FDG-PET/CT for Response Monitoring in Metastatic Breast Cancer: Today, Tomorrow, and Beyond
Abstract
1. Introduction
2. Metastasis, Progression, and Precision Oncology
3. Response Prediction and Response Monitoring
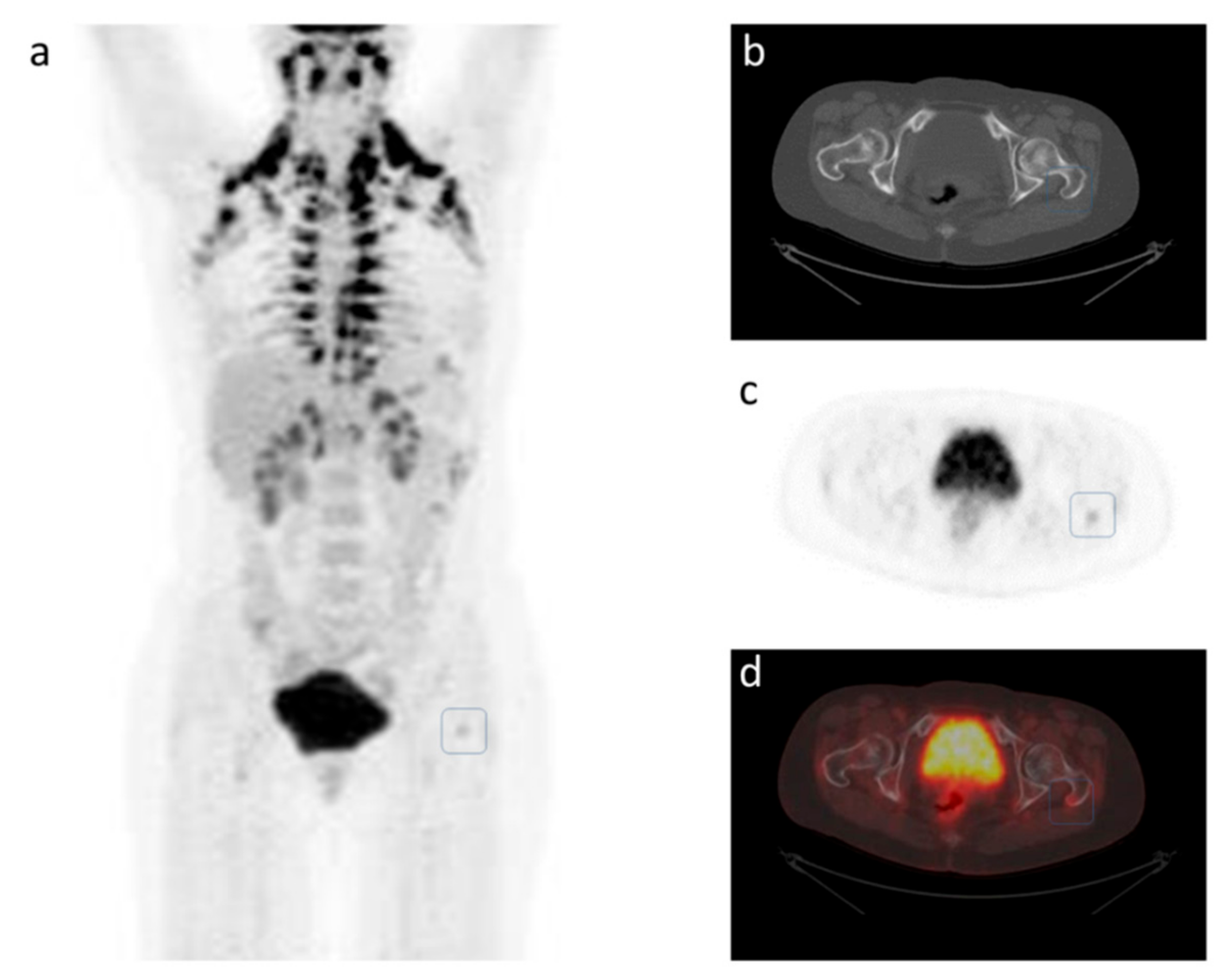
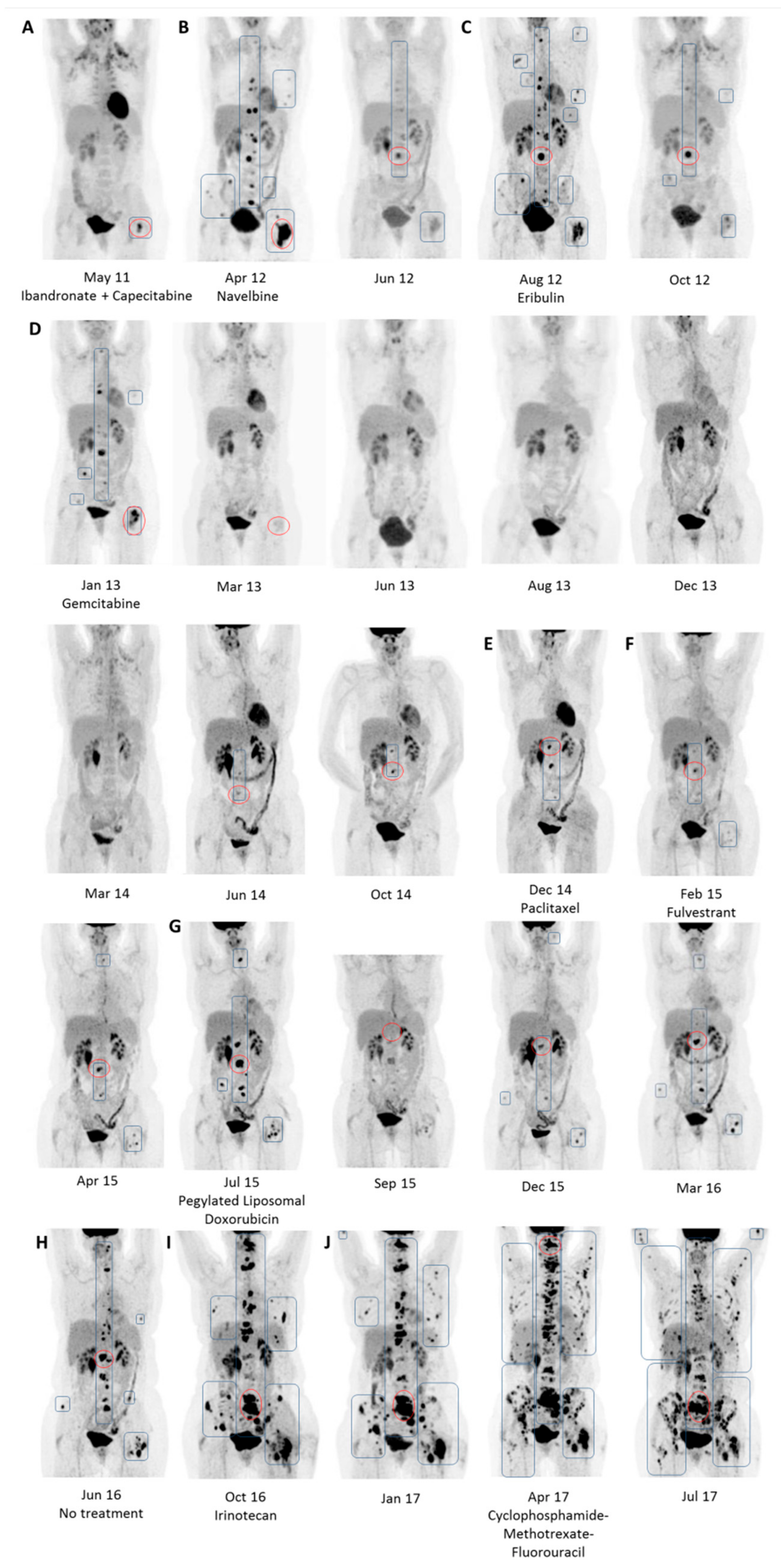
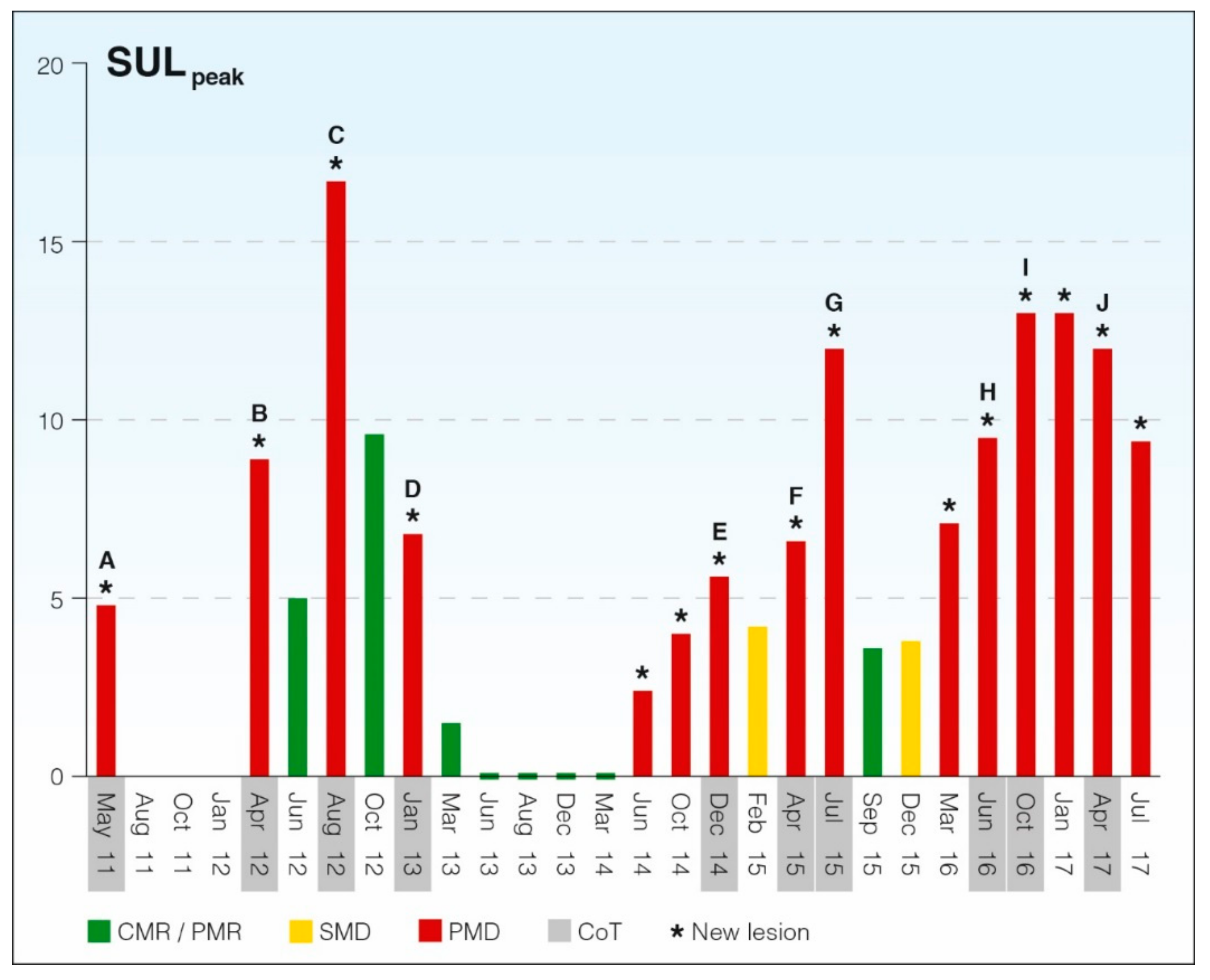
4. A Case Study of Longitudinal Response Monitoring in Metastatic Breast Cancer
5. PERCIST 1.0 for Response Monitoring in Metastatic Breast Cancer
6. Today
7. Tomorrow
8. Beyond
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: Globocan Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, Trastuzumab, and Docetaxel in Her2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised Recist Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; Andre, F.; Harbeck, N.; Aguilar Lopez, B.; Barrios, C.H.; Bergh, J.; et al. 4th Eso-Esmo International Consensus Guidelines for Advanced Breast Cancer (Abc 4) Dagger. Ann. Oncol. 2018, 29, 1634–1657. [Google Scholar] [CrossRef] [PubMed]
- O, J.H.; Lodge, M.A.; Wahl, R.L. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology 2016, 2, 576. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for Pet Response Criteria in Solid Tumors. J. Nucl. Med. 2009, 50, 122S–150S. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, mdz189. [Google Scholar] [CrossRef]
- Alimirzaie, S.; Bagherzadeh, M.; Akbari, M.R. Liquid Biopsy in Breast Cancer: A Comprehensive Review. Clin. Genet. 2019, 95, 643–660. [Google Scholar] [CrossRef]
- Kroigard, A.B.; Larsen, M.J.; Thomassen, M.; Kruse, T.A. Molecular Concordance between Primary Breast Cancer and Matched Metastases. Breast J. 2016, 22, 420–430. [Google Scholar] [CrossRef]
- Gundem, G.; Van Loo, P.; Kremeyer, B.; Alexandrov, L.B.; Tubio, J.M.C.; Papaemmanuil, E.; Brewer, D.S.; Kallio, H.M.L.; Hognas, G.; Annala, M.; et al. The Evolutionary History of Lethal Metastatic Prostate Cancer. Nature 2015, 520, 353–357. [Google Scholar] [CrossRef]
