Inclusion of Platinum Agents in Neoadjuvant Chemotherapy Regimens for Triple-Negative Breast Cancer Patients: Development of GRADE (Grades of Recommendation, Assessment, Development and Evaluation) Recommendation by the Italian Association of Medical Oncology (AIOM)
Abstract
1. Introduction
- National Comprehensive Cancer Network (NCCN) Breast Cancer Guidelines, version 1.2019 [12]: “…the NCCN panel does not recommend addition of carboplatin to neoadjuvant standard chemotherapy for patients with triple-negative breast cancer outside a clinical trial setting”;
- Early breast cancer: European Society of Medical Oncology (ESMO) Clinical Practice Guidelines for diagnosis, treatment and follow up (2019) [13]: “The addition of a platinum compound may be considered in triple-negative tumors and/or in patients with deleterious BRCA1/2 mutations [I, C]” (statement related to neoadjuvant chemotherapy);
- St. Gallen Consensus Conference 2017 [14]: “The Panel clearly recommended against routine use of platinum-based chemotherapy in unselected TNBC cases. In BRCA1/2 associated cancers, the Panel was evenly split on whether to recommend (neo)adjuvant platinum chemotherapy though agreed that such patients should receive alkylating chemotherapy in addition to a taxane and anthracycline”;
- European School of Oncology (ESO)—ESMO consensus guidelines for BC in young women (BCY3) [15]: “In patients with TNBC or BRCA-associated tumors the incorporation of platinum agents increases pCR rates and may be considered when neoadjuvant chemotherapy is indicated. Data on the impact of incremental increases in pCR on long term outcome are not conclusive. The use of platinum derivatives has potential additional impact on fertility and increased toxicity that may compromise standard duration and dosing of systemic treatment, and this needs to be clearly communicated to patients”, Level of Evidence IIA (weak recommendation, high-quality evidence), 77% consensus.
2. Results
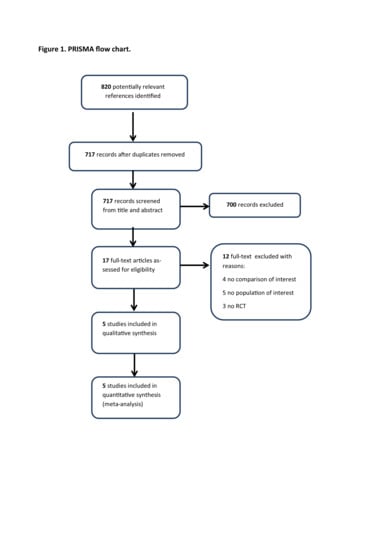
2.1. Search Strategy Results and Details of the Identified Relevant Studies
2.2. Outcomes of Benefit
2.2.1. pCR (ypT0/is ypN0)
2.2.2. DFS/EFS and OS
2.3. Outcomes of Harm
2.3.1. Febrile Neutropenia
2.3.2. Serious Adverse Events
2.3.3. Anemia G3–G4
2.3.4. Thrombocytopenia G3–G4
2.4. EtD (Evidence to Decision) Framework
2.5. Benefit/Harm Balance and Final Recommendation
“In patients with triple-negative breast cancer who are candidates to receive neoadjuvant chemotherapy, the addition of platinum to a standard regimen containing anthracycline and taxane may be taken into consideration”.
3. Discussion
4. Materials and Methods
4.1. The AIOM Guidelines on Breast Cancer Panel
4.2. Development of Clinical Question
- In patients with triple-negative breast cancer candidate to receive neoadjuvant chemotherapy, is the addition of a platinum agent to a taxane and anthracycline-containing regimen recommendable versus a taxane and anthracycline-containing regimen only?
