Type 1 Sodium Calcium Exchanger Forms a Complex with Carbonic Anhydrase IX and Via Reverse Mode Activity Contributes to pH Control in Hypoxic Tumors
Abstract
1. Introduction
2. Results
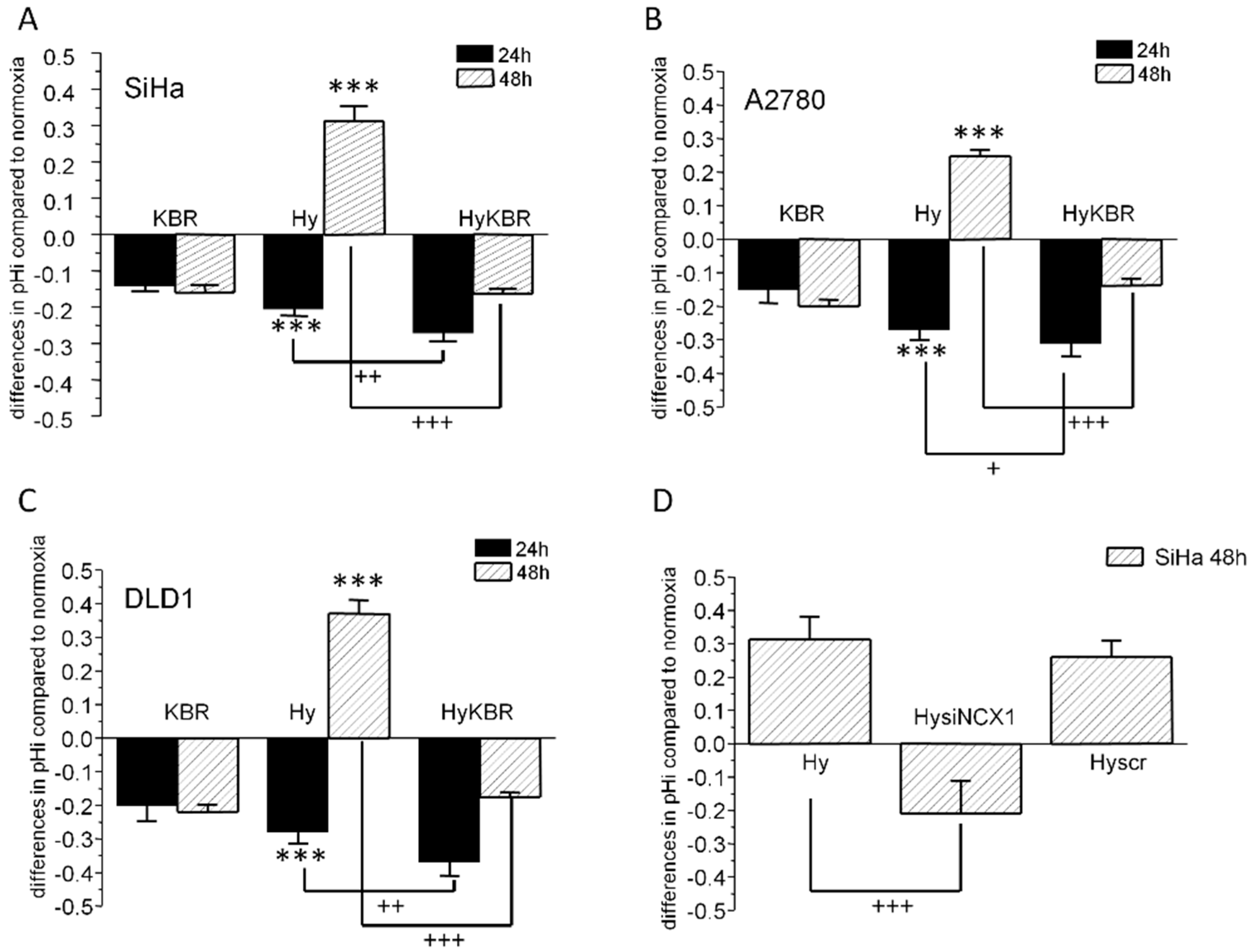
2.1. Changes in Intracellular pH in SiHa, DLD1 and A2780 Cells in Response to KB-R7943 Treatment
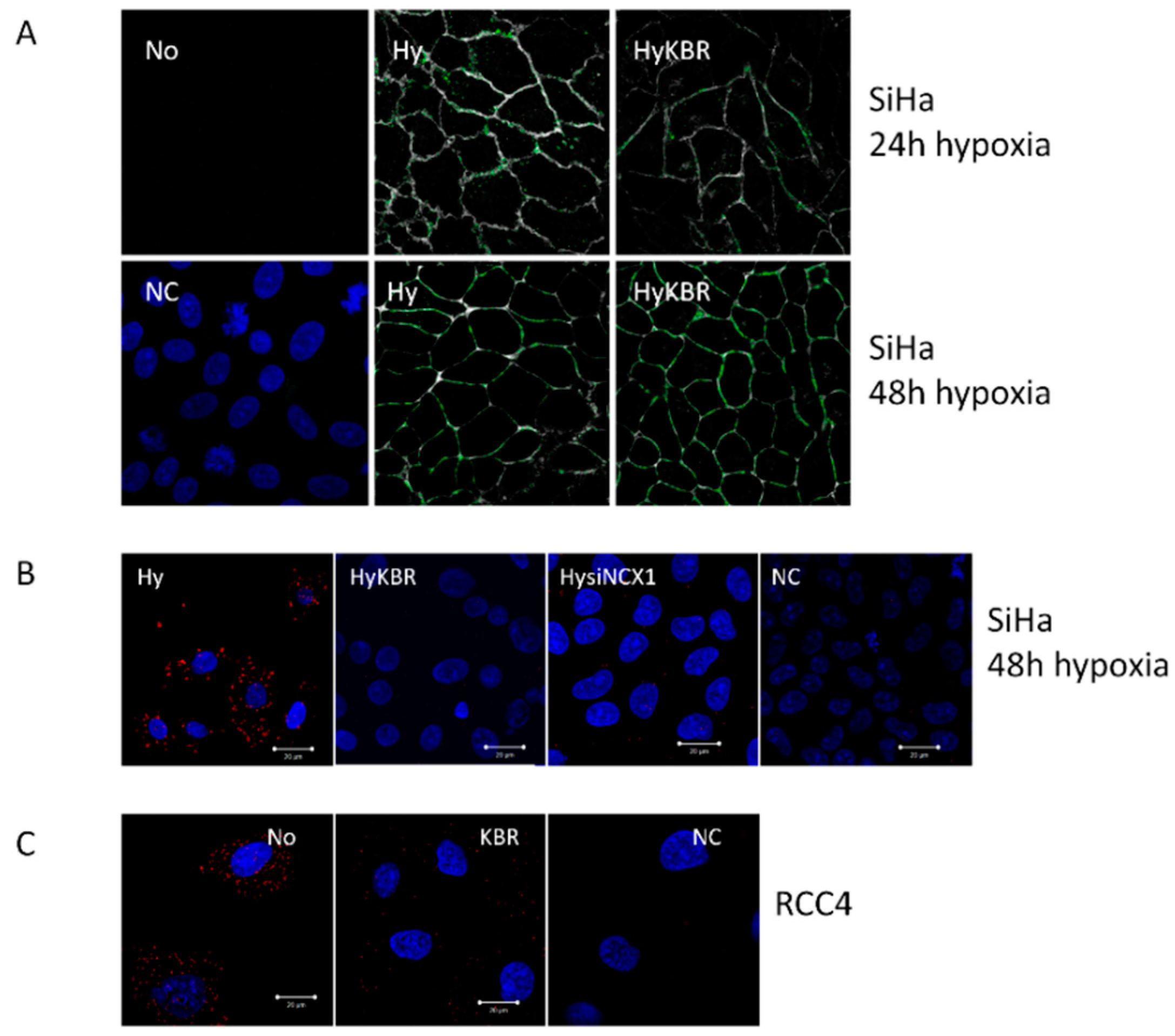
2.2. Co-localization of the NCX1 and CA IX Determined by Proximity Ligation Assay (PLA) in SiHa and RCC4 Cells
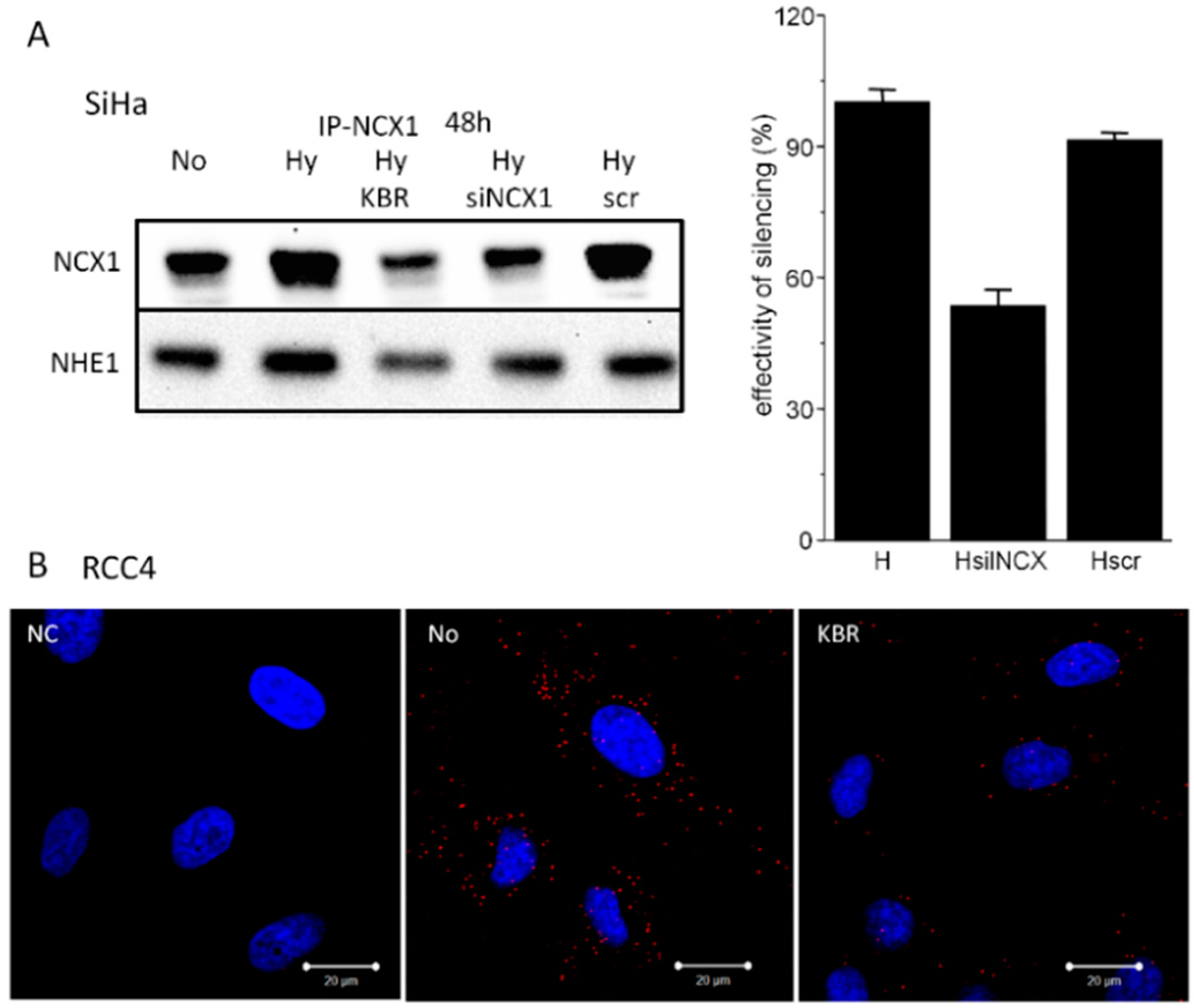
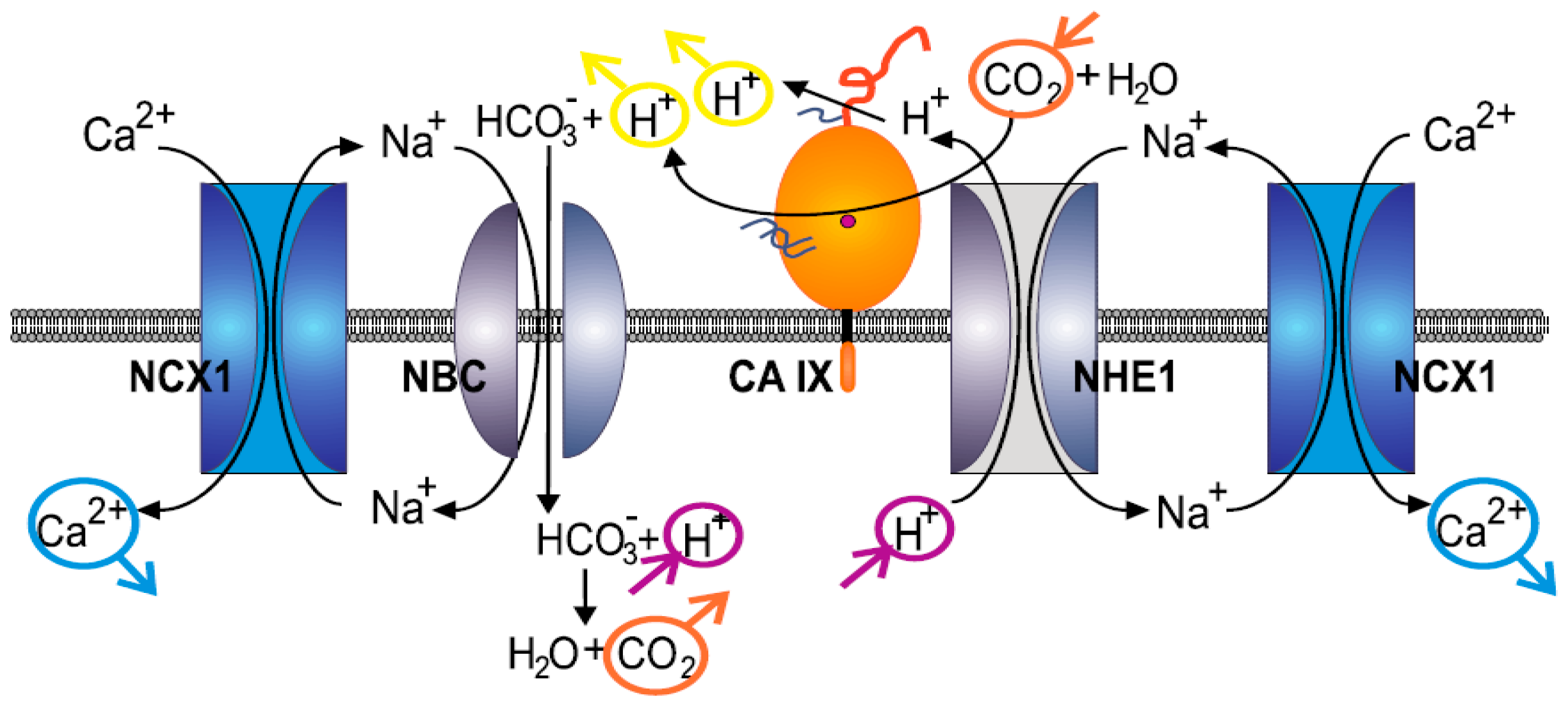
2.3. Formation of NCX1/CA IX/NHE1 Metabolon and Determination of the Cytosolic Ca2+ and Na+ Levels in Hypoxic SiHa, DLD1 and A2780 Cells
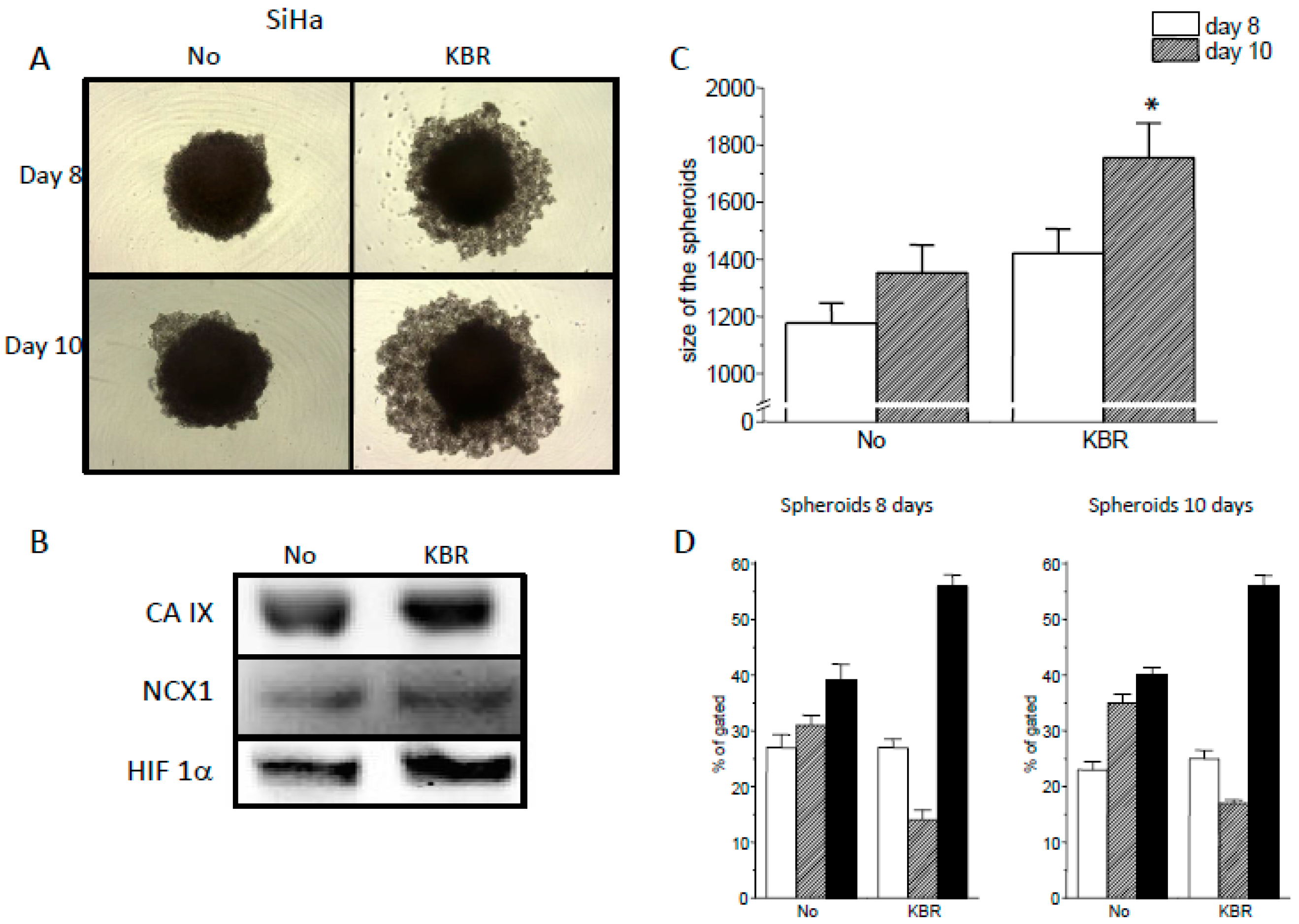
2.4. Role of the NCX1 in Spheroid Formation
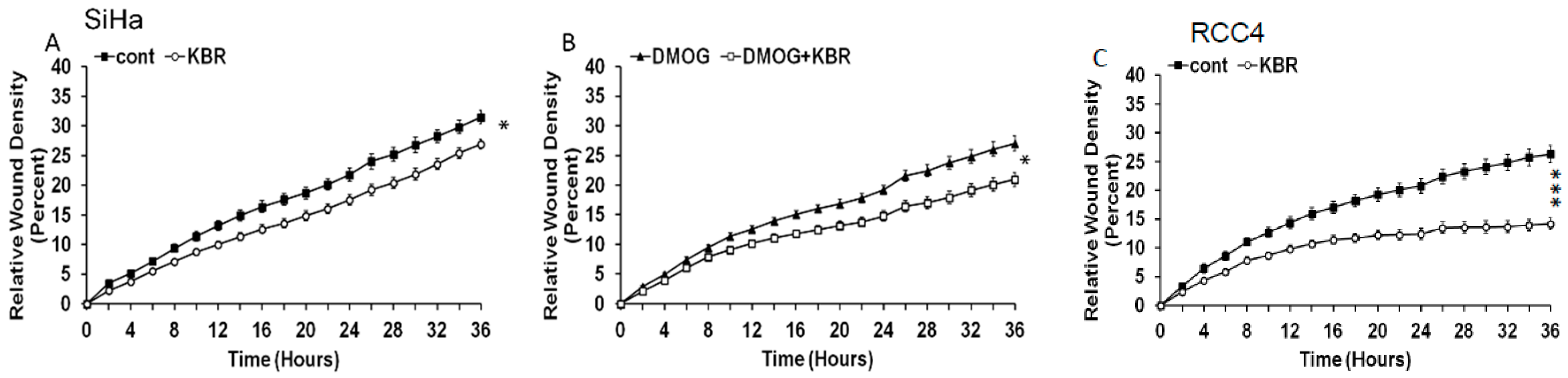
2.5. Effect of the Reverse Mode NCX1 on Cell Migration
3. Discussion
4. Materials and Methods
4.1. Cell Cultivation and Treatment
4.2. Measurement of Intracellular pH
4.3. Immunofluorescence
4.4. Proximity Ligation Assay
4.5. Immunoprecipitation
4.6. Western Blot Analysis
4.7. Cytosolic [Ca2+]i Staining by Fluo-3AM Fluorescent Dye
4.8. Cytosolic [Na+]i Staining by SBFI-AM Fluorescent Dye
4.9. NCX1 Silencing by siRNAs
4.10. Spheroids
4.11. Detection of Apoptosis with Annexin-V-FLUOS
4.12. Cell Migration Assay
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kolosenko, I.; Avnet, S.; Baldini, N.; Viklund, J.; De Milito, A. Therapeutic implications of tumor interstitial acidification. Semin. Cancer Biol. 2017, 14, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T. Cancer: Fundamentals behind pH targeting and the double-edged approach. Onco. Targets Ther. 2016, 9, 6343–6360. