Current State of Liver-Directed Therapies and Combinatory Approaches with Systemic Therapy in Hepatocellular Carcinoma (HCC)
Abstract
1. Introduction
2. Overview on the Current State of Liver Directed Therapy Strategies
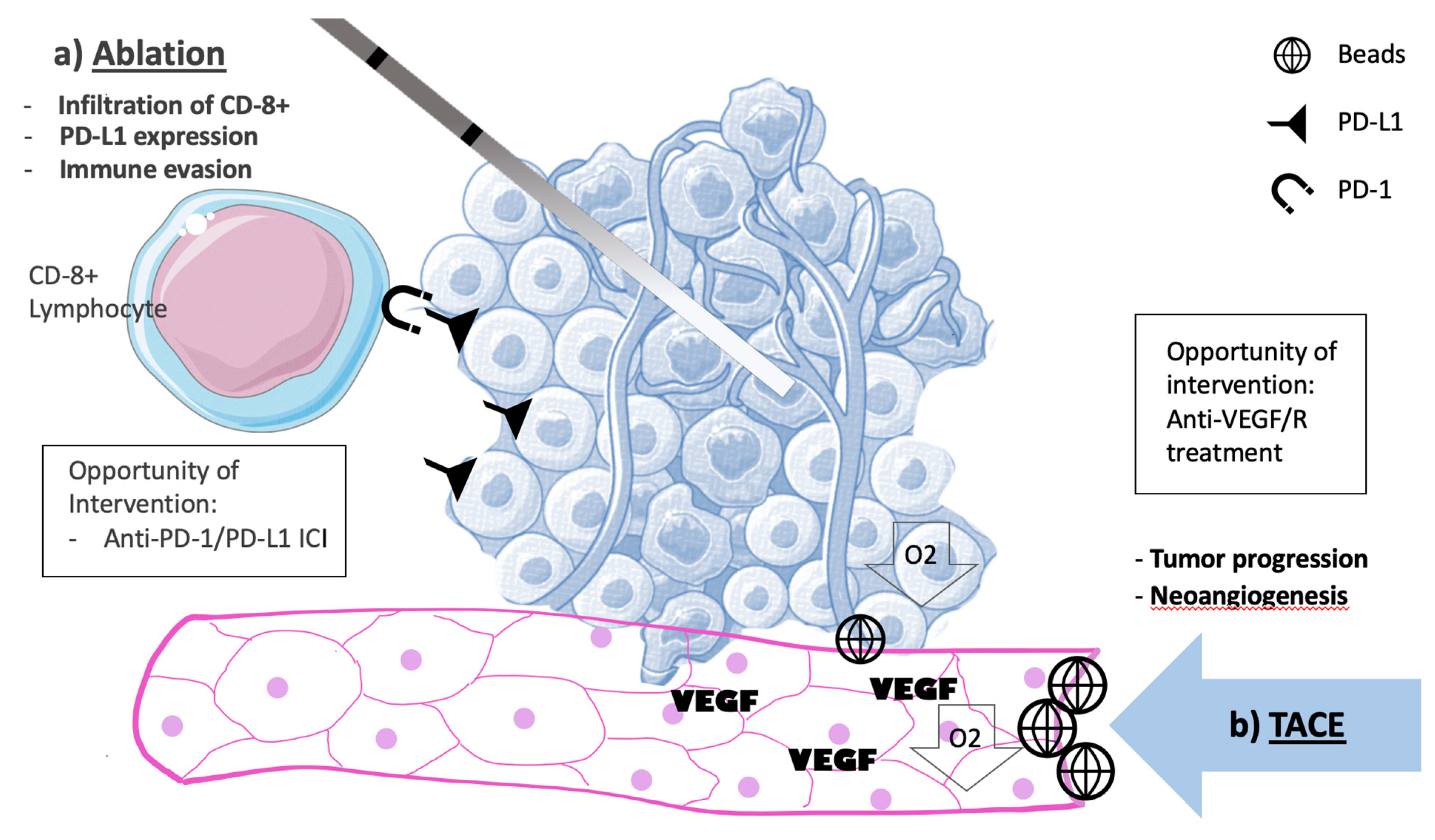
2.1. Percutaneous Ablative Approach
2.2. Stereotactic Body Radiation Therapy
2.3. Trans-Arterial Treatment Techniques
2.4. Radioembolization
2.5. Sequencing
3. Liver Directed Therapies Role Pre-Operatively
4. Liver Directed Therapies Role in Bridging Orthotopic Liver Transplant
5. Role of Liver-Directed Therapies in BCLC-C Patients
6. Molecular Changes Associated with Liver Directed Therapies.
7. Current State of Liver Directed Therapies Associated with TKIs
8. Yttrium-90 vs. Sorafenib
9. Role Liver Directed Therapies Associated with Immunotherapy
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McGuire, S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv. Nutr. 2016, 7, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Vilgrain, V.; Mathieu, D.; Trinchet, J. Hepatocellular carcinoma screening in patients with cirrhosis: a large French multicentric study (HCC). J. Radiol. 2000, 81, 1587–1588. [Google Scholar] [PubMed]
- Robinson, A.; Tavakoli, H.; Cheung, R.; Liu, B.; Bhuket, T.; Wong, R.J. Low Rates of Retention Into Hepatocellular Carcinoma (HCC) Surveillance Program After Initial HCC Screening. J. Clin. Gastroenterol. 2019, 53, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Adhoute, X.; Penaranda, G.; Raoul, J.L.; Edeline, J.; Blanc, J.F.; Pol, B.; Campanile, M.; Perrier, H.; Bayle, O.; Monnet, O.; et al. Barcelona clinic liver cancer nomogram and others staging/scoring systems in a French hepatocellular carcinoma cohort. World J. Gastroenterol. 2017, 23, 2545–2555. [Google Scholar] [CrossRef] [PubMed]
- Njei, B.; Rotman, Y.; Ditah, I.; Lim, J.K. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 2015, 61, 191–199. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, Y.; He, G.; Yu, M.; Zheng, M.; Liu, L.; Zhou, X. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J. Surg. Oncol. 2017, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.; Elbaz, T.; Shousha, H.I.; Mahmoud, S.; Ibrahim, M.; Abdelmaksoud, A.; Nabeel, M. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg. Endosc. 2014, 28, 3429–3434. [Google Scholar] [CrossRef]
- Shibata, T.; Shibata, T.; Maetani, Y.; Isoda, H.; Hiraoka, M. Radiofrequency ablation for small hepatocellular carcinoma: prospective comparison of internally cooled electrode and expandable electrode. Radiology 2006, 238, 346–353. [Google Scholar] [CrossRef]
- Di Costanzo, G.G.; Tortora, R.; D’Adamo, G.; De Luca, M.; Lampasi, F.; Addario, L.; Galeota Lanza, A.; Picciotto, F.P.; Tartaglione, M.T.; Cordone, G.; et al. Radiofrequency ablation versus laser ablation for the treatment of small hepatocellular carcinoma in cirrhosis: a randomized trial. J. Gastroenterol. Hepatol. 2015, 30, 559–565. [Google Scholar] [CrossRef]