4.3. Identification of Outcomes
4.4. Search Strategy and Selection of Evidence
4.5. Quality of Evidence Evaluation
4.6. Evidence to Decision (EtD) Framework
4.7. Benefit/Harm Balance and Clinical Recommendation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A. Research Strategy
- ((((((((((((((“Triple Negative Breast Neoplasms/drug therapy” [Mesh] OR “Triple Negative Breast Neoplasms/pathology” [Mesh]))) OR (“triple negative breast Cancer” [Title/Abstract] OR “triple negative breast neoplasms” [Title/Abstract] OR “triple negative breast tumo*” [Title/Abstract]))))))))
- ((((((((((((((((“Platinum Compounds/therapeutic use” [Mesh]) OR ((((((((“Carboplatin” [Mesh]) OR “Platinum” [Mesh])) OR (Cisplatinum [Text Word] OR carboplatinum [Text Word] OR Platinum [Text Word] OR “platinum compound*” [Text Word] OR “platinum containing regime*” [Text Word] OR biocisplatinum [Text Word] OR platino [Text Word] OR platinol [Text Word] OR paraplatin [Text Word])) OR (((cisplatin [Text Word] OR oxilaplatin [Text Word] OR carboplatin [Text Word]))))))) OR (“Neoadjuvant Therapy” [Mesh]) OR “Neoadjuvant Therapy” [Title/Abstract]))))))
- ((((“Neoadjuvant Therapy” [Mesh]) OR “Neoadjuvant Therapy” [Title/Abstract])))))) OR ((“Anthracyclines” [Mesh]) OR (Anthracycline* [Text Word] OR Daunorubicin [Text Word] OR Doxorubicin [Text Word] OR Idarubicin [Text Word] OR epirubicin [Text Word] OR valrubicin [Text Word] OR Aclarubicin [Text Word] OR Carubicin [Text Word] OR Nogalamycin [Text Word] OR Plicamycin [Text Word])))) AND
- ((((((“Neoadjuvant Therapy” [Mesh]) OR “Neoadjuvant Therapy” [Title/Abstract])))))))) OR ((“Taxoids” [Mesh]) OR (Taxoids [Text Word] OR docetaxel [Text Word] OR paclitaxel [Text Word] OR nab-paclitaxel [Text Word] OR Cabazitaxel [Text Word]))))
- #1 ‘triple negative breast cancer’/exp OR ‘triple negative breast cancer’
- #2 ‘triple negative breast cancer’ OR ‘triple negative breast neoplasms’ OR ‘triple negative breast tumo*’:de,ti,ab
- #3 #1 OR #2
- #4 ‘platinum derivative’/exp OR ‘carboplatin’/exp
- #5 cisplatinum OR carboplatinum OR platinum OR ‘platinum compound*’ OR ‘platinum containing regime*’ OR biocisplatinum OR platino OR platinol OR paraplatin OR cisplatin OR oxilaplatin OR carboplatin:de,ti,ab
- #6 #4 OR #5
- #7 ‘neoadjuvant therapy’/exp
- #8 ‘neoadjuvant therapy’:de,ti,ab
- #9 #7 OR #8
- #10 ‘anthracycline’/exp
- #11 anthracycline* OR daunorubicin OR doxorubicin OR idarubicin OR epirubicin OR valrubicin OR aclarubicin OR carubicin OR nogalamycin OR plicamycin:de,ti,ab
- #12 #10 OR #11
- #13 ‘taxoid’/exp
- #14 taxoids OR docetaxel OR paclitaxel OR nab-paclitaxel OR cabazitaxel:de,ti,ab
- #15 #13 OR #14
- #16 #6 AND #12 AND #15
- #17 #9 OR #16
- #18 #3 AND #17
- #19 ‘crossover procedure’:de OR ‘double-blind procedure’:de OR ‘randomized controlled trial’:de OR ‘single-blind procedure’:de OR random*:de,ab,ti OR factorial*:de,ab,ti OR crossover*:de,ab,ti OR ((cross NEXT/1 over*):de,ab,ti) OR placebo*:de,ab,ti OR ((doubl* NEAR/1 blind*):de,ab,ti) OR ((singl* NEAR/1 blind*):de,ab,ti) OR assign*:de,ab,ti OR allocat*:de,ab,ti OR volunteer*:de,ab,ti
- #20 #18 AND #19
- #21 #18 AND #19 AND ( [article]/lim OR [article in press]/lim) AND [embase]/lim
- #1 MeSH descriptor: [Triple Negative Breast Neoplasms] explode all trees
- #2 (‘triple negative breast cancer’ OR ‘triple negative breast neoplasms’ OR ‘triple negative breast tumo*’):ti,ab,kw (Word variations have been searched)
- #3 #30 OR #31
- #4 MeSH descriptor: [Platinum Compounds] explode all trees
- #5 MeSH descriptor: [Carboplatin] explode all trees