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Gillies, R.J. A microenvironmental model of carcinogenesis. Nat. Rev. Cancer 2008, 8, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Gorbatenko, A.; Olesen, C.W.; Boedtkjer, E.; Pedersen, S.F. Regulation and roles of bicarbonate transporters in cancer. Front. Physiol. 2014, 5, 130. [Google Scholar] [CrossRef] [PubMed]
- Pastorek, J.; Pastorekova, S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: From biology to clinical use. Semin. Cancer Biol. 2015, 31, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Sedlakova, O.; Svastova, E.; Takacova, M.; Kopacek, J.; Pastorek, J.; Pastorekova, S. Carbonic anhydrase IX, a hypoxia-induced catalytic component of the pH regulating machinery in tumors. Front. Physiol. 2014, 4, 400. [Google Scholar] [CrossRef] [PubMed]
- Jamali, S.; Klier, M.; Ames, S.; Barros, L.F.; McKenna, R.; Deitmer, J.W.; Becker, H.M. Hypoxia-induced carbonic anhydrase IX facilitates lactate flux in human breast cancer cells by non-catalytic function. Sci. Rep. 2015, 5, 13605. [Google Scholar] [CrossRef] [PubMed]
- Svastova, E.; Witarski, W.; Csaderova, L.; Kosik, I.; Skvarkova, L.; Hulikova, A.; Zatovicova, M.; Barathova, M.; Kopacek, J.; Pastorek, J.; et al. Carbonic anhydrase IX interacts with bicarbonate transporters in lamellipodia and increases cell migration via its catalytic domain. J. Biol. Chem. 2012, 287, 3392–3402. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Damaghi, M.; Wojtkowiak, J.W.; Gillies, R.J. pH sensing and regulation in cancer. Front. Physiol. 2013, 4, 370. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, A.; Hulikova, A.; Ledaki, I.; Snell, C.; Singleton, D.; Steers, G.; Seden, P.; Jones, D.; Bridges, E.; Wigfield, S.; et al. Disrupting Hypoxia-Induced Bicarbonate Transport Acidifies Tumor Cells and Suppresses Tumor Growth. Cancer Res. 2016, 76, 3744–3755. [Google Scholar] [CrossRef] [PubMed]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Reshkin, S.J.; Cardone, R.A.; Harguindey, S. Na+-H+ exchanger, pH regulation and cancer. Recent. Pat. Anticancer Drug Discov. 2012, 8, 85–99. [Google Scholar] [CrossRef]
- Rios, E.J.; Fallon, M.; Wang, J.; Shimoda, L.A. Chronic hypoxia elevates intracellular pH and activates Na+/H+ exchange in pulmonary arterial smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physio. 2005, 289, L867–L874. [Google Scholar] [CrossRef] [PubMed]
- Parks, S.K.; Cormerais, Y.; Durivault, J.; Pouyssegur, J. Genetic disruption of the pHi-regulating proteins Na+/H+ exchanger 1 (SLC9A1) and carbonic anhydrase 9 severely reduces growth of colon cancer cells. Oncotarget 2017, 8, 10225. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Barber, D.L. A calcineurin homologous protein inhibits GTPase-stimulated Na-H exchange. Proc. Natl. Acad. Sci. USA 1996, 93, 12631–12636. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Xu, X.; Zeng, W.; Diaz, J.; Wojcikiewicz, R.J.; Kuo, T.H.; Wuytack, F.; Racymaekers, L.; Muallem, S. Polarized Expression of Ca2+ Channels in Pancreatic and Salivary Gland Cells Correlation with Initiation and Propagation of [Ca2+] i Waves. J. Biol. Chem. 1977, 272, 15765–15770. [Google Scholar] [CrossRef] [PubMed]