- Giorgio, A.; Di Sarno, A.; De Stefano, G.; Scognamiglio, U.; Farella, N.; Mariniello, A.; Esposito, V.; Coppola, C.; Giorgio, V. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer Res. 2011, 31, 2291–2295. [Google Scholar]
- Lin, S.M.; Lin, C.J.; Lin, C.C.; Hsu, C.W.; Chen, Y.C. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology 2004, 127, 1714–1723. [Google Scholar] [PubMed]
- Shiina, S.; Teratani, T.; Obi, S.; Sato, S.; Tateishi, R.; Fujishima, T.; Ishikawa, T.; Koike, Y.; Yoshida, H.; Kawabe, T.; et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005, 129, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.A.; Allgaier, H.P.; Cioni, D.; Olschewski, M.; Deibert, P.; Crocetti, L.; Frings, H.; Laubenberger, J.; Zuber, I.; Blum, H.E.; et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 2003, 228, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Brunello, F.; Veltri, A.; Carucci, P.; Pagano, E.; Ciccone, G.; Moretto, P.; Sacchetto, P.; Gandini, G.; Rizzetto, M. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand. J. Gastroenterol. 2008, 43, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Yang, W.; Hu, K.; Xie, H.; Hu, K.Q.; Bai, W.; Dong, Z.; Lu, Y.; Zeng, Z.; et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology 2015, 61, 1579–1590. [Google Scholar] [CrossRef]
- Sutter, O.; Calvo, J.; N’Kontchou, G.; Nault, J.C.; Ourabia, R.; Nahon, P.; Ganne-Carrie, N.; Bourcier, V.; Zentar, N.; Bouhafs, F.; et al. Safety and Efficacy of Irreversible Electroporation for the Treatment of Hepatocellular Carcinoma Not Amenable to Thermal Ablation Techniques: A Retrospective Single-Center Case Series. Radiology 2017, 284, 877–886. [Google Scholar] [CrossRef]
- Ferrari, F.S.; Megliola, A.; Scorzelli, A.; Stella, A.; Vigni, F.; Drudi, F.M.; Venezia, D. Treatment of small HCC through radiofrequency ablation and laser ablation. Comparison of techniques and long-term results. Radiol. Med. 2007, 112, 377–393. [Google Scholar] [CrossRef]
- Kutlu, O.C.; Chan, J.A.; Aloia, T.A.; Chun, Y.S.; Kaseb, A.O.; Passot, G.; Yamashita, S.; Vauthey, J.N.; Conrad, C. Comparative effectiveness of first-line radiofrequency ablation versus surgical resection and transplantation for patients with early hepatocellular carcinoma. Cancer 2017, 123, 1817–1827. [Google Scholar] [CrossRef]
- Marelli, L.; Shusang, V.; Buscombe, J.R.; Cholongitas, E.; Stigliano, R.; Davies, N.; Tibballs, J.; Patch, D.; Meyer, T.; Burroughs, A.K. Transarterial injection of (131)I-lipiodol, compared with chemoembolization, in the treatment of unresectable hepatocellular cancer. J. Nucl. Med. 2009, 50, 871–877. [Google Scholar] [CrossRef]
- Oliveri, R.S.; Wetterslev, J.; Gluud, C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst. Rev. 2011, CD004787. [Google Scholar] [CrossRef]