- #6 (cisplatinum OR carboplatinum OR platinum OR ‘platinum compound*’ OR ‘platinum containing regime*’ OR biocisplatinum OR platino OR platinol OR paraplatin OR cisplatin OR oxilaplatin OR carboplatin):ti,ab,kw (Word variations have been searched)
- #7 #33 OR #34 OR #35
- #8 MeSH descriptor: [Anthracyclines] explode all trees
- #9 (anthracycline* OR daunorubicin OR doxorubicin OR idarubicin OR epirubicin OR valrubicin OR aclarubicin OR carubicin OR nogalamycin OR plicamycin):ti,ab,kw (Word variations have been searched)
- #10 #37 OR #38
- #11 MeSH descriptor: [Taxoids] explode all trees
- #12 (taxoids OR docetaxel OR paclitaxel OR nab-paclitaxel OR cabazitaxel):ti,ab,kw (Word variations have been searched)
- #13 #40 OR #41
- #14 MeSH descriptor: [Neoadjuvant Therapy] explode all trees
- #15 #36 AND #39 AND #42
- #16 #44 OR #43
- #17 #45 AND #32
References
- Carey, L.; Winer, E.; Viale, G.; Cameron, D.; Gianni, L. Triple-Negative Breast Cancer: Disease Entity or Title of Convenience? Nat. Rev. Clin. Oncol. 2010, 7, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Aysola, K.; Desai, A.; Welch, C.; Xu, J.; Qin, Y.; Reddy, V.; Matthews, R.; Owens, C.; Okoli, J.; Beech, D.J.; et al. Triple Negative Breast Cancer-an Overview. Hered. Genet. 2013, 2013. [Google Scholar] [CrossRef]
- Carey, L.A.; Dees, E.C.; Sawyer, L.; Gatti, L.; Moore, D.T.; Collichio, F.; Ollila, D.W.; Sartor, C.I.; Graham, M.L.; Perou, C.M. The Triple Negative Paradox: Primary Tumor Chemosensitivity of Breast Cancer Subtypes. Clin. Cancer Res. 2007, 13, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; Andre, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to Neoadjuvant Therapy and Long-Term Survival in Patients with Triple-Negative Breast Cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological Complete Response and Long-Term Clinical Benefit in Breast Cancer: The CTNeoBC Pooled Analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.J.; Ohtani, S.; Im, Y.H.; Lee, E.S.; Yokota, I.; Kuroi, K.; Im, S.A.; Park, B.W.; Kim, S.B.; et al. Adjuvant Capecitabine for Breast Cancer After Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, C.; Rody, A. Neoadjuvant Therapy for Patients with Triple Negative Breast Cancer (TNBC). Rev. Recent. Clin. Trials 2017, 12, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Aleskandarany, M.; Caracappa, D.; Nolan, C.C.; Macmillan, R.D.; Ellis, I.O.; Rakha, E.A.; Green, A.R. DNA Damage Response Markers are Differentially Expressed in BRCA-Mutated Breast Cancers. Breast Cancer Res. Treat. 2015, 150, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.; Nilsson, M.P.; Olsson, E.; George, A.M.; Chen, Y.; Kvist, A.; Torngren, T.; Vallon-Christersson, J.; Hegardt, C.; Hakkinen, J.; et al. Targeted Sequencing of BRCA1 and BRCA2 Across a Large Unselected Breast Cancer Cohort Suggests that One-Third of Mutations are Somatic. Ann. Oncol. 2016, 27, 1532–1538. [Google Scholar] [CrossRef]
- Anders, C.K.; Abramson, V.; Tan, T.; Dent, R. The Evolution of Triple-Negative Breast Cancer: From Biology to Novel Therapeutics. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 34–42. [Google Scholar] [CrossRef]
- Poggio, F.; Bruzzone, M.; Ceppi, M.; Ponde, N.F.; La Valle, G.; Del Mastro, L.; de Azambuja, E.; Lambertini, M. Platinum-Based Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer: A Systematic Review and Meta-Analysis. Ann. Oncol. 2018, 29, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- NCCN. Clinical Practice Guidelines in Oncology-Breast Cancer; Version 1; NCCN: Fort Washington, PA, USA, 2019; Available online: https://www.Nccn.Org/Professionals/Physician_gls/Pdf/Breast.Pdf (accessed on 15 May 2019).