- Quednau, B.D.; Nicoll, D.A.; Philipson, K.D. Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am. J. Physiol. 1997, 272, C1250–C1261. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber, J.I.; Philipson, K.D. Cardiac sodium-calcium exchange and efficient excitation-contraction coupling: Implications for heart disease. Adv. Exp. Med. Biol. 2013, 961, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Hudecova, S.; Kubovcakova, L.; Kvetnansky, R.; Kopacek, J.; Pastorekova, S.; Novakova, M.; Knezl, V.; Tarabova, B.; Lacinova, L.; Sulova, Z.; et al. Modulation of expression of Na+/Ca2+ exchanger in heart of rat and mouse under stress. Acta. Physiol. (Oxf.) 2007, 190, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Kurata, Y.; Hisatome, I.; Shibamoto, T. Roles of sarcoplasmic reticulum Ca2+ cycling and Na+/Ca2+ exchanger in sinoatrial node pacemaking: Insights from bifurcation analysis of mathematical models. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2285–H2300. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, V.; Pignataro, G.; Sirabella, R.; Matrone, C.; Boscia, F.; Scorziello, A.; Sisalli, M.J.; Esposito, E.; Zambrano, N.; Cataldi, M.; et al. Transcriptional regulation of ncx1 gene in the brain. Adv. Exp. Med. Biol. 2013, 961, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Staiano, R.I.; Granata, F.; Secondo, A.; Petraroli, A.; Loffredo, S.; Annunziato, L.; Triggiani, M.; Marone, M. Human macrophages and monocytes express functional Na(+)/Ca(2+) exchangers 1 and 3. Adv. Exp. Med. Biol. 2013, 961, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Hudecova, S.; Lencesova, L.; Csaderova, L.; Sedlak, J.; Bohacova, V.; Laukova, M.; Krizanova, O. Isoproterenol accelerates apoptosis through the over-expression of the sodium/calcium exchanger in HeLa cells. Gen. Physiol. Biophys. 