- Yeung, R.; Beaton, L.; Rackley, T.; Weber, B.; Hamm, J.; Lee, R.; Camborde, M.; Pearson, M.; Duzenli, C.; Loewen, S.K.; et al. Stereotactic Body Radiotherapy for Small Unresectable Hepatocellular Carcinomas. Clin. Oncol. (R. Coll. Radiol.) 2019, 31, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Baumann, B.C.; Wei, J.; Plastaras, J.P.; Lukens, J.N.; Damjanov, N.; Hoteit, M.; Hsu, C.; Levine, M.; Mondschein, J.; Nadolski, G.; et al. Stereotactic Body Radiation Therapy (SBRT) for Hepatocellular Carcinoma: High Rates of Local Control With Low Toxicity. Am. J. Clin. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sanford, N.N.; Pursley, J.; Noe, B.; Yeap, B.Y.; Goyal, L.; Clark, J.W.; Allen, J.N.; Blaszkowsky, L.S.; Ryan, D.P.; Ferrone, C.R.; et al. Protons versus Photons for Unresectable Hepatocellular Carcinoma: Liver Decompensation and Overall Survival. Int. J. Radiat. Oncol. Biol. Phys. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chino, F.; Stephens, S.J.; Choi, S.S.; Marin, D.; Kim, C.Y.; Morse, M.A.; Godfrey, D.J.; Czito, B.G.; Willett, C.G.; Palta, M. The role of external beam radiotherapy in the treatment of hepatocellular cancer. Cancer 2018, 124, 3476–3489. [Google Scholar] [CrossRef] [PubMed]
- Sapir, E.; Tao, Y.; Schipper, M.J.; Bazzi, L.; Novelli, P.M.; Devlin, P.; Owen, D.; Cuneo, K.C.; Lawrence, T.S.; Parikh, N.D.; et al. Stereotactic Body Radiation Therapy as an Alternative to Transarterial Chemoembolization for Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 122–130. [Google Scholar] [CrossRef]
- Chen, L.C.; Chiou, W.Y.; Lin, H.Y.; Lee, M.S.; Lo, Y.C.; Huang, L.W.; Chang, C.M.; Hung, T.H.; Lin, C.W.; Tseng, K.C.; et al. Comparing stereotactic ablative radiotherapy (SABR) versus re-trans-catheter arterial chemoembolization (re-TACE) for hepatocellular carcinoma patients who had incomplete response after initial TACE (TASABR): a randomized controlled trial. BMC Cancer 2019, 19, 275. [Google Scholar] [CrossRef]
- Couri, T.; Pillai, A. Goals and targets for personalized therapy for HCC. Hepatol. Int. 2019, 13, 125–137. [Google Scholar] [CrossRef]
- Meyer, T.; Kirkwood, A.; Roughton, M.; Beare, S.; Tsochatzis, E.; Yu, D.; Davies, N.; Williams, E.; Pereira, S.P.; Hochhauser, D.; et al. A randomised phase II/III trial of 3-weekly cisplatin-based sequential transarterial chemoembolisation vs embolisation alone for hepatocellular carcinoma. Br. J. Cancer 2013, 108, 1252–1259. [Google Scholar] [CrossRef]
- Pitton, M.B.; Kloeckner, R.; Ruckes, C.; Wirth, G.M.; Eichhorn, W.; Worns, M.A.; Weinmann, A.; Schreckenberger, M.; Galle, P.R.; Otto, G.; et al. Randomized comparison of selective internal radiotherapy (SIRT) versus drug-eluting bead transarterial chemoembolization (DEB-TACE) for the treatment of hepatocellular carcinoma. Cardiovasc. Intervent. Radiol. 2015, 38, 352–360. [Google Scholar] [CrossRef]