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Burstein, H.J.; Winer, E.P.; Gnant, M.; Dubsky, P.; Loibl, S.; Colleoni, M.; Regan, M.M.; Piccart-Gebhart, M.; Senn, H.J.; et al. De-Escalating and Escalating Treatments for Early-Stage Breast Cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. 2017, 28, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Paluch-Shimon, S.; Pagani, O.; Partridge, A.H.; Abulkhair, O.; Cardoso, M.J.; Dent, R.A.; Gelmon, K.; Gentilini, O.; Harbeck, N.; Margulies, A.; et al. ESO-ESMO 3rd International Consensus Guidelines for Breast Cancer in Young Women (BCY3). Breast 2017, 35, 203–217. [Google Scholar] [CrossRef] [PubMed]
- The Grade Working Group. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. 2013. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 15 October 2013).
- Loibl, S.; O’Shaughnessy, J.; Untch, M.; Sikov, W.M.; Rugo, H.S.; McKee, M.D.; Huober, J.; Golshan, M.; von Minckwitz, G.; Maag, D.; et al. Addition of the PARP Inhibitor Veliparib Plus Carboplatin Or Carboplatin Alone to Standard Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer (BrighTNess): A Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 497–509. [Google Scholar] [CrossRef]
- Sikov, W.M.; Berry, D.A.; Perou, C.M.; Singh, B.; Cirrincione, C.T.; Tolaney, S.M.; Kuzma, C.S.; Pluard, T.J.; Somlo, G.; Port, E.R.; et al. Impact of the Addition of Carboplatin and/Or Bevacizumab to Neoadjuvant Once-Per-Week Paclitaxel Followed by Dose-Dense Doxorubicin and Cyclophosphamide on Pathologic Complete Response Rates in Stage II to III Triple-Negative Breast Cancer: CALGB 40603 (Alliance). J. Clin. Oncol. 2015, 33, 13–21. [Google Scholar] [PubMed]
- Von Minckwitz, G.; Schneeweiss, A.; Loibl, S.; Salat, C.; Denkert, C.; Rezai, M.; Blohmer, J.U.; Jackisch, C.; Paepke, S.; Gerber, B.; et al. Neoadjuvant Carboplatin in Patients with Triple-Negative and HER2-Positive Early Breast Cancer (GeparSixto; GBG 66): A Randomised Phase 2 Trial. Lancet Oncol. 2014, 15, 747–756. [Google Scholar] [CrossRef]
- Alba, E.; Chacon, J.I.; Lluch, A.; Anton, A.; Estevez, L.; Cirauqui, B.; Carrasco, E.; Calvo, L.; Segui, M.A.; Ribelles, N.; et al. A Randomized Phase II Trial of Platinum Salts in Basal-Like Breast Cancer Patients in the Neoadjuvant Setting. Results from the GEICAM/2006-03, Multicenter Study. Breast Cancer Res. Treat. 2012, 136, 487–493. [Google Scholar] [CrossRef]
- Ando, M.; Yamauchi, H.; Aogi, K.; Shimizu, S.; Iwata, H.; Masuda, N.; Yamamoto, N.; Inoue, K.; Ohono, S.; Kuroi, K.; et al. Randomized Phase II Study of Weekly Paclitaxel with and without Carboplatin Followed by Cyclophosphamide/Epirubicin/5-Fluorouracil as Neoadjuvant Chemotherapy for Stage II/IIIA Breast Cancer without HER2 Overexpression. Breast Cancer Res. Treat. 2014, 145, 401–409. [Google Scholar] [CrossRef]
- Sikov, W.M.; Berry, D.A.; Perou, C.M.; Singh, B.; Cirrincione, C.T.; Tolaney, S.M.; Somlo, G.; Port, E.R.; Qamar, R.; Sturtz, K.; et al. Event-Free and overall Survival Following Neoadjuvant Weekly Paclitaxel and Dose-Dense AC +/− Carboplatin and/Or Bevacizumab in Triple-Negative Breast Cancer: Outcomes from CALGB 40603 (Alliance). Cancer Res. 2016, 76. [Google Scholar] [CrossRef]
- Hahnen, E.; Lederer, B.; Hauke, J.; Loibl, S.; Krober, S.; Schneeweiss, A.; Denkert, C.; Fasching, P.A.; Blohmer, J.U.; Jackisch, C.; et al. Germline Mutation Status, Pathological Complete Response, and Disease-Free Survival in Triple-Negative Breast Cancer: Secondary Analysis of the GeparSixto Randomized Clinical Trial. JAMA Oncol. 2017, 3, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- 163PD—Long-Term Survival Analysis of the Randomized Phase II Trial Investigating the Addition of Carboplatin to Neoadjuvant Therapy for Triple-Negative (TNBC) and HER2-Positive Early Breast Cancer (GeparSixto). Available online: https://oncologypro.esmo.org/Meeting-Resources/ESMO-2017-Congress/Long-term-survival-analysis-of-the-randomized-phase-II-trial-investigating-the-addition-of-carboplatin-to-neoadjuvant-therapy-for-triple-negative-TNBC-and-HER2-positive-early-breast-cancer-GeparSixto (accessed on 15 June 2019).
