Untargeted Assessment of Tumor Fractions in Plasma for Monitoring and Prognostication from Metastatic Breast Cancer Patients Undergoing Systemic Treatment
Abstract
1. Introduction
2. Results
2.1. Study Population
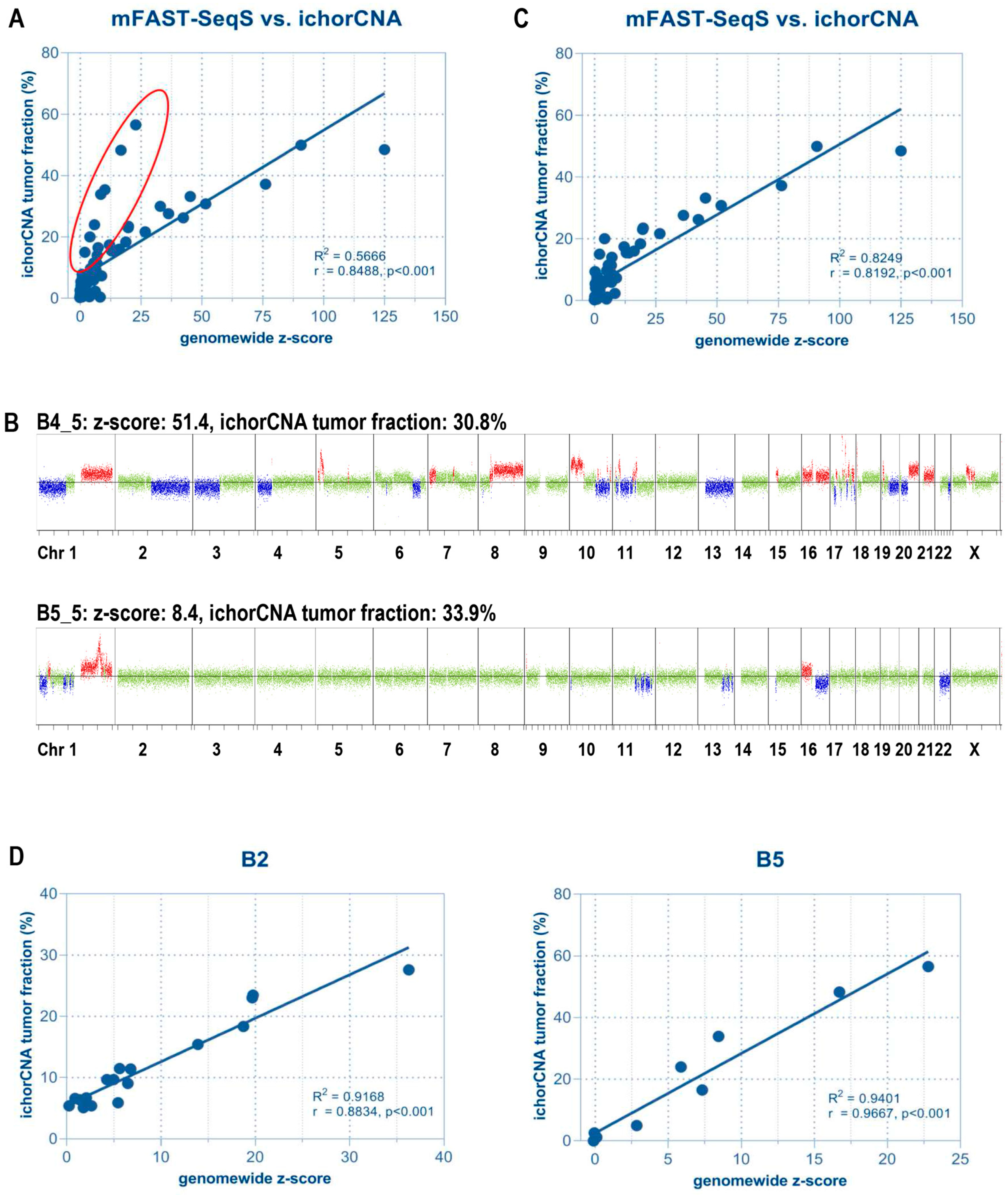
2.2. Untargeted Assessment of Tumor Fraction
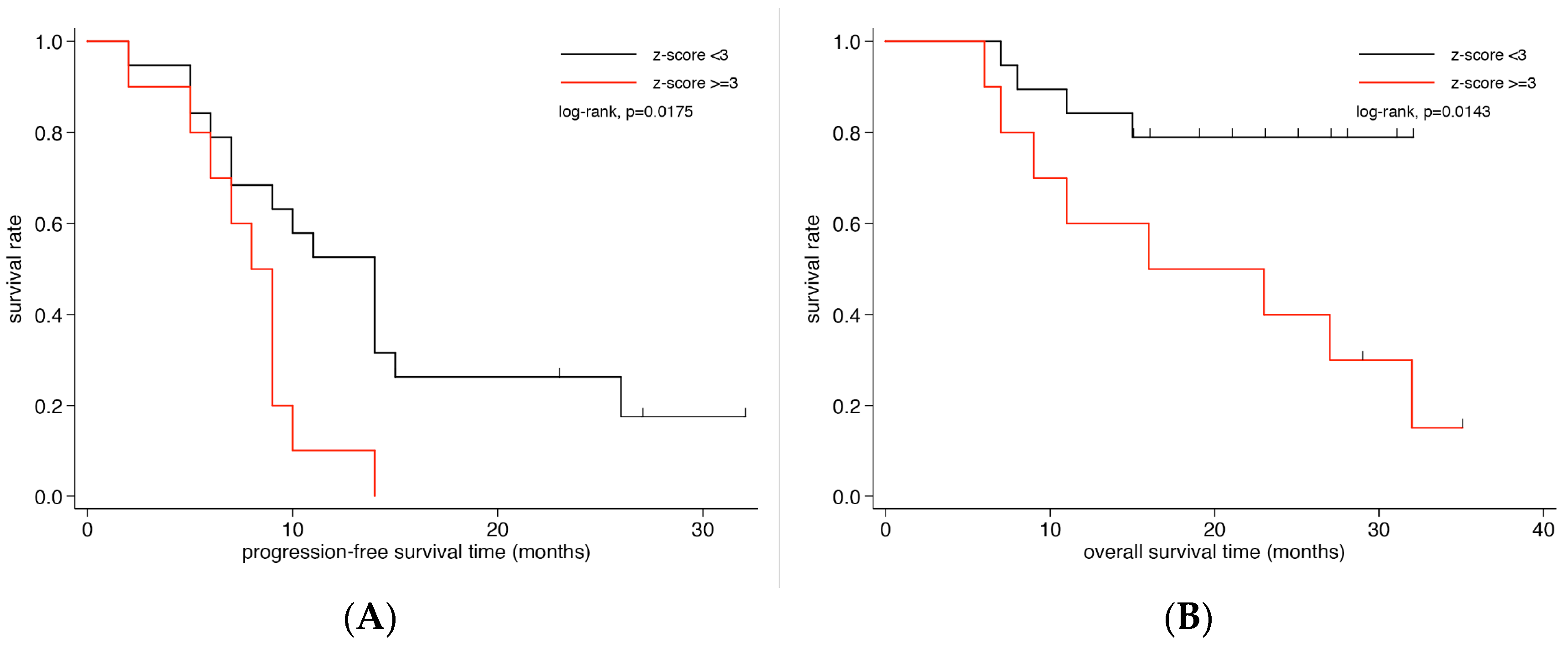
3. Prognostic Impact of ctDNA, CTCs and Tumor Markers on Survival
4. Association of Tumor Fractions, CTC Detection Rate, Tumor Marker and Clinicopathological Characteristics
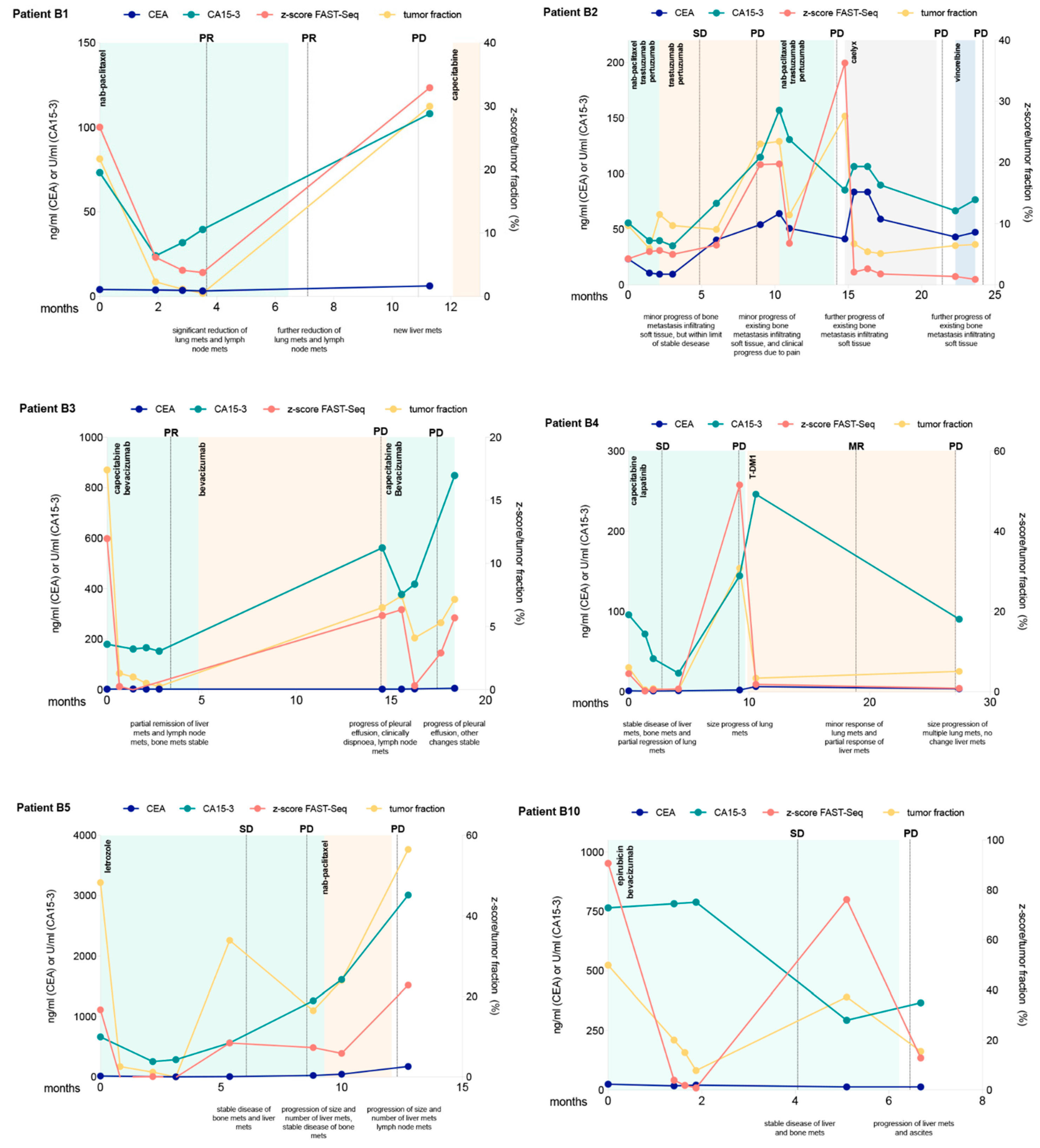
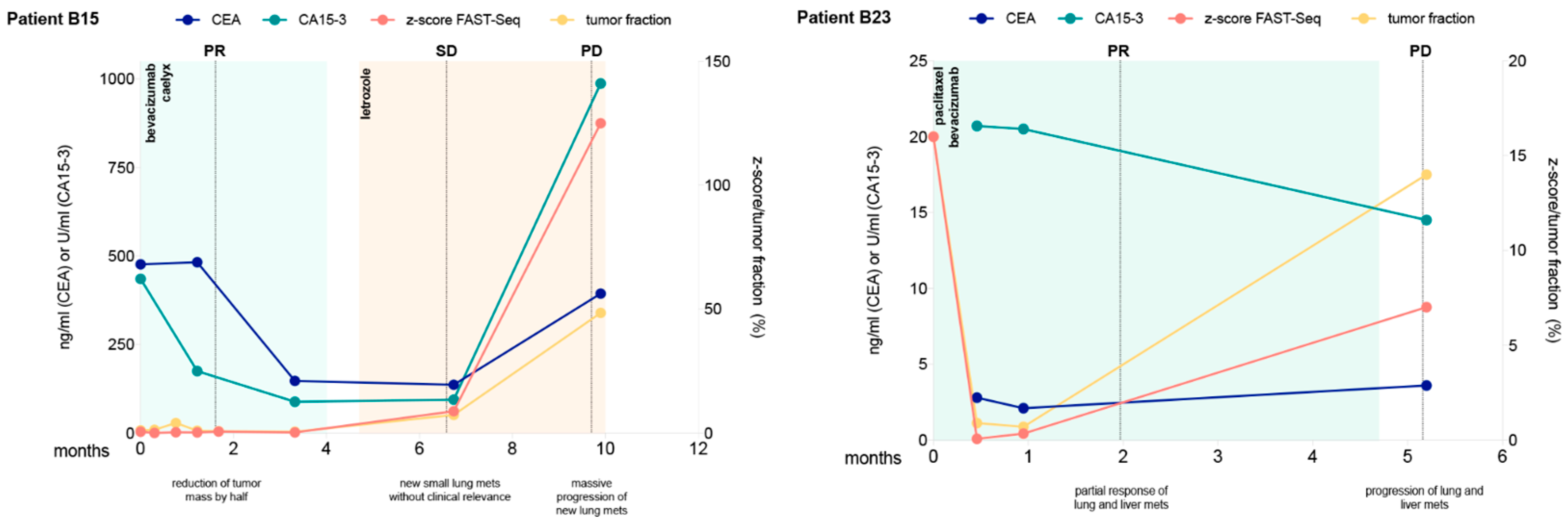
5. Serial Monitoring of the Genome-Wide z-Score of ctDNA
6. Discussion
7. Materials and Methods
7.1. Patients and Sample Collections
7.2. Preparation of Plasma DNA
7.3. Modified Fast Aneuploid Screening Test-Sequencing System (mFAST-SeqS)
7.4. Plasma-Seq
7.5. Enumeration of CTC
7.6. Statistical Analysis
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; Conte, P.; Rosso, R.; Orlandini, C.; Bruzzi, P. Survival of metastatic breast carcinoma patients over a 20-year period: A retrospective analysis based on individual patient data from six consecutive studies. Cancer 2005, 104, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Laessig, D.; Nagel, D.; Heinemann, V.; Untch, M.; Kahlert, S.; Bauerfeind, I.; Stieber, P. Importance of cea and ca 15-3 during disease progression in metastatic breast cancer patients. Anticancer Res. 2007, 27, 1963–1968. [Google Scholar] [PubMed]
- Lauro, S.; Trasatti, L.; Bordin, F.; Lanzetta, G.; Bria, E.; Gelibter, A.; Reale, M.G.; Vecchione, A. Comparison of cea, mca, ca 15-3 and ca 27-29 in follow-up and monitoring therapeutic response in breast cancer patients. Anticancer Res. 1999, 19, 3511–3515. [Google Scholar] [PubMed]
- Stieber, P.; Lässig, D.; Untch, M.; Nagel, D.; Heinemann, V. How can cea and ca15-3 be used for estimation of the clinical status and effectiveness of therapy during metastatic breast cancer? J. Clin. Oncol. 2004, 22, 755. [Google Scholar] [CrossRef]
