Long-Term Dynamics of Three Dimensional Telomere Profiles in Circulating Tumor Cells in High-Risk Prostate Cancer Patients Undergoing Androgen-Deprivation and Radiation Therapy
Abstract
1. Introduction
2. Results
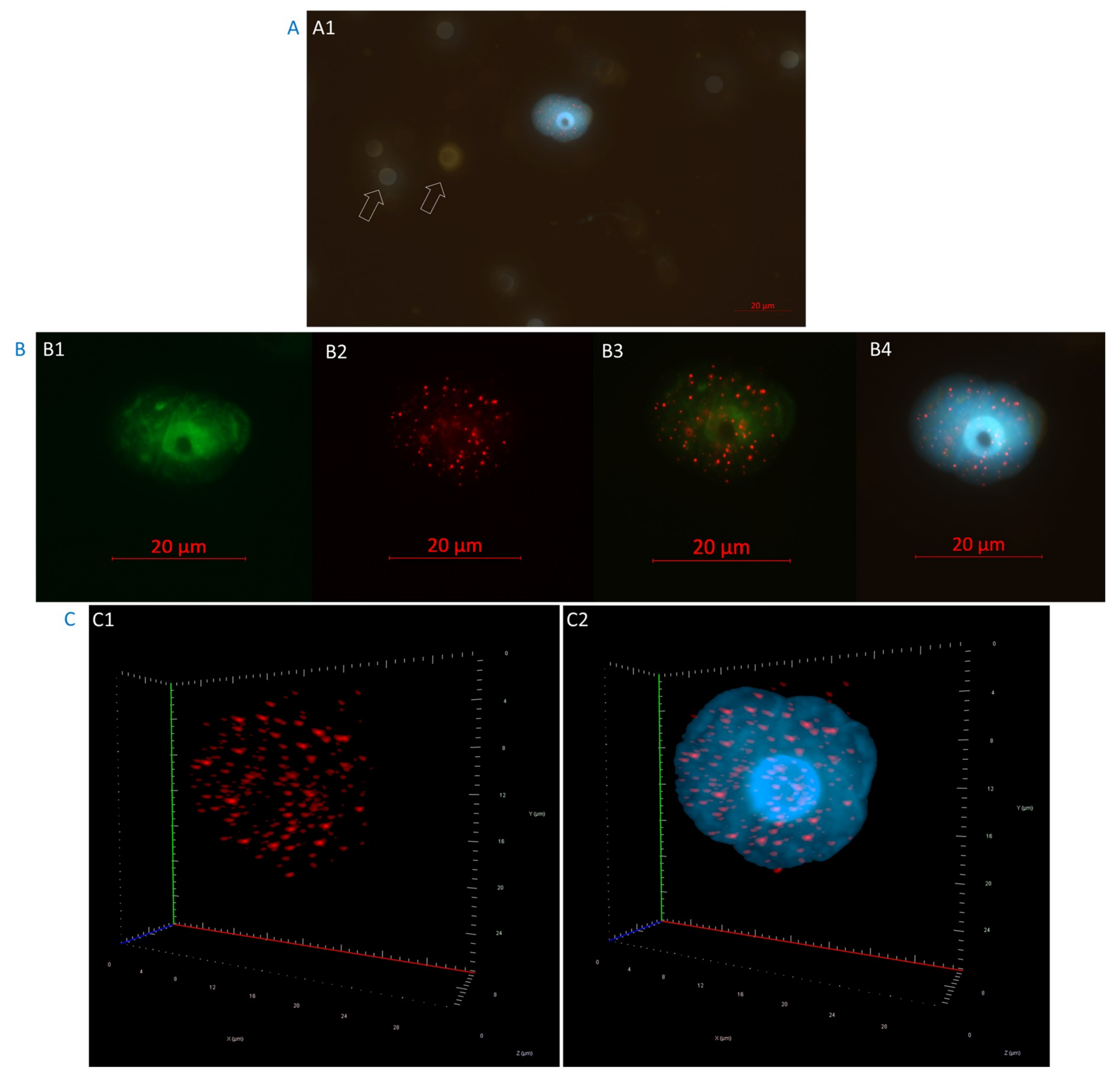
2.1. High-Risk Prostate Cancer CTCs were Selected Based on Their Androgen Receptor Staining
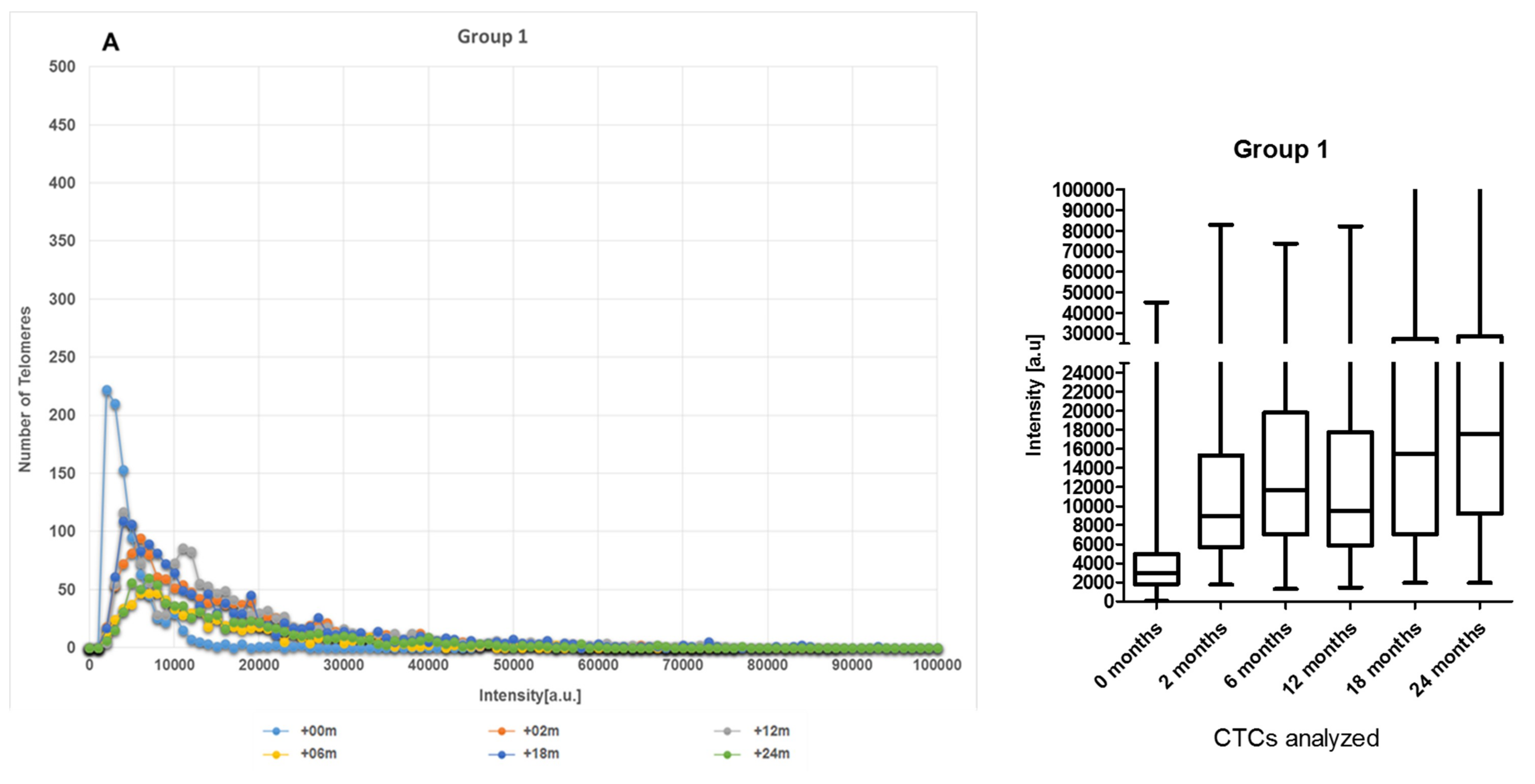
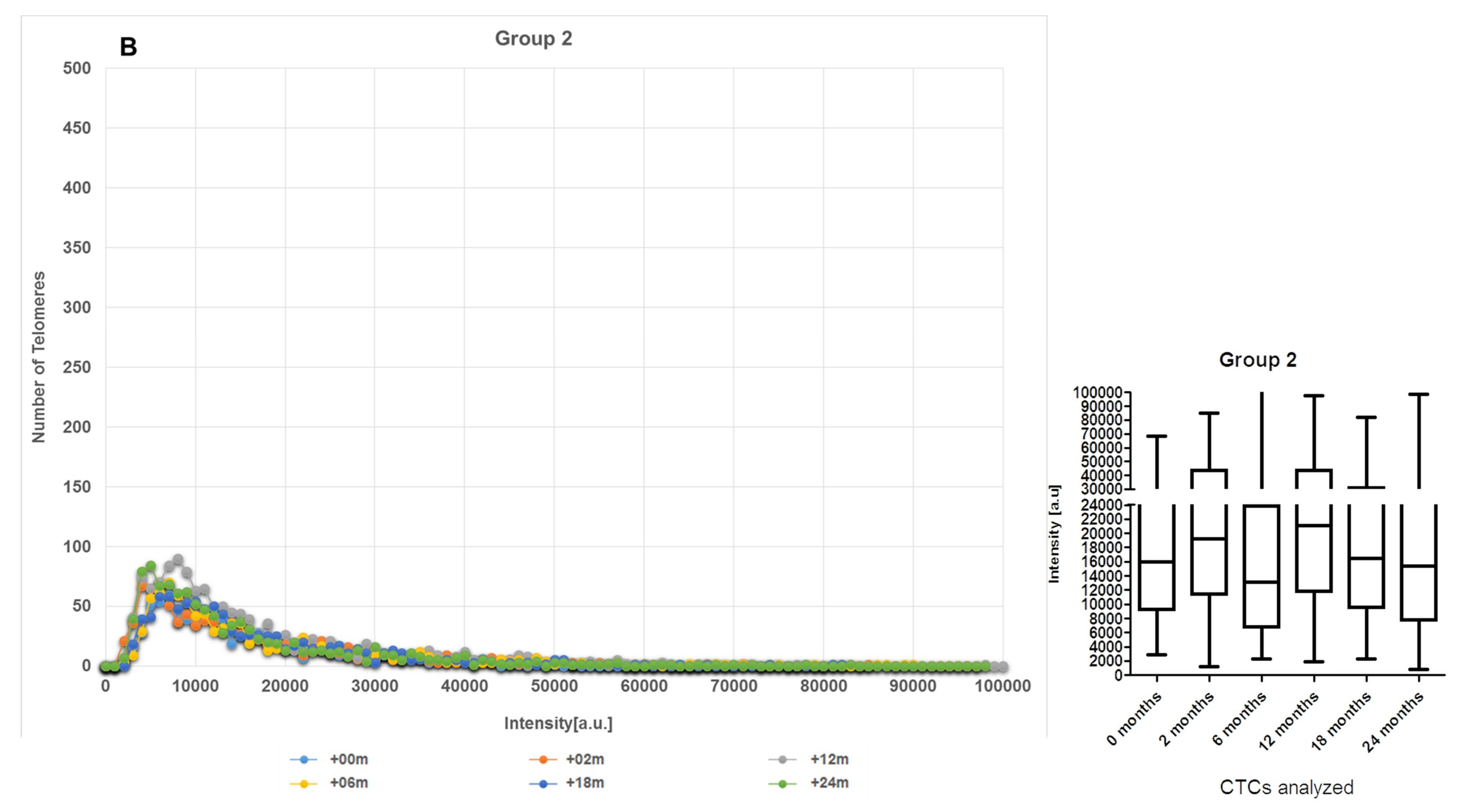
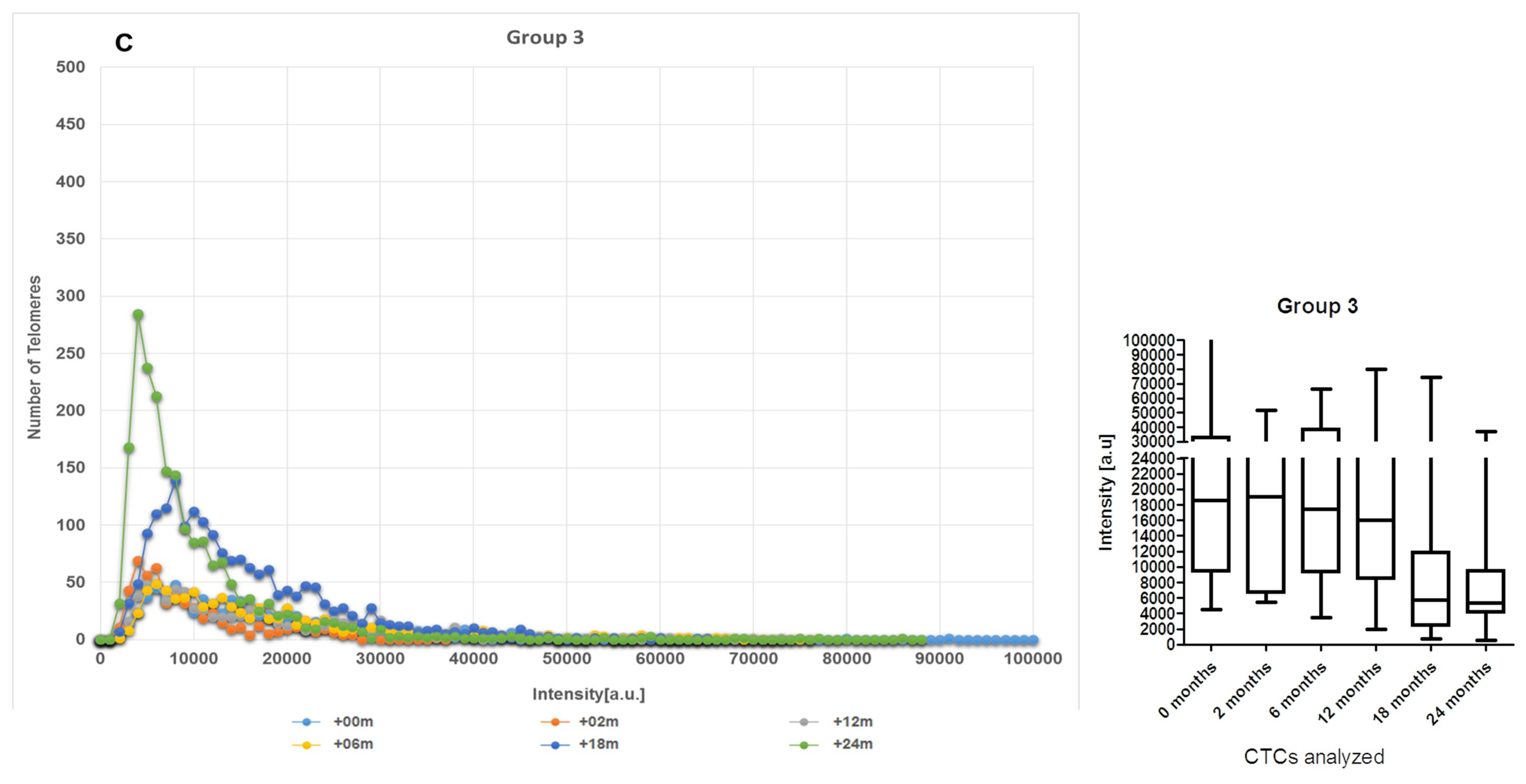
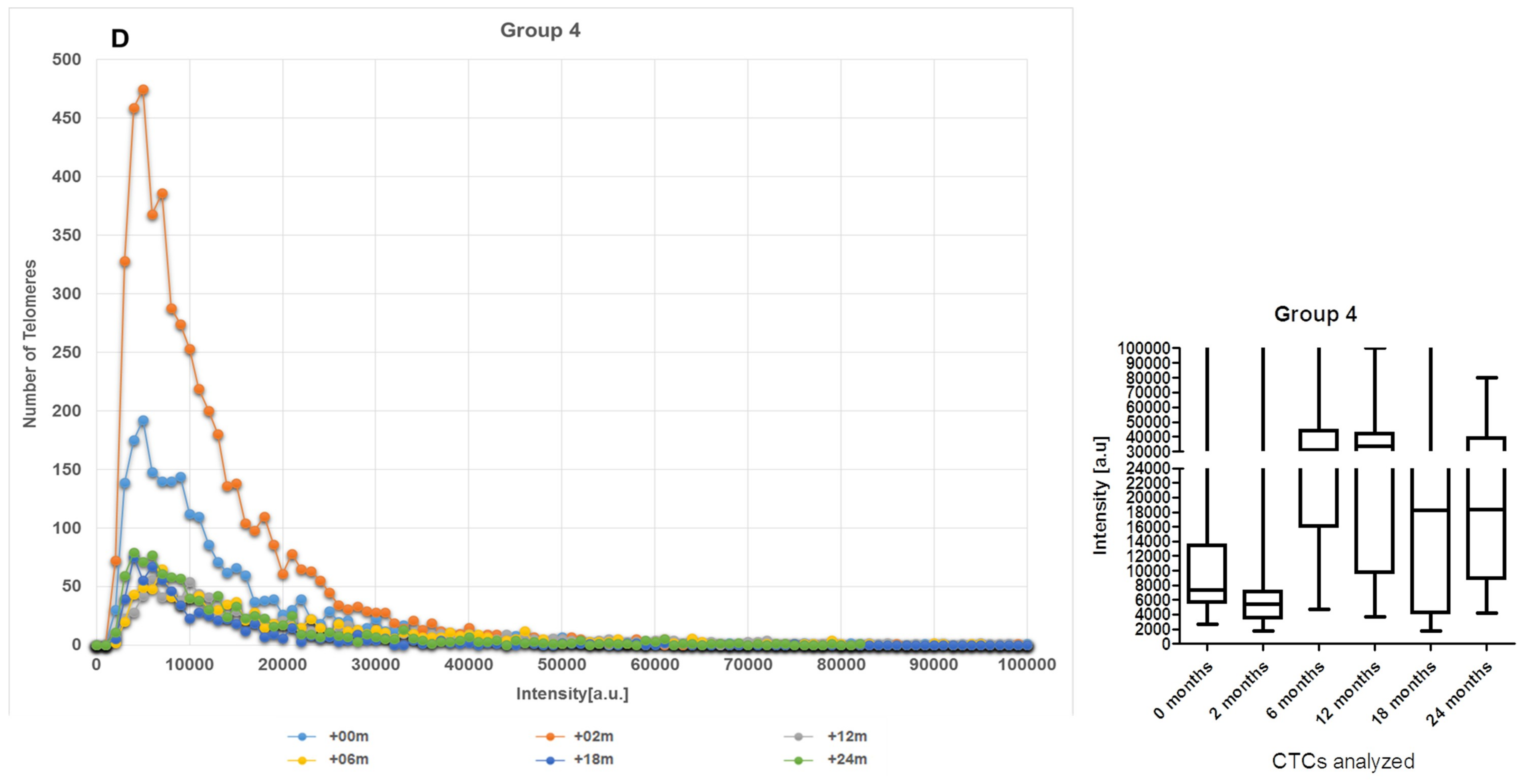
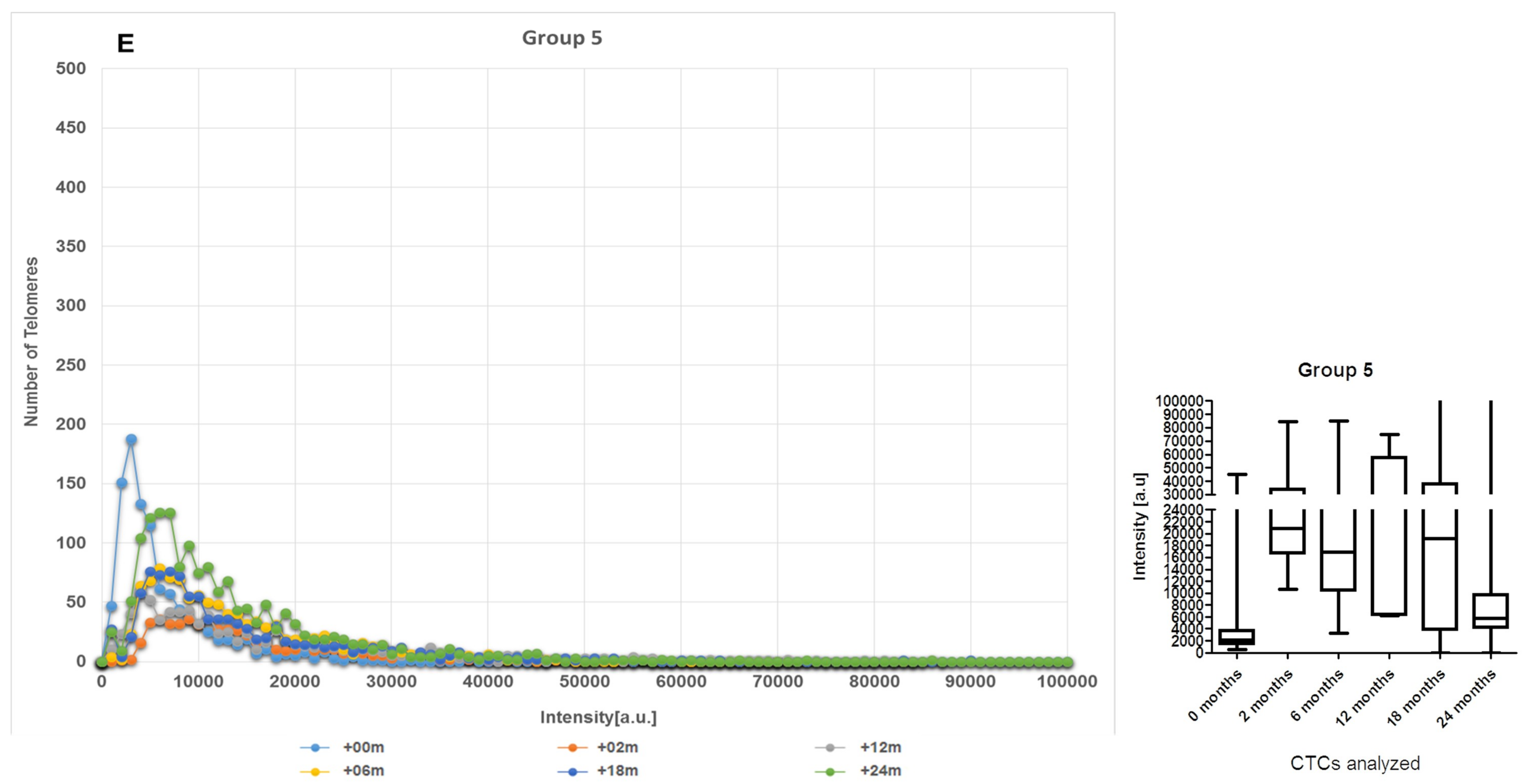
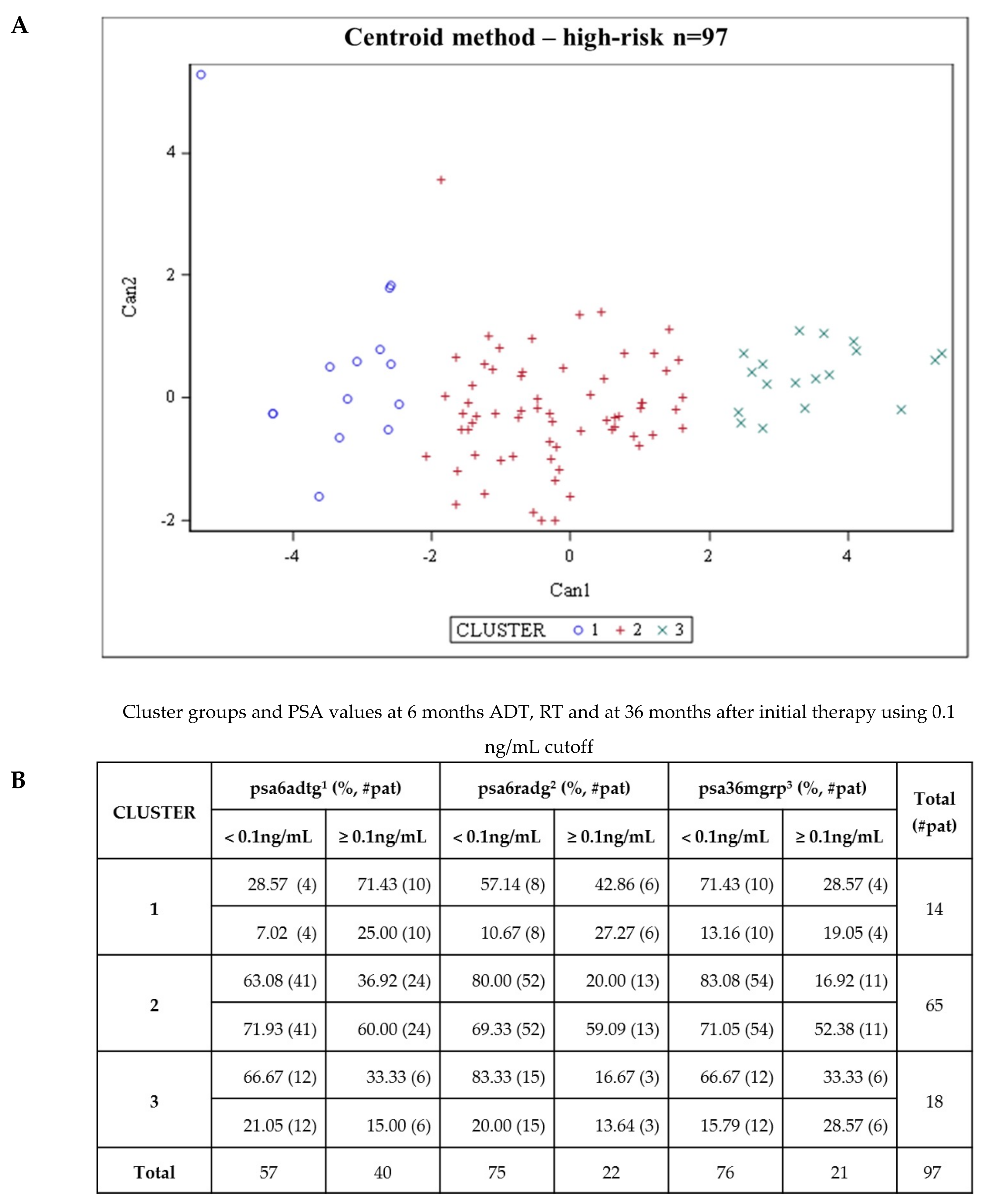
2.2. CTCs Dynamics of the 3D Telomere Architecture Stratified Patients into 5 Distinct Subgroups
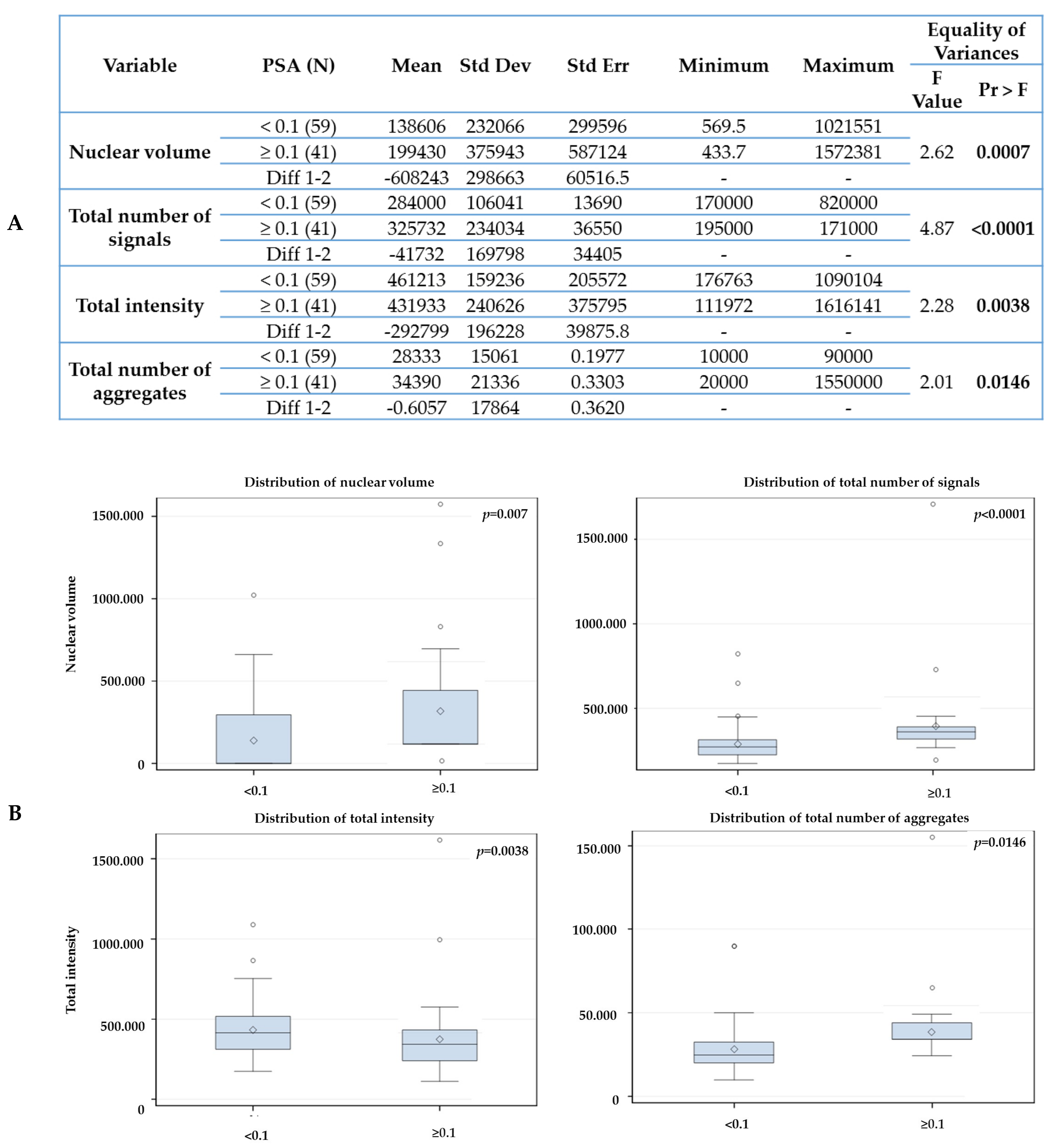
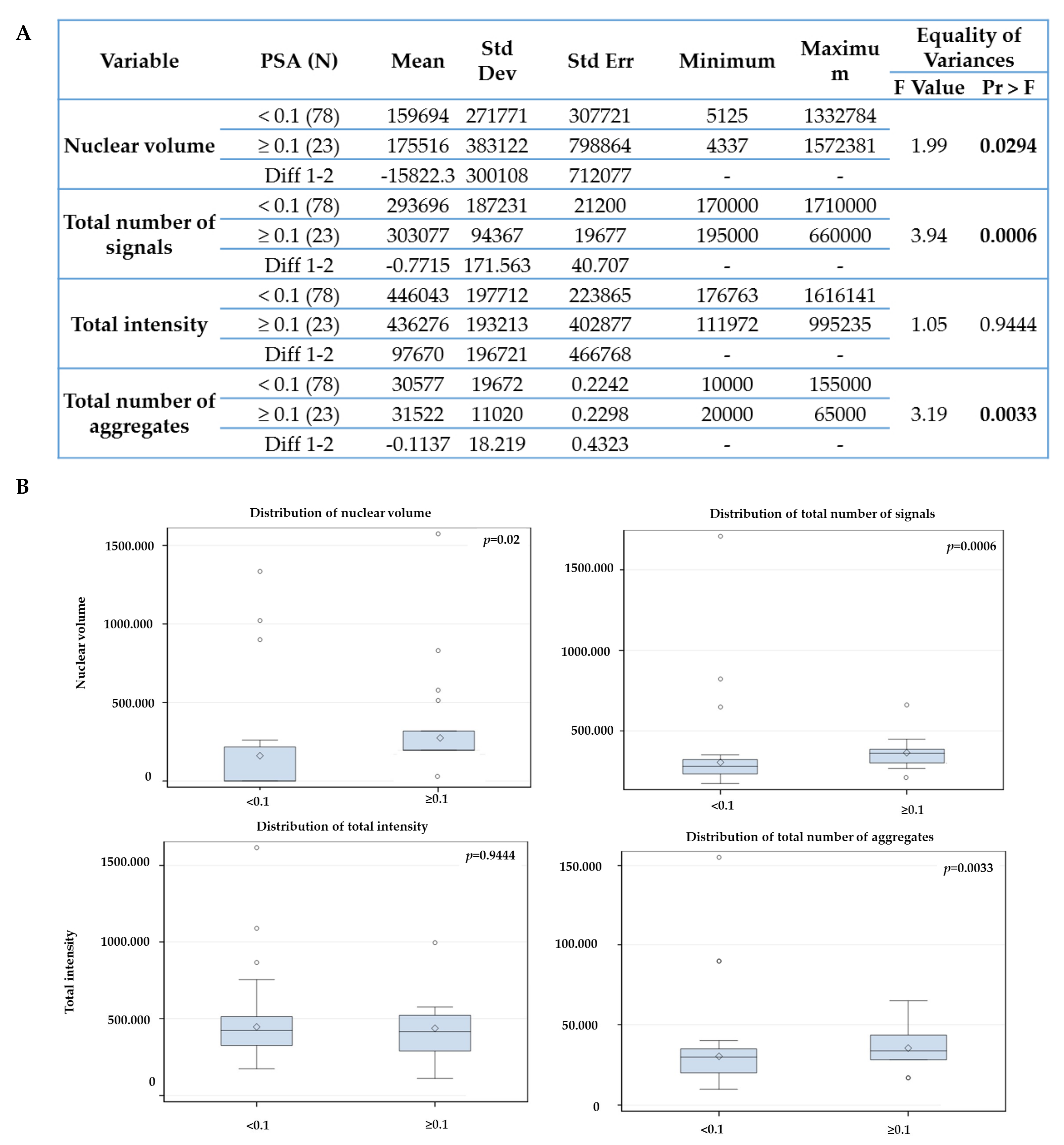
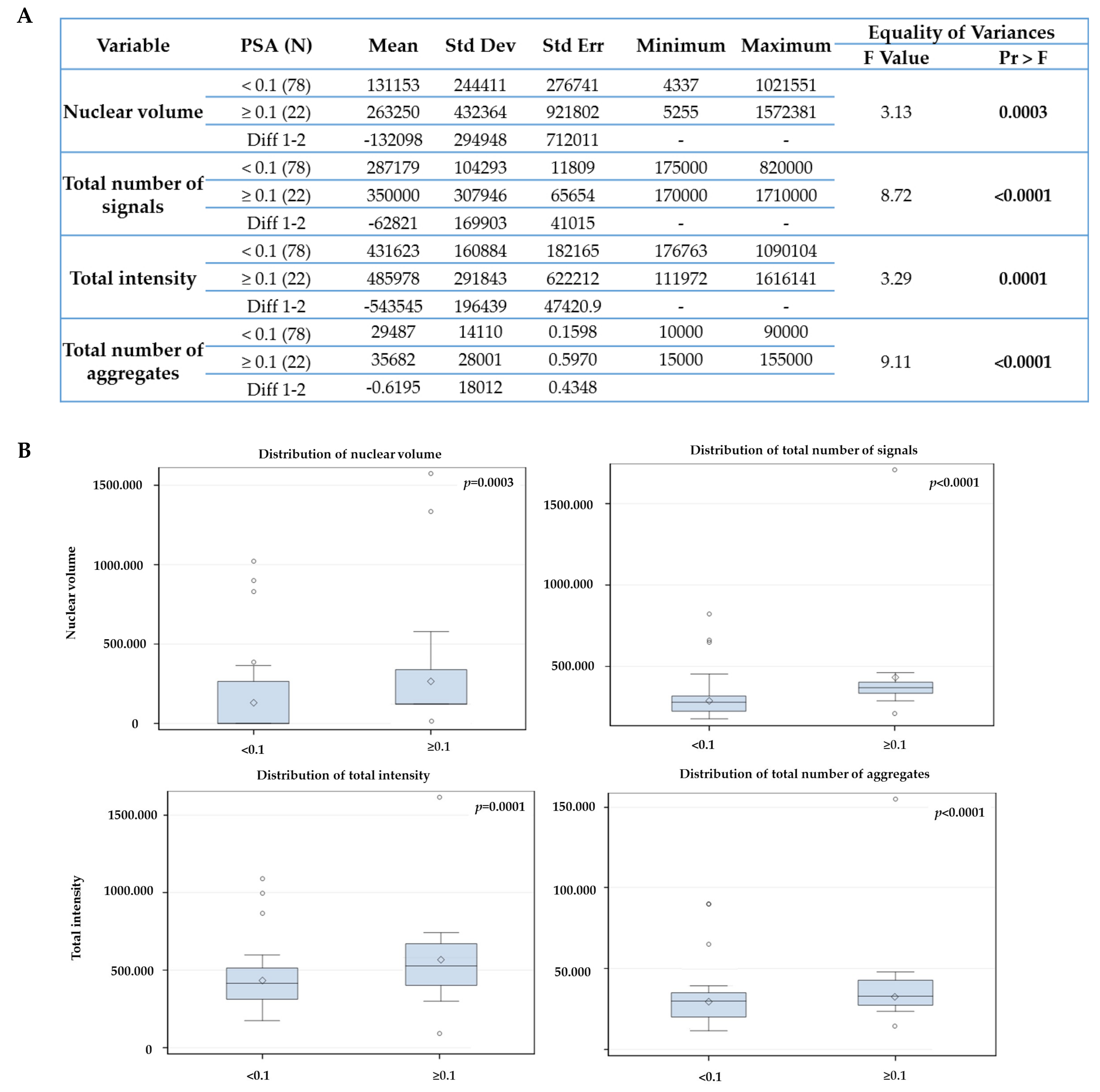
2.3. The Telomere Parameters Can Predict PSA Increase at 6 Months of ADT, RT and at 36 Months after Initial Therapy
3. Discussion
4. Materials and Methods
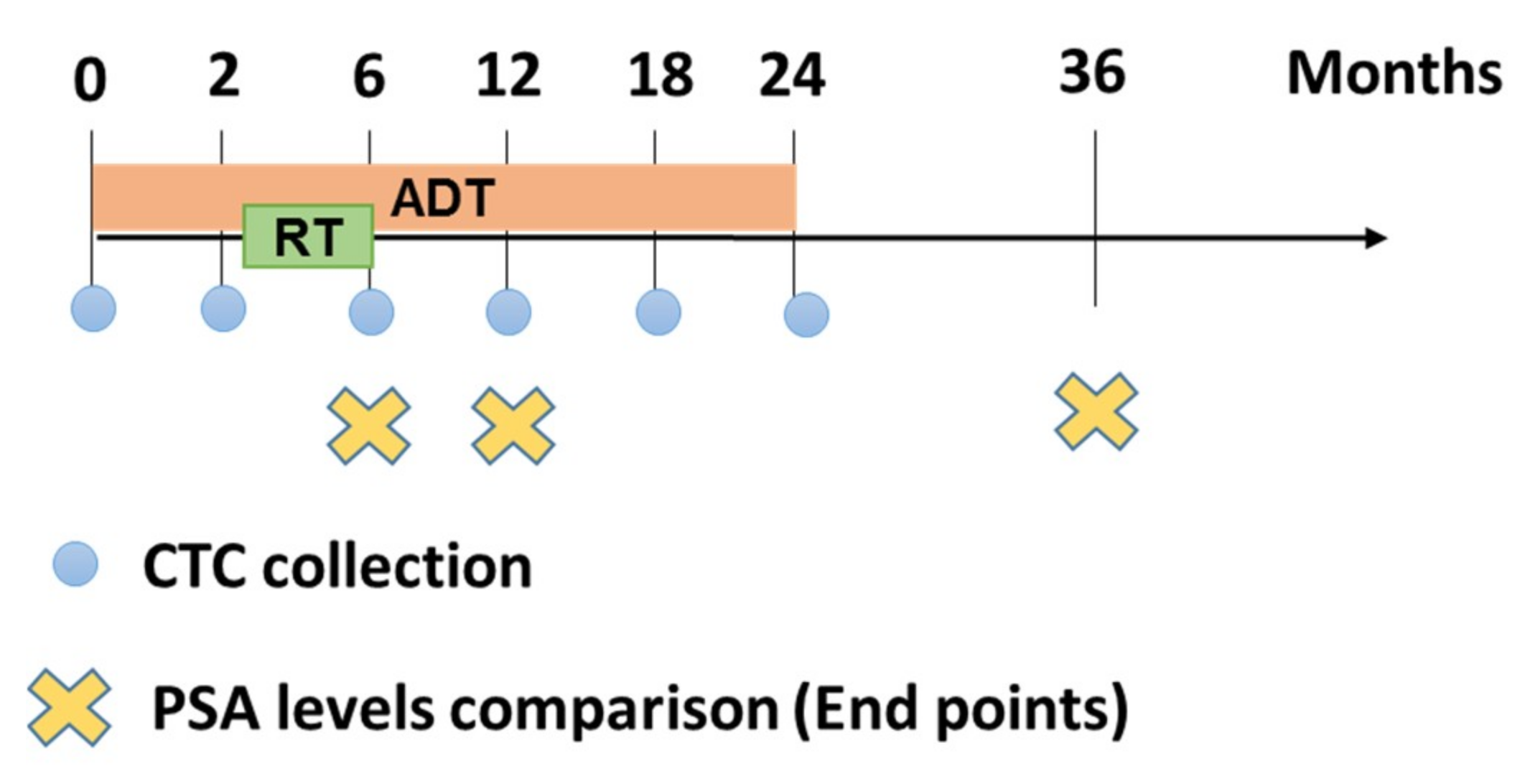
4.1. Patient Samples, Treatment and Study Design
4.2. CTC Isolation and Androgen Receptor (AR) Staining
4.3. Telomere Three-dimensional Quantitative Fluorescent in situ Hybridization (3D-QFISH)
4.4. Imaging & Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohler, J.L.; Armstrong, A.J.; Bahnson, R.R.; D’Amico, A.V.; Davis, B.J.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; Horwitz, E.M.; et al. Prostate cancer, version 1. 2016. J. Natl. Compr. Cancer Netw. 2016, 14, 19–30. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Baskin-Bey, E.S.; Watson, M.; Worsfold, A.; Rider, A.; Tombal, B. Treatment patterns and characteristics of European patients with castration-resistant prostate cancer. BMC Urol. 2013, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Merseburger, A.S.; Alcaraz, A.; von Klot, C.A. Androgen deprivation therapy as backbone therapy in the management of prostate cancer. Onco Targets Ther. 2016, 9, 7263–7274. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.L.; Martin, N.E.; Choeurng, V.; Palmer-Aronsten, B.; Kolisnik, T.; Beard, C.J.; Orio, P.F.; Nezolosky, M.D.; Chen, Y.W.; Shin, H.; et al. Utilization of biopsy-based genomic classifier to predict distant metastasis after definitive radiation and short-course ADT for intermediate and high-risk prostate cancer. Prostate Cancer Prostatic Dis. 2017, 20, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Roach, M.; Hanks, G.; Thames, H.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. 2006, 65, 965–974. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.V.; Chen, M.-H.; De Castro, M.; Loffredo, M.; Lamb, D.S.; Steigler, A.; Kantoff, P.W.; Denham, J.W. Surrogate endpoints for prostate cancer-specific mortality after radiotherapy and androgen suppression therapy in men with localised or locally advanced prostate cancer: An analysis of two randomised trials. Lancet Oncol. 2012, 13, 189–195. [Google Scholar] [CrossRef]
- De Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.; Pienta, K.J.; Raghavan, D. Circulating Tumor Cells Predict Survival Benefit from Treatment in Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef]
- Scher, H.I.; Jia, X.; de Bono, J.S.; Fleisher, M.; Pienta, K.J.; Raghavan, D.; Heller, G. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: A reanalysis of IMMC38 trial data. Lancet Oncol. 2009, 10, 233–239. [Google Scholar] [CrossRef]
- Olmos, D.; Arkenau, H.T.; Ang, J.E.; Ledaki, I.; Attard, G.; Carden, C.P.; Reid, A.H.; A’Hern, R.; Fong, P.C.; Oomen, N.B.; et al. Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): A single-centre experience. Ann. Oncol. 2009, 20, 27–33. [Google Scholar] [CrossRef]
- Danila, D.C.; Heller, G.; Gignac, G.A.; Gonzalez-Espinoza, R.; Anand, A.; Tanaka, E.; Lilja, H.; Schwartz, L.; Larson, S.; Fleisher, M.; et al. Circulating Tumor Cell Number and Prognosis in Progressive Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2007, 13, 7053–7058. [Google Scholar] [CrossRef]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Saylor, P.J.; Michaelson, M.D.; Rothenberg, S.M.; Smas, M.E.; Miyamoto, D.T.; Gurski, C.A.; Xie, W.; Maheswaran, S.; Haber, D.A.; et al. A dose-ranging study of cabozantinib in men with castration-resistant prostate cancer and bone metastases. Clin. Cancer Res. 2013, 19, 3088–3094. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-F.; Ho, H.; Lichterman, J.; Lu, Y.-T.; Zhang, Y.; Garcia, M.A.; Chen, S.-F.; Liang, A.-J.; Hodara, E.; Zhau, H.E.; et al. Sub-classification of prostate cancer circulating tumor cells (CTCs) by nuclear size reveals very-small nuclear CTCs in patients with visceral metastases. Cancer 2015, 121, 3240–3251. [Google Scholar] [CrossRef] [PubMed]
- Dorff, T.B.; Groshen, S.; Tsao-Wei, D.D.; Xiong, S.; Gross, M.E.; Vogelzang, N.; Quinn, D.I.; Pinski, J.K. A Phase II Trial of a Combination Herbal Supplement for Men with Biochemically Recurrent Prostate Cancer. Prostate Cancer Prostatic Dis. 2014, 17, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Budna-Tukan, J.; Świerczewska, M.; Mazel, M.; Cieślikowski, W.A.; Ida, A.; Jankowiak, A.; Antczak, A.; Nowicki, M.; Pantel, K.; Azria, D.; et al. Analysis of Circulating Tumor Cells in Patients with Non-Metastatic High-Risk Prostate Cancer before and after Radiotherapy Using Three Different Enumeration Assays. Cancers (Basel) 2019, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Zu, Y. Detecting circulating tumor cells: Current challenges and new trends. Theranostics 2013, 3, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yin, B.; Wang, X.; Yu, P.; Duan, X.; Liu, C.; Wang, B.; Tao, Z. Circulating tumor cells in prostate cancer: Precision diagnosis and therapy. Oncol. Lett. 2017, 14, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Awe, J.A.; Saranchuk, J.; Drachenberg, D.; Mai, S. Filtration-based enrichment of circulating tumor cells from all prostate cancer risk groups. Urol. Oncol. 2017, 35, 300–309. [Google Scholar] [CrossRef]
- Wark, L.; Klonisch, T.; Awe, J.; LeClerc, C.; Dyck, B.; Quon, H.; Mai, S. Dynamics of three-dimensional telomere profiles of circulating tumor cells in patients with high-risk prostate cancer who are undergoing androgen deprivation and radiation therapies. Urol. Oncol. 2017, 35, 112.e1–112.e11. [Google Scholar] [CrossRef]