- Omarini, C.; Guaitoli, G.; Pipitone, S.; Moscetti, L.; Cortesi, L.; Cascinu, S.; Piacentini, F. Neoadjuvant Treatments in Triple-Negative Breast Cancer Patients: Where we are Now and Where we are Going. Cancer. Manag. Res. 2018, 10, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.M.; Shalaby, A.; O’Loughlin, M.; Keane, N.; Webber, M.J.; Kerin, M.J.; Keane, M.M.; Glynn, S.A.; Callagy, G.M. Outcome for Triple Negative Breast Cancer in a Retrospective Cohort with an Emphasis on Response to Platinum-Based Neoadjuvant Therapy. Breast Cancer Res. Treat. 2019, 174, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.; Tovey, H.; Cheang, M.C.U.; Kernaghan, S.; Kilburn, L.; Gazinska, P.; Owen, J.; Abraham, J.; Barrett, S.; Barrett-Lee, P.; et al. Carboplatin in BRCA1/2-Mutated and Triple-Negative Breast Cancer BRCAness Subgroups: The TNT Trial. Nat. Med. 2018, 24, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.P.; Richardson, A.L.; Eklund, A.C.; Wang, Z.C.; Szallasi, Z.; Li, Q.; Juul, N.; Leong, C.O.; Calogrias, D.; Buraimoh, A.; et al. Efficacy of Neoadjuvant Cisplatin in Triple-Negative Breast Cancer. J. Clin. Oncol. 2010, 28, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Byrski, T.; Gronwald, J.; Huzarski, T.; Grzybowska, E.; Budryk, M.; Stawicka, M.; Mierzwa, T.; Szwiec, M.; Wisniowski, R.; Siolek, M.; et al. Pathologic Complete Response Rates in Young Women with BRCA1-Positive Breast Cancers After Neoadjuvant Chemotherapy. J. Clin. Oncol. 2010, 28, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Caramelo, O.; Silva, C.; Caramelo, F.; Frutuoso, C.; Almeida-Santos, T. The Effect of Neoadjuvant Platinum-Based Chemotherapy in BRCA Mutated Triple Negative Breast Cancers -Systematic Review and Meta-Analysis. Hered. Cancer Clin. Pract. 2019, 17. [Google Scholar] [CrossRef]
- Robson, M. Selecting Patients with Triple Negative Breast Cancer for Platinum-Based Therapy: We Still Haven’T found what we’Re Looking For. Ann. Oncol. 2018, 29, 1609–1610. [Google Scholar] [CrossRef]
- Munzone, E.; Curigliano, G.; Burstein, H.J.; Winer, E.P.; Goldhirsch, A. CMF Revisited in the 21st Century. Ann. Oncol. 2012, 23, 305–311. [Google Scholar] [CrossRef]
- Berrada, N.; Delaloge, S.; Andre, F. Treatment of Triple-Negative Metastatic Breast Cancer: Toward Individualized Targeted Treatments Or Chemosensitization? Ann. Oncol. 2010, 21. [Google Scholar] [CrossRef]
- Associazione Italiana di Oncologia Medica. Available online: www.aiom.it (accessed on 15 June 2019).