- Shiomi-Mouri, Y.; Kousaka, J.; Ando, T.; Tetsuka, R.; Nakano, S.; Yoshida, M.; Fujii, K.; Akizuki, M.; Imai, T.; Fukutomi, T.; et al. Clinical significance of circulating tumor cells (ctcs) with respect to optimal cut-off value and tumor markers in advanced/metastatic breast cancer. Breast Cancer 2016, 23, 120–127. [Google Scholar] [CrossRef]
- Bidard, F.C.; Mathiot, C.; Delaloge, S.; Brain, E.; Giachetti, S.; de Cremoux, P.; Marty, M.; Pierga, J.Y. Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann. Oncol. 2010, 21, 729–733. [Google Scholar] [CrossRef]
- Fehm, T.; Sauerbrei, W. Information from ctc measurements for metastatic breast cancer prognosis-we should do more than selecting an “optimal cut point”. Breast Cancer Res. Treat. 2010, 122, 219–220. [Google Scholar] [CrossRef][Green Version]
- Hayes, D.F.; Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Miller, M.C.; Matera, J.; Allard, W.J.; Doyle, G.V.; Terstappen, L.W. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin. Cancer Res. 2006, 12, 4218–4224. [Google Scholar] [CrossRef]
- Olsson, E.; Winter, C.; George, A.; Chen, Y.; Howlin, J.; Tang, M.H.; Dahlgren, M.; Schulz, R.; Grabau, D.; van Westen, D.; et al. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol. Med. 2015, 7, 1034–1047. [Google Scholar] [CrossRef]
- Shaw, J.A.; Guttery, D.S.; Hills, A.; Fernandez-Garcia, D.; Page, K.; Rosales, B.M.; Goddard, K.S.; Hastings, R.K.; Luo, J.; Ogle, O.; et al. Mutation analysis of cell-free DNA and single circulating tumor cells in metastatic breast cancer patients with high circulating tumor cell counts. Clin. Cancer Res. 2017, 23, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224ra224. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Egleston, B.L.; Hajage, D.; Bland, J.; Hortobagyi, G.N.; Reuben, J.M.; Pierga, J.Y.; Cristofanilli, M.; Bidard, F.C. Establishment and validation of circulating tumor cell-based prognostic nomograms in first-line metastatic breast cancer patients. Clin. Cancer Res. 2013, 19, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Pierga, J.Y.; Hajage, D.; Bachelot, T.; Delaloge, S.; Brain, E.; Campone, M.; Dieras, V.; Rolland, E.; Mignot, L.; Mathiot, C.; et al. High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann. Oncol. 2012, 23, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef]