- Weide, R.; Feiten, S.; Friesenhahn, V.; Heymanns, J.; Kleboth, K.; Thomalla, J.; van Roye, C.; Koppler, H. Metastatic Breast Cancer: Prolongation of Survival in Routine Care Is Restricted to Hormone-Receptor- and Her2-Positive Tumors. Springerplus 2014, 3, 535. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goh, K.I.; Cusick, M.E.; Valle, D.; Childs, B.; Vidal, M.; Barabasi, A.L. The Human Disease Network. Proc. Natl. Acad. Sci. USA 2007, 104, 8685–8690. [Google Scholar] [CrossRef] [PubMed]
- Horak, P.; Klink, B.; Heining, C.; Groschel, S.; Hutter, B.; Frohlich, M.; Uhrig, S.; Hubschmann, D.; Schlesner, M.; Eils, R.; et al. Precision Oncology Based on Omics Data: The Nct Heidelberg Experience. Int. J. Cancer 2017, 141, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Buono, G.; Gerratana, L.; Bulfoni, M.; Provinciali, N.; Basile, D.; Giuliano, M.; Corvaja, C.; Arpino, G.; Del Mastro, L.; De Placido, S.; et al. Circulating Tumor DNA Analysis in Breast Cancer: Is It Ready for Prime-Time? Cancer Treat. Rev. 2019, 73, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Kroigard, A.B.; Larsen, M.J.; Brasch-Andersen, C.; Laenkholm, A.V.; Knoop, A.S.; Jensen, J.D.; Bak, M.; Mollenhauer, J.; Thomassen, M.; Kruse, T.A. Genomic Analyses of Breast Cancer Progression Reveal Distinct Routes of Metastasis Emergence. Sci. Rep. 2017, 7, 43813. [Google Scholar] [CrossRef] [PubMed]
- Munkley, J.; Elliott, D.J. Hallmarks of Glycosylation in Cancer. Oncotarget 2016, 7, 35478–35489. [Google Scholar] [CrossRef] [PubMed]
- Kwee, T.C.; Basu, S.; Saboury, B.; Ambrosini, V.; Torigian, D.A.; Alavi, A. A New Dimension of FDG-PET Interpretation: Assessment of Tumor Biology. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1158–1170. [Google Scholar] [CrossRef]
- Benard, F.; Sterman, D.; Smith, R.J.; Kaiser, L.R.; Albelda, S.M.; Alavi, A. Prognostic Value of FDG PET Imaging in Malignant Pleural Mesothelioma. J. Nucl. Med. 1999, 40, 1241–1245. [Google Scholar]
- Kitajima, K.; Miyoshi, Y.; Yamano, T.; Odawara, S.; Higuchi, T.; Yamakado, K. Prognostic Value of FDG-PET and DWI in Breast Cancer. Ann. Nucl. Med. 2018, 32, 44–53. [Google Scholar] [CrossRef]