- Avan, A.; Postma, T.J.; Ceresa, C.; Avan, A.; Cavaletti, G.; Giovannetti, E.; Peters, G.J. Platinum-Induced Neurotoxicity and Preventive Strategies: Past, Present, and Future. Oncologist 2015, 20, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Coello, P.; Oxman, A.D.; Moberg, J.; Brignardello-Petersen, R.; Akl, E.A.; Davoli, M.; Treweek, S.; Mustafa, R.A.; Vandvik, P.O.; Meerpohl, J.; et al. GRADE Evidence to Decision (EtD) Frameworks: A Systematic and Transparent Approach to Making Well Informed Healthcare Choices. 2: Clinical Practice Guidelines. BMJ 2016, 353, i2089. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, M.C.; Kerkvliet, K.; Spithoff, K.; AGREE Next Steps Consortium. The AGREE Reporting Checklist: A Tool to Improve Reporting of Clinical Practice Guidelines. BMJ 2016, 352, i1152. [Google Scholar] [CrossRef] [PubMed]
- McAndrew, N.; DeMichele, A. Neoadjuvant Chemotherapy Considerations in Triple-Negative Breast Cancer. J. Target. Ther. Cancer. 2018, 7, 52–69. [Google Scholar] [PubMed]
| Study Name | Phase | n TNBC | Treatment Arms |
|---|---|---|---|
| BrighTNess [15] * | III | 338 | P 80 mg/mq qw for 12 w + Cb AUC 6 q3w for 4 courses → AC (60 mg/mq and 600 mg/mq) q3w for 4 courses P 80 mg/mq qw for 12 w + Cb AUC 6 q3w for 4 courses → AC (60 mg/mq and 600 mg/mq) q3w for 4 courses |
| CALGB 40603 [16] § | II | 454 | P 80 mg/mq qw for 12 w + Cb AUC6 q3w for 4 courses +/− Bev 10 mg/kg q2w for 9 courses° → ddAC (60 mg/mq and 600 mg/mq q2w for 4 courses, with myeloid growth factor support) P 80 mg/mq qw for 12 w +/− Bev 10 mg/kg q2w for 9 courses° → ddAC (60 mg/mq and 600 mg/mq q2w for 4 courses, with myeloid growth factor support) |
| GeparSixto GBG66 [17] ^ | II | 315 | P 80 mg/mq qw + nplD 220 mg/mq qw + Bev 15 mg/kg q3w + Cb AUC 2.0 or 1.5 qw for 18 w P 80 mg/mq qw + nplD 220 mg/mq qw + Bev 15 mg/kg q3w for 18 w |
| GEICAM/2006-3 [18] | II | 93 | EC (90 mg/mq and 600 mg/mq q3w for 4 courses) → Doc 75 mg/mq q3w for 4 courses + Cb AUC6 q3w for 4 courses EC (90 mg/mq and 600 mg/mq q3w for 4 courses) → Doc 100 mg/mq q3w for 4 courses |
| UMIN000003355 [19] ^ | II | 75 | P 80 mg/mq qw for 12 w + Cb AUC5 q3w for 4 courses → CEF (500 mg/mq, 100 mg/mq and 500 mg/mq) q3w for 4 courses P 80 mg/mq qw for 12 w → CEF (500 mg/mq, 100 mg/mq and 500 mg/mq) q3w for 4 courses |
| Certainty Assessment | No of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Platinum Added to Taxane- and Anthracycline-Based Neoadjuvant Chemotherapy | Taxane- and Anthracycline-Based Neoadjuvant Chemotherapy only | Relative (95% CI) | Absolute (95% CI) | ||
| Overall survival (follow up: range 39 months to 47.3 months) | ||||||||||||
| 3 a | randomised trials | not serious | serious b | not serious | serious c | none d | 33/384 (8.6%) e | 36/385 (9.4%) e | HR 0.86 (0.46 to 1.63) | 1 fewer per 100 (from 5 fewer to 5 more) | ⨁⨁◯ LOW | CRITICAL |
| DFS/EFS (follow up: range 39 months to 47.3 months) | ||||||||||||
| 4 f | randomised trials | serious g | serious h | not serious | serious i | publication bias STRONGLY suspected j | 75/384 (19.5%) e | 100/385 (26.0%) e | HR 0.72 (0.49 to 1.06) | 7 fewer per 100 (from 12 fewer to 1 more) | ⨁◯◯◯ VERY LOW | CRITICAL |
| Pathological complete response rate (assessed with: no residual invasive tumour in both the breast and the axilla: i.e., ypT0/is pN0) | ||||||||||||
| 5 k | randomised trials | serious l | serious m | not serious | not serious | none | 338/623 (54.3%) e | 229/611 (37.5%) e | RR 1.45 (1.28 to 1.64) | 17 more per 100 (from 10 more to 24 more) | ⨁⨁◯◯ LOW | CRITICAL |