- Murtaza, M.; Dawson, S.J.; Pogrebniak, K.; Rueda, O.M.; Provenzano, E.; Grant, J.; Chin, S.F.; Tsui, D.W.Y.; Marass, F.; Gale, D.; et al. Multifocal clonal evolution characterized using circulating tumour DNA in a case of metastatic breast cancer. Nat. Commun. 2015, 6, 8760. [Google Scholar] [CrossRef]
- Murtaza, M.; Dawson, S.J.; Tsui, D.W.; Gale, D.; Forshew, T.; Piskorz, A.M.; Parkinson, C.; Chin, S.F.; Kingsbury, Z.; Wong, A.S.; et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 2013, 497, 108–112. [Google Scholar] [CrossRef]
- Heitzer, E.; Ulz, P.; Geigl, J.B. Circulating tumor DNA as a liquid biopsy for cancer. Clin. Chem. 2015, 61, 112–123. [Google Scholar] [CrossRef]
- Kleppe, M.; Levine, R.L. Tumor heterogeneity confounds and illuminates: Assessing the implications. Nat. Med. 2014, 20, 342–344. [Google Scholar] [CrossRef]
- Leary, R.J.; Sausen, M.; Kinde, I.; Papadopoulos, N.; Carpten, J.D.; Craig, D.; O’Shaughnessy, J.; Kinzler, K.W.; Parmigiani, G.; Vogelstein, B.; et al. Detection of chromosomal alterations in the circulation of cancer patients with whole-genome sequencing. Sci. Transl. Med. 2012, 4, 162ra154. [Google Scholar] [CrossRef]
- Chan, K.C. Cancer genome scanning in plasma: Detection of tumor-associated copy number aberrations, single-nucleotide variants, and tumoral heterogeneity by massively parallel sequencing. Clin. Chem. 2013, 59, 211–224. [Google Scholar] [CrossRef]
- Heitzer, E.; Auer, M.; Hoffmann, E.M.; Pichler, M.; Gasch, C.; Ulz, P.; Lax, S.; Waldispuehl-Geigl, J.; Mauermann, O.; Mohan, S.; et al. Establishment of tumor-specific copy number alterations from plasma DNA of patients with cancer. Int. J. Cancer 2013, 133, 346–356. [Google Scholar] [CrossRef]