- Baun, C.; Falch, K.; Gerke, O.; Hansen, J.; Nguyen, T.; Alavi, A.; Hoilund-Carlsen, P.F.; Hildebrandt, M.G. Quantification of FDG-PET/CT with Delayed Imaging in Patients with Newly Diagnosed Recurrent Breast Cancer. BMC Med. Imaging 2018, 18, 11. [Google Scholar] [CrossRef]
- Hildebrandt, M.G.; Gerke, O.; Baun, C.; Falch, K.; Hansen, J.A.; Farahani, Z.A.; Petersen, H.; Larsen, L.B.; Duvnjak, S.; Buskevica, I.; et al. [18f]Fluorodeoxyglucose (FDG)-Positron Emission Tomography (PET)/Computed Tomography (CT) in Suspected Recurrent Breast Cancer: A Prospective Comparative Study of Dual-Time-Point FDG-PET/CT, Contrast-Enhanced CT, and Bone Scintigraphy. J. Clin. Oncol. 2016, 34, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Riedl, C.C.; Pinker, K.; Ulaner, G.A.; Ong, L.T.; Baltzer, P.; Jochelson, M.S.; McArthur, H.L.; Gonen, M.; Dickler, M.; Weber, W.A. Comparison of FDG-PET/CT and Contrast-Enhanced CT for Monitoring Therapy Response in Patients with Metastatic Breast Cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Catalano, O.A.; Nicolai, E.; Rosen, B.R.; Luongo, A.; Catalano, M.; Iannace, C.; Guimaraes, A.; Vangel, M.G.; Mahmood, U.; Soricelli, A.; et al. Comparison of CE-FDG-PET/CT with CE-FDG-PET/MR in the evaluation of osseous metastases in breast cancer patients. Br. J. Cancer 2015, 112, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Fledelius, J.; Khalil, A.; Hjorthaug, K.; Frokiaer, J. Inter-observer agreement improves with PERCIST 1.0 as opposed to qualitative evaluation in non-small cell lung cancer patients evaluated with F-18-FDG PET/CT early in the course of chemo-radiotherapy. EJNMMI Res. 2016, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Vach, W.; Hoilund-Carlsen, P.F.; Fischer, B.M.; Gerke, O.; Weber, W. How to Study Optimal Timing of PET/CT for Monitoring of Cancer Treatment. Am. J. Nucl. Med. Mol. Imaging 2011, 1, 54–62. [Google Scholar] [PubMed]
- Gerke, O.; Ehlers, K.; Motschall, E.; Hoilund-Carlsen, P.F.; Vach, W. PET/CT-Based Response Evaluation in Cancer-a Systematic Review of Design Issues. Mol. Imaging Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Pinker, K.; Riedl, C.C.; Ong, L.; Jochelson, M.; Ulaner, G.A.; McArthur, H.; Dickler, M.; Gonen, M.; Weber, W.A. The Impact That Number of Analyzed Metastatic Breast Cancer Lesions Has on Response Assessment by 18f-FDG PET/CT Using PERCIST. J. Nucl. Med. 2016, 57, 1102–1104. [Google Scholar] [CrossRef][Green Version]