| Febrile neutropenia | ||||||||||||
| 5 k | randomised trials | not serious | not serious | not serious n,o | serious i | none | 63/701 (9.0%) | 44/695 (6.3%) | RR 1.42 (0.98 to 2.06) | 3 more per 100 (from 0 fewer to 7 more) | ⨁⨁⨁◯ MODERATE | CRITICAL |
| Anemia grade 3/4 | ||||||||||||
| 5 k | randomised trials | not serious | not serious | not serious n,o | serious i | none | 99/701 (14.1%) | 2/695 (0.3%) | RR 49.08 (12.15 to 198.20) | 14 more per 100 (from 3 more to 57 more) | ⨁⨁⨁◯ MODERATE | IMPORTANT |
| Seriuos adverse events | ||||||||||||
| 3 p | randomised trials | serious q | not serious r | not serious n,o | not serious | none | 174/566 (30.7%) | 134/558 (24.0%) | RR 1.28 (1.06 to 1.55) | 7 more per 100 (from 1 more to 13 more) | ⨁⨁⨁◯ MODERATE | CRITICAL |
| Thrombocytopenia grade 3/4 | ||||||||||||
| 5 k | randomised trials | not serious | not serious s | not serious n,o | serious i | none | 79/701 (11.3%) | 5/695 (0.8%) | RR 15.66 (6.38 to 38.44) | 11 more per 100 (from 4 more to 27 more) | ⨁⨁⨁◯ MODERATE | IMPORTANT |
| CLINICAL QUESTION: In patients with triple-negative breast cancer who were to receive neoadjuvant chemotherapy, was the addition of a platinum agent to a taxane and anthracycline-containing regimen recommendable versus a taxane and anthracycline-containing regimen only? | |||
| Recommendation: In patients with triple-negative breast cancer, receiving neoadjuvant chemotherapy, the addition of platinum to a standard regimen containing anthracycline and taxane may be taken into consideration | |||
| Strength of recommendation: CONDITIONAL POSITIVE | |||
| Quality of evidence: Outcomes of benefit: Very Low; Outcomes of harm: Moderate | |||
| Reasons/comments on the benefit/harm balance Outcomes of benefit. The Panel identified as “critical” the following outcomes of benefit: pCR, DFS/EFS and OS. Five randomized controlled trials (RCTs) with the same anthracycline and taxane-based chemotherapy backbone in the platinum (carboplatin) and no-platinum arms were considered for the assessment of the clinical question [17,18,19,20,21] All five studies reported pCR [17,18,19,20,21] and only two studies reported survival data [18,19,22,23,24]. The risk of pCR was 54 every 100 patients with carboplatin and 37 every 100 patients without carboplatin (RR 1.45, 95% CI 1.28–1.64). The quality of evidence in support of pCR outcome was low (potential bias due to the possible lack of blinded outcome assessor and heterogeneity with I-squared 55%). There was no significant difference in favor of the addition of platinum for: DFS/EFS (HR 0.72, 95% CI 0.49–1.06) and OS (HR 0.86, 95%CI 0.46–1.63). Although the available evidence was insufficient to detect a significant difference in survival (low number of events and short follow-up), considering the difference in pCR, the panel judged as “moderate” the substantiality of the desirable effects derived from the addition of platinum to anthracycline and taxane-based neoadjuvant chemotherapy. Outcomes of harm. The Panel identified the following outcomes of harms: febrile neutropenia, anemia (grade 3–4), serious adverse event (SAE), thrombocytopenia (grade 3–4). Only febrile neutropenia and SAE were considered “critical outcomes”. The addition of platinum to a standard anthracycline and taxane-based chemotherapy was not associated with a significant difference in the risk of febrile neutropenia (RR 1.42, 95% CI 0.98 to 2.06). The addition of platinum significantly increased the risk of SAE (RR 1.28, 95% CI 1.06 to 1.55), grade 3–4 anemia (RR 49.08, 95% CI 12.15 to 198.20), and grade 3–4 thrombocytopenia (RR 15.66, 95% CI 6.38 to 38.44). The panel also considered the adverse events occurring in the BrighTNess study in the second treatment segment (anthracycline-based) that were not included in the overall assessment. The Panel, considering the heterogeneity of treatment schedules applied in the different studies that might have influenced the incidence of adverse events, judged as “small” the substantiality of the undesirable effects. Although the survival impact of the addition of platinum remains undefined, the Panel voted for the benefit/harm balance as uncertain-favorable, considering the increase in pCR rates and the acceptable safety costs. | |||
| Vote on benefit/harm balance | |||
| Favorable | Uncertain (Favorable) | Uncertain (Unfavorable) | Unfavorable |
| 10 | 1 | ||
| Vote on strength of recommendation | |||
| Strong Positive | Weak Positive | Weak Negative | Strong Negative |
| 10 | 1 | ||
| Implications for future research: a longer follow-up of those studies that report DFS/EFS and/or OS as secondary endpoint is necessary in order to define the survival impact of the addition of platinum. | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieci, M.V.; Del Mastro, L.; Cinquini, M.; Montemurro, F.; Biganzoli, L.; Cortesi, L.; Zambelli, A.; Criscitiello, C.; Levaggi, A.; Conte, B.; et al. Inclusion of Platinum Agents in Neoadjuvant Chemotherapy Regimens for Triple-Negative Breast Cancer Patients: Development of GRADE (Grades of Recommendation, Assessment, Development and Evaluation) Recommendation by the Italian Association of Medical Oncology (AIOM). Cancers 2019, 11, 1137. https://doi.org/10.3390/cancers11081137
Dieci MV, Del Mastro L, Cinquini M, Montemurro F, Biganzoli L, Cortesi L, Zambelli A, Criscitiello C, Levaggi A, Conte B, et al. Inclusion of Platinum Agents in Neoadjuvant Chemotherapy Regimens for Triple-Negative Breast Cancer Patients: Development of GRADE (Grades of Recommendation, Assessment, Development and Evaluation) Recommendation by the Italian Association of Medical Oncology (AIOM). Cancers. 2019; 11(8):1137. https://doi.org/10.3390/cancers11081137
Chicago/Turabian StyleDieci, Maria Vittoria, Lucia Del Mastro, Michela Cinquini, Filippo Montemurro, Laura Biganzoli, Laura Cortesi, Alberto Zambelli, Carmen Criscitiello, Alessia Levaggi, Benedetta Conte, and et al. 2019. "Inclusion of Platinum Agents in Neoadjuvant Chemotherapy Regimens for Triple-Negative Breast Cancer Patients: Development of GRADE (Grades of Recommendation, Assessment, Development and Evaluation) Recommendation by the Italian Association of Medical Oncology (AIOM)" Cancers 11, no. 8: 1137. https://doi.org/10.3390/cancers11081137
APA StyleDieci, M. V., Del Mastro, L., Cinquini, M., Montemurro, F., Biganzoli, L., Cortesi, L., Zambelli, A., Criscitiello, C., Levaggi, A., Conte, B., Calabrese, M., Fiorentino, A., Marchiò, C., Tinterri, C., Fittipaldo, V. A., Pappagallo, G., & Gori, S. (2019). Inclusion of Platinum Agents in Neoadjuvant Chemotherapy Regimens for Triple-Negative Breast Cancer Patients: Development of GRADE (Grades of Recommendation, Assessment, Development and Evaluation) Recommendation by the Italian Association of Medical Oncology (AIOM). Cancers, 11(8), 1137. https://doi.org/10.3390/cancers11081137