- Graham, M.K.; Meeker, A. Telomeres and telomerase in prostate cancer development and therapy. Nat. Rev. Urol. 2017, 14, 607–619. [Google Scholar] [CrossRef]
- Heaphy, C.M.; Gaonkar, G.; Peskoe, S.B.; Joshu, C.E.; De Marzo, A.M.; Lucia, M.S.; Goodman, P.J.; Lippman, S.M.; Thompson, I.M.; Platz, E.A.; et al. Prostate stromal cell telomere shortening is associated with risk of prostate cancer in the placebo arm of the Prostate Cancer Prevention Trial. Prostate 2015, 75, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Richardson, M.; Reddy, V.; Menon, M.; Barrack, E.R.; Reddy, G.P.; Kim, S.H. Structural and functional association of androgen receptor with telomeres in prostate cancer cells. Aging (Albany NY) 2013, 5, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Richardson, M.; Chinnakannu, K.; Bai, V.U.; Menon, M.; Barrack, E.R.; Reddy, G.P. Androgen receptor interacts with telomeric proteins in prostate cancer cells. J. Biol. Chem. 2010, 285, 10472–10476. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.S.; Yeap, B.B.; Hoermann, R.; Hui, J.; Beilby, J.P.; Grossmann, M. Effects of androgen deprivation therapy on telomere length. Clin. Endocrinol. 2017, 87, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Zelefsky, M.J.; Lyass, O.; Fuks, Z.; Wolfe, T.; Burman, C.; Ling, C.C.; Leibel, S.A. Predictors of improved outcome for patients with localized prostate cancer treated with neoadjuvant androgen ablation therapy and three-dimensional conformal radiotherapy. J. Clin. Oncol. 1998, 16, 3380–3385. [Google Scholar] [CrossRef] [PubMed]
- Lamb, D.S.; Denham, J.W.; Joseph, D.; Matthews, J.; Atkinson, C.; Spry, N.A.; Duchesne, G.; Ebert, M.; Steigler, A.; Delahunt, B.; et al. A Comparison of the Prognostic Value of Early PSA Test-Based Variables Following External Beam Radiotherapy, With or Without Preceding Androgen Deprivation: Analysis of Data From the TROG 96.01 Randomized Trial. Int. J. Radiat. Oncol. 2011, 79, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Benchikh, E.; Fegoun, A.; Villers, A.; Moreau, J.L.; Richaud, P.; Rebillard, X.; Beuzeboc, P. PSA and follow-up after treatment of prostate cancer. Prog. Urol. 2008, 18, 137–144. [Google Scholar] [CrossRef] [PubMed]