- Ulaner, G.A. PET/CT for Patients with Breast Cancer: Where Is the Clinical Impact? AJR Am. J. Roentgenol. 2019, 213, 254–265. [Google Scholar] [CrossRef]
- Devriese, J.; Beels, L.; Maes, A.; Van de Wiele, C.; Pottel, H. Impact of Pet Reconstruction Protocols on Quantification of Lesions That Fulfil the Percist Lesion Inclusion Criteria. EJNMMI Phys. 2018, 5, 35. [Google Scholar] [CrossRef]
- O, J.H.; Wahl, R.L. PERCIST in Perspective. Nucl. Med. Mol. Imaging 2018, 52, 1–4. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The Bridge between Medical Imaging and Personalized Medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Sepucha, K.R.; Ozanne, E.M.; Partridge, A.H.; Moy, B. Is There a Role for Decision Aids in Advanced Breast Cancer? Med. Decis. Making 2009, 29, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Spronk, I.; Burgers, J.S.; Schellevis, F.G.; van Vliet, L.M.; Korevaar, J.C. The Availability and Effectiveness of Tools Supporting Shared Decision Making in Metastatic Breast Cancer Care: A Review. BMC Palliat. Care 2018, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, S.J.; Wallace, A.; Williams, B.R.; Turkman, Y.; Williams, C.P.; Bhatia, S.; Knight, S.; Rocque, G.B. Trust but Verify: Exploring the Role of Treatment-Related Information and Patient-Physician Trust in Shared Decision Making among Patients with Metastatic Breast Cancer. J. Cancer Educ. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, G.L.; Lamberts, E.; Wimana, Z.; Garcia, C.; Emonts, P.; Ameye, L.; Stroobants, S.; Huizing, M.; Aftimos, P.; Tol, J.; et al. Molecular Imaging as a Tool to Investigate Heterogeneity of Advanced HER2-Positive Breast Cancer and to Predict Patient Outcome under Trastuzumab Emtansine (T-Dm1): The Zephir Trial. Ann. Oncol. 2016, 4, 619–624. [Google Scholar] [CrossRef]