- Heitzer, E.; Ulz, P.; Belic, J.; Gutschi, S.; Quehenberger, F.; Fischereder, K.; Benezeder, T.; Auer, M.; Pischler, C.; Mannweiler, S.; et al. Tumor-associated copy number changes in the circulation of patients with prostate cancer identified through whole-genome sequencing. Genome Med. 2013, 5, 30. [Google Scholar] [CrossRef]
- Heidary, M.; Auer, M.; Ulz, P.; Heitzer, E.; Petru, E.; Gasch, C.; Riethdorf, S.; Mauermann, O.; Lafer, I.; Pristauz, G.; et al. The dynamic range of circulating tumor DNA in metastatic breast cancer. Breast Cancer Res. 2014, 16, 421. [Google Scholar] [CrossRef]
- Mohan, S.; Heitzer, E.; Ulz, P.; Lafer, I.; Lax, S.; Auer, M.; Pichler, M.; Gerger, A.; Eisner, F.; Hoefler, G.; et al. Changes in colorectal carcinoma genomes under anti-egfr therapy identified by whole-genome plasma DNA sequencing. PLoS Genet. 2014, 10, e1004271. [Google Scholar] [CrossRef]
- Belic, J.; Graf, R.; Bauernhofer, T.; Cherkas, Y.; Ulz, P.; Waldispuehl-Geigl, J.; Perakis, S.; Gormley, M.; Patel, J.; Li, W.; et al. Genomic alterations in plasma DNA from patients with metastasized prostate cancer receiving abiraterone or enzalutamide. Int. J. Cancer 2018, 143, 1236–1248. [Google Scholar] [CrossRef]
- Ulz, P.; Belic, J.; Graf, R.; Auer, M.; Lafer, I.; Fischereder, K.; Webersinke, G.; Pummer, K.; Augustin, H.; Pichler, M.; et al. Whole-genome plasma sequencing reveals focal amplifications as a driving force in metastatic prostate cancer. Nat. Commun. 2016, 7, 12008. [Google Scholar] [CrossRef]
- Adalsteinsson, V.A.; Ha, G.; Freeman, S.S.; Choudhury, A.D.; Stover, D.G.; Parsons, H.A.; Gydush, G.; Reed, S.C.; Rotem, D.; Rhoades, J.; et al. Scalable whole-exome sequencing of cell-free DNA reveals high concordance with metastatic tumors. Nat. Commun. 2017, 8, 1324. [Google Scholar] [CrossRef]
- Belic, J.; Koch, M.; Ulz, P.; Auer, M.; Gerhalter, T.; Mohan, S.; Fischereder, K.; Petru, E.; Bauernhofer, T.; Geigl, J.B.; et al. Rapid identification of plasma DNA samples with increased ctdna levels by a modified fast-seqs approach. Clin. Chem. 2015, 61, 838–849. [Google Scholar] [CrossRef]