- DeSitter, I.; Guerrouahen, B.S.; Benali-Furet, N.; Wechsler, J.; Jänne, P.A.; Kuang, Y.; Yanagita, M.; Wang, L.; Berkowitz, J.A.; Distel, R.J.; et al. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res. 2011, 31, 427–441. [Google Scholar]
- Miyamoto, D.T.; Lee, R.J.; Stott, S.L.; Ting, D.T.; Wittner, B.S.; Ulman, M.; Smas, M.E.; Lord, J.B.; Brannigan, B.W.; Trautwein, J.; et al. Androgen receptor signaling in circulating tumor cells as a marker of hormon- ally responsive prostate cancer. Cancer Discov. 2012, 2, 995–1003. [Google Scholar] [CrossRef]
- Meeker, A.K.; Hicks, J.L.; Iacobuzio-Donahue, C.A.; Montgomery, E.A.; Westra, W.H.; Chan, T.Y.; Ronnett, B.M.; De Marzo, A.M. Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin. Cancer Res. 2004, 10, 3317–3326. [Google Scholar] [CrossRef]
- Schaefer, L.H.; Schuster, D.; Herz, H. Generalized approach for accelerated maximum likelihood based image restoration applied to three-dimensional fluorescence microscopy. J. Microsc. 2001, 204, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Hugosson, J.; Carlsson, S.; Aus, G.; Bergdahl, S.; Khatami, A.; Lodding, P.; Pihl, C.G.; Stranne, J.; Holmberg, E.; Liljia, H. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010, 11, 725–732. [Google Scholar] [CrossRef]
- Schröder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.; Ciatto, S.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Lilja, H.; Zappa, M.; et al. Screening and Prostate-Cancer Mortality in a Randomized European Study. N. Engl. J. Med. 2009, 360, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Andriole, G.L.; Crawford, E.D.; Grubb, R.L.; Buys, S.S.; Chia, D.; Church, T.R.; Fouad, M.N.; Gelmann, E.P.; Kvale, P.A.; Reding, D.J.; et al. Mortality Results from a Randomized Prostate-Cancer Screening Trial. N. Engl. J. Med. 2009, 360, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Stamey, T.A.; McNeal, J.E.; Yemoto, C.M.; Sigal, B.M.; Johnstone, I.M. Biological Determinants of Cancer Progression in Men With Prostate Cancer. JAMA 1999, 281, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Vermolen, B.J.; Garini, Y.; Mai, S.; Mougey, V.; Fest, T.; Chuang, A.Y.