- Mabed, M.; Esmaeel, M.; El-Khodary, T.; Awad, M.; Amer, T. A randomized controlled trial of transcatheter arterial chemoembolization with lipiodol, doxorubicin and cisplatin versus intravenous doxorubicin for patients with unresectable hepatocellular carcinoma. Eur. J. Cancer Care 2009, 18, 492–499. [Google Scholar] [CrossRef]
- Pleguezuelo, M.; Marelli, L.; Misseri, M.; Germani, G.; Calvaruso, V.; Xiruochakis, E.; Manousou, P.; Burroughs, A.K. TACE versus TAE as therapy for hepatocellular carcinoma. Expert Rev. Anticancer Ther. 2008, 8, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Real, M.I.; Montana, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Sola, R.; et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002, 359, 1734–1739. [Google Scholar] [CrossRef]
- Lo, C.M.; Ngan, H.; Tso, W.K.; Liu, C.L.; Lam, C.M.; Poon, R.T.; Fan, S.T.; Wong, J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002, 35, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.B.; Ma, L.; Wang, X.B.; Bai, T.; Ye, J.Z.; Zhong, J.H.; Li, L.Q. Transarterial embolization with or without chemotherapy for advanced hepatocellular carcinoma: a systematic review. Tumour Biol. 2014, 35, 8451–8459. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.; Tapete, G.; Simonetti, N.; Sellitri, R.; Natali, V.; Melissari, S.; Cabibbo, G.; Biscaglia, L.; Bresci, G.; Giacomelli, L. Transarterial chemoembolization for the treatment of hepatocellular carcinoma: a review. J. Hepatocell Carcinoma 2017, 4, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Lammer, J.; Malagari, K.; Vogl, T.; Pilleul, F.; Denys, A.; Watkinson, A.; Pitton, M.; Sergent, G.; Pfammatter, T.; Terraz, S.; et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc. Intervent. Radiol. 2010, 33, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.; Bargellini, I.; Bertini, M.; Bozzi, E.; Romano, A.; Petruzzi, P.; Tumino, E.; Ginanni, B.; Federici, G.; Cioni, R.; et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J. Vasc. Interv. Radiol. 2011, 22, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Kloeckner, R.; Weinmann, A.; Prinz, F.; Pinto dos Santos, D.; Ruckes, C.; Dueber, C.; Pitton, M.B. Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer 2015, 15, 465. [Google Scholar] [CrossRef]
- Kallini, J.R.; Gabr, A.; Salem, R.; Lewandowski, R.J. Transarterial Radioembolization with Yttrium-90 for the Treatment of Hepatocellular Carcinoma. Adv. Ther. 2016, 33, 699–714. [Google Scholar] [CrossRef]
- Riaz, A.; Gates, V.L.; Atassi, B.; Lewandowski, R.J.; Mulcahy, M.F.; Ryu, R.K.; Sato, K.T.; Baker, T.; Kulik, L.; Gupta, R.; et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 163–171. [Google Scholar] [CrossRef]