2013, 32, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Hudecova, S.; Lencesova, L.; Csaderova, L.; Sirova, M.; Cholujova, D.; Cagala, M.; Kopacek, J.; Dobrota, D.; Pastorekova, S.; Krizanova, O. Chemically mimicked hypoxia modulates gene expression and protein levels of the sodium calcium exchanger in HEK 293 cell line via HIF-1α. Gen. Physiol. Biophys. 2011, 30, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Khananshvili, D. Sodium-calcium exchangers (NCX): Molecular hallmarks underlying the tissue-specific and systemic functions. Pflugers. Arch. 2014, 466, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Roome, C.J.; Power, E.M.; Empson, R.M. Transient reversal of the sodium/calcium exchanger boosts presynaptic calcium and synaptic transmission at a cerebellar synapse. J. Neurophysiol. 2013, 109, 1669–1680. [Google Scholar] [CrossRef]
- Poburko, D.; Fameli, N.; Kuo, K.H.; van Breemen, C. Ca2+ signaling in smooth muscle: TRPC6, NCX and LNats in nanodomains. Channels (Austin) 2008, 2, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ye, L.; Liu, J.; Hong, H. Hypoxia-induced cytosolic calcium influx is mediated primarily by the reverse mode of Na+/Ca2+ exchanger in smooth muscle cells of fetal small pulmonary arteries. J. Obstet. Gynaecol. Res. 2014, 40, 1578–1583. [Google Scholar] [CrossRef]
- Nashida, T.; Takuma, K.; Fukuda, S.; Kawasaki, T.; Takahashi, T.; Baba, A.; Ago, Y.; Matsuda, T. The specific Na(+)/Ca(2+) exchange inhibitor SEA0400 prevents nitric oxide-induced cytotoxicity in SH-SY5Y cells. Neurochem. Int. 2011, 59, 51–58. [Google Scholar] [CrossRef]