-C.; Wark, L.; Young, I.T.; Chuang, T.C.-Y.; Chuang, T.C. Characterizing the three-dimensional organization of telomeres. Cytom. Part A 2005, 67, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Kolostova, K.; Zhang, Y.; Hoffman, R.M.; Bobek, V. In Vitro Culture and Characterization of Human Lung Cancer Circulating Tumor Cells Isolated by Size Exclusion from an Orthotopic Nude-Mouse Model Expressing Fluorescent Protein. J. Fluoresc. 2014, 24, 1531–1536. [Google Scholar] [CrossRef]
- Chen, C.L.; Mahalingam, D.; Osmulski, P.; Jadhav, R.R.; Wang, C.M.; Leach, R.J.; Chang, T.C.; Weitman, S.D.; Kumar, A.P.; Sun, L.; et al. Single cell analysis of circulating tumor cells identifies cumulative expression patterns of EMT-related genes in metastatic prostate cancer. Prostate 2013, 73, 813–826. [Google Scholar] [CrossRef]
- Raimondi, C.; Nicolazzo, C.; Gradilone, A. Circulating tumor cells isolation: The “post-EpCAM era”. Chin. J. Cancer Res. 2015, 27, 461–470. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Functional studies on viable circulating tumor cells. Clin. Chem. 2016, 62, 328–334. [Google Scholar] [CrossRef]
- Paris, P.L.; Kobayashi, Y.; Zhao, Q.; Zeng, W.; Sridharan, S.; Fan, T.; Adler, H.L.; Yera, E.R.; Zarrabi, M.; Zucker, S.; et al. Functional phenotyping and genotyping of circulating tumor cells from patients with castration resistant prostate cancer. Cancer Lett. 2009, 277, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gregory, S.G.; Garcia-Blanco, M.A.; Armstrong, A.J. Using circulating tumor cells to inform on prostate cancer biology and clinical utility. Crit. Rev. Clin. Lab. Sci. 2015, 52, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Helzer, K.T.; Barnes, H.E.; Day, L.; Harvey, J.; Billings, P.R.; Forsyth, A. Circulating Tumor Cells Are Transcriptionally Similar to the Primary Tumor in a Murine Prostate Model. Cancer Res. 2009, 69, 7860–7866. [Google Scholar] [CrossRef] [PubMed]