- Gong, C.; Yang, Z.; Sun, Y.; Zhang, J.; Zheng, C.; Wang, L.; Zhang, Y.; Xue, J.; Yao, Z.; Pan, H.; et al. A Preliminary Study of (18)F-FES PET/CT in Predicting Metastatic Breast Cancer in Patients Receiving Docetaxel or Fulvestrant with Docetaxel. Sci. Rep. 2017, 7, 6584. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, M.A.; Nannini, M.; Maleddu, A.; Fanti, S.; Ambrosini, V.; Nanni, C.; Boschi, S.; Biasco, G. Conventional and Novel PET Tracers for Imaging in Oncology in the Era of Molecular Therapy. Cancer Treat. Rev. 2008, 34, 103–121. [Google Scholar] [CrossRef]
- Dalm, S.; Verzijlbergen, U.J.F.; De Jong, M. Review: Receptor Targeted Nuclear Imaging of Breast Cancer. Int. J. Mol. Sci. 2017, 18, 260. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Hyman, D.M.; Lyashchenko, S.K.; Lewis, J.S.; Carrasquillo, J.A. 89Zr-Trastuzumab PET/CT for Detection of Human Epidermal Growth Factor Receptor 2-Positive Metastases in Patients With Human Epidermal Growth Factor Receptor 2-Negative Primary Breast Cancer. Clin. Nucl. Med. 2017, 42, 912–917. [Google Scholar] [CrossRef]
- Yordanova, A.; Eppard, E.; Kurpig, S.; Bundschuh, R.A.; Schonberger, S.; Gonzalez-Carmona, M.; Feldmann, G.; Ahmadzadehfar, H.; Essler, M. Theranostics in Nuclear Medicine Practice. Onco. Targets Ther. 2017, 10, 4821–4828. [Google Scholar] [CrossRef]
| Quantification Measure | Suggested quantification measure | SULpeak for the hottest tumor, which is not necessarily the same tumor on scans over time (one-lesion method) |
| Suggested quantification measure | SULpeak of up to five target lesions in hottest tumors, up to two per organ (five-lesions method) | |
| Measurability Criteria at Baseline | Disease under study must be FDG-avid, meaning that SULpeak should be ≥ 1 and ≥ 1.5 × SULmean, liver + 2 SD SULmean, liver | |
| Assessabilty Criteria at Follow-Up | Scanner criteria | Same scanner or scanner model at same site should be used |
| Same reconstruction should be applied | ||
| Properly calibrated scanners should be used | ||
| Patient criteria | Patients should fast for at least 4 h | |
| Serum glucose levels should be < 200 mg/dL | ||