- Long, Z.; Chen, B.; Liu, Q.; Zhao, J.; Yang, Z.; Dong, X.; Xia, L.; Huang, S.; Hu, X.; Song, B.; et al. The reverse-mode NCX1 activity inhibitor KB-R7943 promotes prostate cancer cell death by activating the JNK pathway and blocking autophagic flux. Oncotarget 2016, 7, 42059–42070. [Google Scholar] [CrossRef] [PubMed]
- Sennoune, S.R.; Santos, J.M.; Hussain, F.; Martínez-Zaguilán, R. Sodium calcium exchanger operates in the reverse mode in metastatic human melanoma cells. Cell Mol. Biol. (Noisy-le-Grand) 2015, 6, 40–49. [Google Scholar]
- Szadvari, I.; Hudecova, S.; Chovancova, B.; Matuskova, M.; Cholujova, D.; Lencesova, L.; Valerian, D.; Ondrias, K.; Babula, P.; Krizanova, K. Sodium/calcium exchanger is involved in apoptosis induced by H2S in tumor cells through decreased levels of intracellular pH. Nitric Oxide 2019, 87, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, M.; McSheehy, P.M.; Griffiths, J.R.; Bashford, C.L. Causes and consequences of tumour acidity and implications for treatment. Mol. Med. Today. 2000, 6, 15–19. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Beasley, N.; Watson, P.H.; Campo, L.; Chia, S.K.; English, R.; Pastorek, J.; Sly, S.W.; Ratcliffe, P.; Harris, A.L. Expression of the hypoxia-inducible and tumor-associated carbonic anhydrases in ductal carcinoma in situ of the breast. Am. J. Pathol. 2001, 158, 1011–1019. [Google Scholar] [CrossRef]