- Thalgott, M.; Rack, B.; Maurer, T.; Souvatzoglou, M.; Eiber, M.; Kreß, V.; Heck, M.M.; Andergassen, U.; Nawroth, R.; Gschwend, J.E.; et al. Detection of circulating tumor cells in different stages of prostate cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.W.; Nakanishi, H.; Kumar, V.S.; Bhadkamkar, V.A.; McCormack, R.; Fritsche, H.A.; Handy, B.; Gornet, T.; Babaian, R.J. Circulating Tumor Cells in Peripheral Blood Samples From Patients With Increased Serum Prostate Specific Antigen: Initial Results in Early Prostate Cancer. J. Urol. 2008, 179, 2187–2191. [Google Scholar] [CrossRef] [PubMed]
- Okegawa, T.; Ninomiya, N.; Masuda, K.; Nakamura, Y.; Tambo, M.; Nutahara, K. AR-V7 in circulating tumor cells cluster as a predictive biomarker of abiraterone acetate and enzalutamide treatment in castration-resistant prostate cancer patients. Prostate 2018, 78, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Gadji, M.; Adebayo, J.; Rodrigues, P.; Kumar, R.; Houston, D.S.; Klewes, L.; Dièye, T.N.; Rego, E.M.; Passetto, R.F.; de Oliveira, F.M.; et al. Profiling threedimensional nuclear telomeric architecture of myelodysplastic syndromes and acute myeloid leukemia defines patient subgroups. Clin. Cancer Res. 2012, 18, 3293–3304. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Pozzo, A.; De Souza, D.C.; Schmid-Braz, A.T.; De Azambuja, A.P.; Ferraz-Aguiar, T.; Borgonovo, T.; Mai, S. 3D Telomere Structure Analysis to Detect Genomic Instability and Cytogenetic Evolution in Myelodysplastic Syndromes. Cells 2019, 8, 304. [Google Scholar] [CrossRef]
- Caria, P.; Dettori, T.; Frau, D.V.; Lichtenzstejn, D.; Pani, F.; Vanni, R.; Mai, S. Characterizing the three-dimensional organization of telomeres in papillary thyroid carcinoma cells. J. Cell Physiol. 2019, 234, 5175–5185. [Google Scholar] [CrossRef]
- Williams, S. Surrogate endpoints in early prostate cancer research. Transl. Androl. Urol. 2018, 7, 472–482. [Google Scholar] [CrossRef]