| FDG criteria | Difference in injected dose should be ≤ 20% | |
| Difference in injection-to-scan-time between baseline and follow-up scan should be ≤ 15 min | ||
| Injection-to-scan-time should be within 50–70 min | ||
| Background activity criteria | Difference in SULmean, liver < 20% and < 0.3 SUL units | |
| Response Categories at Follow-Up | Follow-up scans are suggested to be compared to the baseline/pretreatment scan | |
| Complete Metabolic Response | Complete resolution of cancer-suspect lesions. Any previous lesion should have FDG uptake less than SULmean, liver and be indistinguishable from background. No new lesions appeared. | |
| Partial Metabolic Response | Decrease in SULpeak of hottest tumor ≥ 30% and at least 0.8 SUL units. No new lesions appeared. No increase in size greater than 30%. No unequivocal progression of a non-target lesion (increase in SULpeak or size greater than 30%). | |
| Stable Metabolic Disease | Increase or decrease of SULpeak of less than 30%. No new lesions appeared. No increase in size greater than 30%. No unequivocal progression of a non-target lesion. | |
| Progressive Metabolic Disease | Increase in SULpeak of hottest tumor of ≥ 30% and of at least 0.8 SUL units, or development of one or more new lesions in a pattern suspect of cancer. | |
| Scan Month | Baseline/Pre- Treatment Scan (*) | SULmean, liver | ITST/Min | Matrix Size | New Lesion | SULpeak Target Lesion | Response Category |
|---|---|---|---|---|---|---|---|
| May 11 | Yes (A) | 1.51 | 64 | 128 | Yes | 4.78 | BL |
| Apr 12 | Yes (B) | 2.03 | 60 | 128 | Yes | 8.89 | PMD |
| Jun 12 | 1.66 | 82 | 128 | No | 4.99 | PMR | |
| Aug 12 | Yes (C) | 1.92 | 74 | 128 | Yes | 16.67 | PMD |
| Oct 12 | 1.35 | 73 | 128 | No | 9.58 | PMR | |
| Jan 13 | Yes (D) | 1.68 | 61 | 128 | Yes | 6.81 | PMD |
| Mar 13 | 1.68 | 64 | 128 | No | 1.47 | PMR | |
| Jun 13 | 1.85 | 68 | 128 | No | - | CMR | |
| Aug 13 | 1.92 | 58 | 256 | No | - | CMR | |
| Dec 13 | 2.07 | 58 | 256 | No | - | CMR | |
| Mar 14 | 1.80 | 111 | 256 | No | - | CMR | |
| Jun 14 | 1.95 | 58 | 256 | Yes | 2.42 | PMD | |
| Oct 14 | 1.93 | 67 | 256 | Yes | 4.03 | PMD | |