- Stover, D.G.; Parsons, H.A.; Ha, G.; Freeman, S.S.; Barry, W.T.; Guo, H.; Choudhury, A.D.; Gydush, G.; Reed, S.C.; Rhoades, J.; et al. Association of cell-free DNA tumor fraction and somatic copy number alterations with survival in metastatic triple-negative breast cancer. J. Clin. Oncol. 2018, 36, 543–553. [Google Scholar] [CrossRef]
- Annala, M.; Vandekerkhove, G.; Khalaf, D.; Taavitsainen, S.; Beja, K.; Warner, E.W.; Sunderland, K.; Kollmannsberger, C.; Eigl, B.J.; Finch, D.; et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 2018, 8, 444–457. [Google Scholar] [CrossRef]
- Lee, R.J.; Gremel, G.; Marshall, A.; Myers, K.A.; Fisher, N.; Dunn, J.A.; Dhomen, N.; Corrie, P.G.; Middleton, M.R.; Lorigan, P.; et al. Circulating tumor DNA predicts survival in patients with resected high-risk stage ii/iii melanoma. Ann. Oncol. 2018, 29, 490–496. [Google Scholar] [CrossRef]
- Tie, J.; Kinde, I.; Wang, Y.; Wong, H.L.; Roebert, J.; Christie, M.; Tacey, M.; Wong, R.; Singh, M.; Karapetis, C.S.; et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann. Oncol. 2015, 26, 1715–1722. [Google Scholar] [CrossRef]
- Yates, L.R.; Knappskog, S.; Wedge, D.; Farmery, J.H.R.; Gonzalez, S.; Martincorena, I.; Alexandrov, L.B.; Van Loo, P.; Haugland, H.K.; Lilleng, P.K.; et al. Genomic evolution of breast cancer metastasis and relapse. Cancer Cell 2017, 32, 169–184.e7. [Google Scholar] [CrossRef]
- de Wit, S.; Rossi, E.; Weber, S.; Tamminga, M.; Manicone, M.; Swennenhuis, J.F.; Groothuis-Oudshoorn, C.G.M.; Vidotto, R.; Facchinetti, A.; Zeune, L.L.; et al. Single tube liquid biopsy for advanced non-small cell lung cancer. Int. J. Cancer 2019, 144, 3127–3137. [Google Scholar] [CrossRef]
- Dawson, S.J.; Tsui, D.W.; Murtaza, M.; Biggs, H.; Rueda, O.M.; Chin, S.F.; Dunning, M.J.; Gale, D.; Forshew, T.; Mahler-Araujo, B.; et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N. Engl. J. Med. 2013, 368, 1199–1209. [Google Scholar] [CrossRef]