- Bryant, A.K.; D’Amico, A.V.; Nguyen, P.L.; Einck, J.P.; Kane, C.J.; McKay, R.R.; Simpson, D.R.; Mundt, A.J.; Murphy, J.D.; Rose, B.S. Three-month posttreatment prostate-specific antigen level as a biomarker of treatment response in patients with intermediate-risk or high-risk prostate cancer treated with androgen deprivation therapy and radiotherapy. Cancer 2018, 124, 2939–2947. [Google Scholar] [CrossRef] [PubMed]
- Schröder, F.H.; Carter, H.B.; Wolters, T.; Bergh, R.C.V.D.; Gosselaar, C.; Bangma, C.H.; Roobol, M.J. Early Detection of Prostate Cancer in 2007. Eur. Urol. 2008, 53, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Hultdin, M.; Grönlund, E.; Norrback, K.; Eriksson-Lindström, E.; Just, T.; Roos, G. Telomere analysis by fluorescence in situ hybridization and flow cytometry. Nucleic Acids Res. 1998, 26, 3651–3656. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wark, L.; Quon, H.; Ong, A.; Drachenberg, D.; Rangel-Pozzo, A.; Mai, S. Long-Term Dynamics of Three Dimensional Telomere Profiles in Circulating Tumor Cells in High-Risk Prostate Cancer Patients Undergoing Androgen-Deprivation and Radiation Therapy. Cancers 2019, 11, 1165. https://doi.org/10.3390/cancers11081165
Wark L, Quon H, Ong A, Drachenberg D, Rangel-Pozzo A, Mai S. Long-Term Dynamics of Three Dimensional Telomere Profiles in Circulating Tumor Cells in High-Risk Prostate Cancer Patients Undergoing Androgen-Deprivation and Radiation Therapy. Cancers. 2019; 11(8):1165. https://doi.org/10.3390/cancers11081165
Chicago/Turabian StyleWark, Landon, Harvey Quon, Aldrich Ong, Darrel Drachenberg, Aline Rangel-Pozzo, and Sabine Mai. 2019. "Long-Term Dynamics of Three Dimensional Telomere Profiles in Circulating Tumor Cells in High-Risk Prostate Cancer Patients Undergoing Androgen-Deprivation and Radiation Therapy" Cancers 11, no. 8: 1165. https://doi.org/10.3390/cancers11081165
APA StyleWark, L., Quon, H., Ong, A., Drachenberg, D., Rangel-Pozzo, A., & Mai, S. (2019). Long-Term Dynamics of Three Dimensional Telomere Profiles in Circulating Tumor Cells in High-Risk Prostate Cancer Patients Undergoing Androgen-Deprivation and Radiation Therapy. Cancers, 11(8), 1165. https://doi.org/10.3390/cancers11081165