| Dec 14 | Yes (E) | 1.82 | 59 | 256 | Yes | 5.59 | PMD |
| Feb 15 | 1.94 | 62 | 256 | No | 4.16 | PMR | |
| Apr 15 | Yes (F) | 2.04 | 66 | 256 | Yes | 6.59 | PMD |
| Jul 15 | Yes (G) | 2.06 | 58 | 256 | Yes | 12.01 | PMD |
| Sep 15 | 1.91 | 70 | 256 | No | 3.59 | PMR | |
| Dec 15 | 1.99 | 54 | 256 | No | 3.82 | PMR | |
| Mar 16 | 1.80 | 57 | 256 | Yes | 7.08 | PMD | |
| Jun 16 | Yes (H) | 1.81 | 66 | 256 | Yes | 9.46 | PMD |
| Oct 16 | Yes (I) | 1.71 | 60 | 256 | Yes | 13.07 | PMD |
| Jan 17 | 1.93 | 65 | 256 | Yes | 13.09 | PMD | |
| Apr 17 | Yes (J) | 1.44 | 64 | 256 | Yes | 11.93 | PMD |
| Jul 17 | 2.03 | 61 | 256 | Yes | 9.44 | PMD |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hildebrandt, M.G.; Lauridsen, J.F.; Vogsen, M.; Holm, J.; Vilstrup, M.H.; Braad, P.-E.; Gerke, O.; Thomassen, M.; Ewertz, M.; Høilund-Carlsen, P.F.; et al. FDG-PET/CT for Response Monitoring in Metastatic Breast Cancer: Today, Tomorrow, and Beyond. Cancers 2019, 11, 1190. https://doi.org/10.3390/cancers11081190
Hildebrandt MG, Lauridsen JF, Vogsen M, Holm J, Vilstrup MH, Braad P-E, Gerke O, Thomassen M, Ewertz M, Høilund-Carlsen PF, et al. FDG-PET/CT for Response Monitoring in Metastatic Breast Cancer: Today, Tomorrow, and Beyond. Cancers. 2019; 11(8):1190. https://doi.org/10.3390/cancers11081190
Chicago/Turabian StyleHildebrandt, Malene Grubbe, Jeppe Faurholdt Lauridsen, Marianne Vogsen, Jorun Holm, Mie Holm Vilstrup, Poul-Erik Braad, Oke Gerke, Mads Thomassen, Marianne Ewertz, Poul Flemming Høilund-Carlsen, and et al. 2019. "FDG-PET/CT for Response Monitoring in Metastatic Breast Cancer: Today, Tomorrow, and Beyond" Cancers 11, no. 8: 1190. https://doi.org/10.3390/cancers11081190
APA StyleHildebrandt, M. G., Lauridsen, J. F., Vogsen, M., Holm, J., Vilstrup, M. H., Braad, P.-E., Gerke, O., Thomassen, M., Ewertz, M., Høilund-Carlsen, P. F., & The Centre for Personalized Response Monitoring in Oncology (PREMIO). (2019). FDG-PET/CT for Response Monitoring in Metastatic Breast Cancer: Today, Tomorrow, and Beyond. Cancers, 11(8), 1190. https://doi.org/10.3390/cancers11081190








