk I, Frey B, Gaipl U, Jurányi Z, et al. Radiotherapy-Induced Changes in the Systemic Immune and Inflammation Parameters of Head and Neck Cancer Patients. Cancers. 2019; 11(9):1324. https://doi.org/10.3390/cancers11091324
Chicago/Turabian StyleBalázs, Katalin, Enikő Kis, Christophe Badie, Enikő Noémi Bogdándi, Serge Candéias, Lourdes Cruz Garcia, Iwona Dominczyk, Benjamin Frey, Udo Gaipl, Zsolt Jurányi, and et al. 2019. "Radiotherapy-Induced Changes in the Systemic Immune and Inflammation Parameters of Head and Neck Cancer Patients" Cancers 11, no. 9: 1324. https://doi.org/10.3390/cancers11091324
APA StyleBalázs, K., Kis, E., Badie, C., Bogdándi, E. N., Candéias, S., Cruz Garcia, L., Dominczyk, I., Frey, B., Gaipl, U., Jurányi, Z., Kocsis, Z. S., Rutten, E. A., Sáfrány, G., Widlak, P., & Lumniczky, K. (2019). Radiotherapy-Induced Changes in the Systemic Immune and Inflammation Parameters of Head and Neck Cancer Patients. Cancers, 11(9), 1324. https://doi.org/10.3390/cancers11091324