- Liu, Z.; Zeng, W.; Liu, C.; Wang, S.; Xiong, Y.; Guo, Y.; Li, Y.; Sun, S.; Chen, T.; Maimaiti, Y.; et al. Diagnostic accuracy of ultrasonographic features for lymph node metastasis in papillary thyroid microcarcinoma: A single-center retrospective study. World J. Surg. Oncol. 2017, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Cong, D.; Zhu, W.; Kuo, J.S.; Hu, S.; Sun, D. Ion transporters in brain tumors. Curr. Med. Chem. 2015, 22, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- De Giusti, V.C.; Ciancio, M.C.; Orlowski, A.; Aiello, E.A. Modulation of the cardiac sodium/bicarbonate cotransporter by the renin angiotensin aldosterone system: Pathophysiological consequences. Front. Physiol. 2014, 4, 411. [Google Scholar] [CrossRef][Green Version]
- McDonald, P.C.; Winum, J.Y.; Supuran, C.T.; Dedhar, S. Recent developments in targeting carbonic anhydrase IX for cancer therapeutics. Oncotarget 2012, 3, 84–97. [Google Scholar] [CrossRef]
- Kemény, L.V.; Schnúr, A.; Czepán, M.; Jr Rakonczay, Z.; Gál, E.; Lonovics, J.; Lázár, Y.; Simonka, Z.; Venglovecz, V.; Maléth, J.; et al. Na+/Ca2+ exchangers regulate the migration and proliferation of human gastric myofibroblasts. Am. J. Physiol. Gastrointest Liver Physiol. 2013, 305, G552–G563. [Google Scholar] [CrossRef]
- Noda, M.; Ifuku, M.; Mori, Y.; Verkhratsky, A. Calcium influx through reversed NCX controls migration of microglia. Adv. Exp. Med. Biol. 2013, 961, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Sathish, V.; Delmotte, P.F.; Thompson, M.A.; Pabelick, C.M.; Sieck, G.C.; Prakash, Y.S. Sodium-calcium exchange in intracellular calcium handling of human airway smooth muscle. PLoS ONE 2011, 6, e23662. [Google Scholar] [CrossRef] [PubMed]