- De Mattos-Arruda, L.; Weigelt, B.; Cortes, J.; Won, H.H.; Ng, C.K.; Nuciforo, P.; Bidard, F.C.; Aura, C.; Saura, C.; Peg, V.; et al. Capturing intra-tumor genetic heterogeneity by de novo mutation profiling of circulating cell-free tumor DNA: A proof-of-principle. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1729–1735. [Google Scholar] [CrossRef]
- Douville, C.; Springer, S.; Kinde, I.; Cohen, J.D.; Hruban, R.H.; Lennon, A.M.; Papadopoulos, N.; Kinzler, K.W.; Vogelstein, B.; Karchin, R. Detection of aneuploidy in patients with cancer through amplification of long interspersed nucleotide elements (lines). Proc. Natl. Acad. Sci. USA 2018, 115, 1871–1876. [Google Scholar] [CrossRef]
- Lin, H.K.; Zheng, S.; Williams, A.J.; Balic, M.; Groshen, S.; Scher, H.I.; Fleisher, M.; Stadler, W.; Datar, R.H.; Tai, Y.C.; et al. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin. Cancer Res. 2010, 16, 5011–5018. [Google Scholar] [CrossRef]
- Zheng, S.; Lin, H.; Liu, J.Q.; Balic, M.; Datar, R.; Cote, R.J.; Tai, Y.C. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J. Chromatogr. A 2007, 1162, 154–161. [Google Scholar] [CrossRef]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
| Category | Number | % |
|---|---|---|
| Total | 29 | |
| Age at the time of sampling (years) | ||
| Median and range | 56 (50–68) | |
| Menopausal status | ||
| Premenopausal | 15 | 51.7 |
| Postmenopausal | 14 | 48.3 |
| Histologic type | ||
| IDC/NST | 25 | 86.2 |
| ILC | 2 | 6.9 |
| Mixed type | 1 | 3.4 |
| Other | 1 | 3.4 |
| Tumor grading (at primary diagnosis) | ||
| G1 | 0 | 0 |
| G2 | 12 | 41.4 |
| G3 | 16 | 55.2 |
| Unknown | 1 | 3.4 |
| Tumor size (primary tumor) | ||
| pT1 | 10 | 34.5 |
| pT2 | 9 | 31 |
| pT3/pT4 | 4 | 13.8 |
| Unknown | 6 | 20.7 |
| Lymph node status | ||
| N0 | 10 | 34.5 |
| N1–3 | 12 | 41.4 |
| Unknown | 7 | 24.1 |
| ER status | ||
| Negative | 5 | 17.2 |
| Positive | 24 | 82.8 |
| PR status | ||
| Negative | 9 | 31 |
| Positive | 20 | 69 |
| Her2 status | ||
| Negative | 21 | 72.4 |
| Positive | 8 | 27.6 |
| Subtype (primary tumor) | ||
| HR+/Her2− | 18 | 62.1 |
| HR−/Her2− | 3 | 10.3 |