- Markova, J.; Hudecova, S.; Soltysova, A.; Sirova, M.; Csaderova, L.; Lencesova, L.; Ondrias, K.; Krizanova, O. Sodium/calcium exchanger is upregulated by sulfide signaling, forms complex with the β1 and β3 but not β2 adrenergic receptors, and induces apoptosis. Pflugers. Arch. 2014, 466, 1329–1342. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liskova, V.; Hudecova, S.; Lencesova, L.; Iuliano, F.; Sirova, M.; Ondrias, K.; Pastorekova, S.; Krizanova, O. Type 1 Sodium Calcium Exchanger Forms a Complex with Carbonic Anhydrase IX and Via Reverse Mode Activity Contributes to pH Control in Hypoxic Tumors. Cancers 2019, 11, 1139. https://doi.org/10.3390/cancers11081139
Liskova V, Hudecova S, Lencesova L, Iuliano F, Sirova M, Ondrias K, Pastorekova S, Krizanova O. Type 1 Sodium Calcium Exchanger Forms a Complex with Carbonic Anhydrase IX and Via Reverse Mode Activity Contributes to pH Control in Hypoxic Tumors. Cancers. 2019; 11(8):1139. https://doi.org/10.3390/cancers11081139
Chicago/Turabian StyleLiskova, Veronika, Sona Hudecova, Lubomira Lencesova, Filippo Iuliano, Marta Sirova, Karol Ondrias, Silvia Pastorekova, and Olga Krizanova. 2019. "Type 1 Sodium Calcium Exchanger Forms a Complex with Carbonic Anhydrase IX and Via Reverse Mode Activity Contributes to pH Control in Hypoxic Tumors" Cancers 11, no. 8: 1139. https://doi.org/10.3390/cancers11081139
APA StyleLiskova, V., Hudecova, S., Lencesova, L., Iuliano, F., Sirova, M., Ondrias, K., Pastorekova, S., & Krizanova, O. (2019). Type 1 Sodium Calcium Exchanger Forms a Complex with Carbonic Anhydrase IX and Via Reverse Mode Activity Contributes to pH Control in Hypoxic Tumors. Cancers, 11(8), 1139. https://doi.org/10.3390/cancers11081139