| Her2+ | 8 | 27.6 |
| Bone metastases | ||
| No | 9 | 31 |
| Yes | 20 | 69 |
| Lung metastases | ||
| No | 16 | 55.2 |
| Yes | 13 | 44.8 |
| Liver metastases | ||
| No | 19 | 65.5 |
| Yes | 10 | 34.5 |
| Other metastases | ||
| No | 7 | 24.1 |
| Yes | 22 | 75.9 |
| Number of metastatic sites | ||
| One | 9 | 31 |
| Multiple | 20 | 69 |
| Number of previous therapy lines for metastatic disease | ||
| 0 | 18 | 62.1 |
| 1 | 4 | 13.8 |
| ≥2 | 7 | 24.1 |
| OS status | ||
| Alive | 19 | 65.5 |
| Dead | 10 | 34.5 |
| PFS status | ||
| No progress | 6 | 20.7 |
| Progress | 23 | 79.3 |
| CTC ≥ 1 | ||
| No | 12 | 41.4 |
| Yes | 17 | 58.6 |
| CTC ≥ 5 | ||
| No | 21 | 72.4 |
| Yes | 8 | 27.6 |
| Z-score ≥ 3 | ||
| No | 18 | 65.5 |
| Yes | 10 | 34.5 |
| CTC (≥1) or z-score (≥3) positive | ||
| No | 10 | 34.5 |
| Yes | 19 | 65.5 |
| All Samples (n = 127) | z-Score | CTC Count | CEA | CA15-3 |
|---|---|---|---|---|
| z-score | - | 0.02 (n = 97) | 0.26 (n = 112) | 0.25 (n = 112) |
| CTC count | 0.02 (n = 97) | - | 0.01 (n = 84) | 0.30 (n = 84) |
| CEA | 0.26 (n = 112) | 0.01 (n = 84) | - | 0.41 (n = 111) |
| CA15-3 | 0.25 (n = 112) | 0.30 (n = 84) | 0.41 (n = 111) | - |
| Variable | z-Score < 3 | z-Score > = 3 | p Value | CTC <5 | CTC > = 5 | p Value |
|---|---|---|---|---|---|---|
| Menopausal status | 1.000 | 0.215 | ||||
| Premenopausal | 10 | 5 | 9 | 6 | ||
| Postmenopausal | 9 | 5 | 12 | 2 | ||
| Histology | 0.592 | 0.304 | ||||
| IDC/NST | 17 | 8 | 17 | 8 | ||
| others | 2 | 2 | 4 | 0 | ||
| Tumor grading | 0.714 | 0.697 | ||||
| Grade 1/2 | 8 | 5 | 10 | 3 | ||
| Grade 3 | 11 | 5 | 11 | 5 | ||
| Tumor size (pT) # | 0.252 | 0.121 | ||||
| pT1 | 7 | 3 | 9 | 1 | ||
| pT2 | 3 | 6 | 5 | 4 | ||
| pT3/4 | 3 | 1 | 4 | 0 | ||
| Lymph nodes (pN) * | 1.000 | 1.000 | ||||
| pN0 | 5 | 5 | 8 | 2 | ||
| pN1-3 | 7 | 5 | 9 | 3 | ||
| ER status | 0.306 | 0.597 | ||||
| Negative | 2 | 3 | 3 | 2 | ||
| Positive | 17 | 7 | 18 | 6 | ||
| PR status | 1.000 | 1.000 | ||||
| Negative | 6 | 3 | 7 | 2 | ||
| Positive | 13 | 7 | 14 | 6 | ||
| HER2 status | 0.675 | 1.000 | ||||
| Negative | 13 | 8 | 15 | 6 | ||
| Positive | 6 | 2 | 6 | 2 | ||
| Subtype (primary tumor) | 0.065 | 1.000 | ||||
| HR+/Her2− | 13 | 5 | 13 | 5 | ||
| HR−/Her2− | 0 | 3 | 2 | 1 | ||
| Her2+ | 6 | 2 | 6 | 2 | ||
| Bone metastases | 1.000 | 0.371 | ||||
| No | 6 | 3 | 8 | 1 | ||
| Yes | 13 | 7 | 13 | 7 | ||
| Lung metastases | 0.270 | 0.406 | ||||
| No | 12 | 4 | 13 | 3 | ||
| Yes | 7 | 6 | 8 | 5 | ||
| Liver metastases | 0.244 | 0.001 | ||||
| No | 14 | 5 | 18 | 1 | ||
| Yes | 5 | 5 | 3 | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suppan, C.; Brcic, I.; Tiran, V.; Mueller, H.D.; Posch, F.; Auer, M.; Ercan, E.; Ulz, P.; Cote, R.J.; Datar, R.H.; et al. Untargeted Assessment of Tumor Fractions in Plasma for Monitoring and Prognostication from Metastatic Breast Cancer Patients Undergoing Systemic Treatment. Cancers 2019, 11, 1171. https://doi.org/10.3390/cancers11081171
Suppan C, Brcic I, Tiran V, Mueller HD, Posch F, Auer M, Ercan E, Ulz P, Cote RJ, Datar RH, et al. Untargeted Assessment of Tumor Fractions in Plasma for Monitoring and Prognostication from Metastatic Breast Cancer Patients Undergoing Systemic Treatment. Cancers. 2019; 11(8):1171. https://doi.org/10.3390/cancers11081171
Chicago/Turabian StyleSuppan, Christoph, Iva Brcic, Verena Tiran, Hannah D Mueller, Florian Posch, Martina Auer, Erkan Ercan, Peter Ulz, Richard J Cote, Ram H Datar, and et al. 2019. "Untargeted Assessment of Tumor Fractions in Plasma for Monitoring and Prognostication from Metastatic Breast Cancer Patients Undergoing Systemic Treatment" Cancers 11, no. 8: 1171. https://doi.org/10.3390/cancers11081171
APA StyleSuppan, C., Brcic, I., Tiran, V., Mueller, H. D., Posch, F., Auer, M., Ercan, E., Ulz, P., Cote, R. J., Datar, R. H., Dandachi, N., Heitzer, E., & Balic, M. (2019). Untargeted Assessment of Tumor Fractions in Plasma for Monitoring and Prognostication from Metastatic Breast Cancer Patients Undergoing Systemic Treatment. Cancers, 11(8), 1171. https://doi.org/10.3390/cancers11081171