- Ozkan, Z.G.; Poyanli, A.; Ucar, A.; Kuyumcu, S.; Akyuz, F.; Keskin, S.; Saglam, S.; Yilmaz, E.; Karaca, C.; Turkmen, C. Favorable survival time provided with radioembolization in hepatocellular carcinoma patients with and without portal vein thrombosis. Cancer Biother. Radiopharm. 2015, 30, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Thurston, K.G. Radioembolization with 90yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 2: special topics. J. Vasc. Interv. Radiol. 2006, 17, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.E.; Abou-Alfa, G.K.; Geschwind, J.F.; Krishnan, S.; Salem, R.; Venook, A.P.; American Hepato-Pancreato-Biliary, A.; Society of Surgical, O.; Society for Surgery of the Alimentary, T. Nonoperative therapies for combined modality treatment of hepatocellular cancer: expert consensus statement. HPB (Oxford) 2010, 12, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Gabr, A.; Riaz, A.; Mora, R.; Ali, R.; Abecassis, M.; Hickey, R.; Kulik, L.; Ganger, D.; Flamm, S.; et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology 2018, 68, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Gaba, R.C.; Lewandowski, R.J.; Kulik, L.M.; Riaz, A.; Ibrahim, S.M.; Mulcahy, M.F.; Ryu, R.K.; Sato, K.T.; Gates, V.; Abecassis, M.M.; et al. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann. Surg. Oncol. 2009, 16, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Raoul, J.L.; Guyader, D.; Bretagne, J.F.; Duvauferrier, R.; Bourguet, P.; Bekhechi, D.; Deugnier, Y.M.; Gosselin, M. Randomized controlled trial for hepatocellular carcinoma with portal vein thrombosis: intra-arterial iodine-131-iodized oil versus medical support. J. Nucl. Med. 1994, 35, 1782–1787. [Google Scholar]
- Golfieri, R. SIR-Spheres yttrium-90 radioembolization for the treatment of unresectable liver cancers. Hepatic Oncol. 2014, 1, 265–283. [Google Scholar] [CrossRef]
- Raoul, J.L.; Guyader, D.; Bretagne, J.F.; Heautot, J.F.; Duvauferrier, R.; Bourguet, P.; Bekhechi, D.; Deugnier, Y.M.; Gosselin, M. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology 1997, 26, 1156–1161. [Google Scholar] [CrossRef]
- Mantry, P.S.; Mehta, A.; Madani, B.; Mejia, A.; Shahin, I. Selective internal radiation therapy using yttrium-90 resin microspheres in patients with unresectable hepatocellular carcinoma: a retrospective study. J. Gastrointest. Oncol. 2017, 8, 799–807. [Google Scholar] [CrossRef]
- Yang, Y.; Si, T. Yttrium-90 transarterial radioembolization versus conventional transarterial chemoembolization for patients with hepatocellular carcinoma: a systematic review and meta-analysis. Cancer Biol. Med. 2018, 15, 299–310. [Google Scholar] [CrossRef]
- Kolligs, F.T.; Bilbao, J.I.; Jakobs, T.; Inarrairaegui, M.; Nagel, J.M.; Rodriguez, M.; Haug, A.; D’Avola, D.; op den Winkel, M.; Martinez-Cuesta, A.; et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int. 2015, 35, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Gordon, A.C.; Mouli, S.; Hickey, R.; Kallini, J.; Gabr, A.; Mulcahy, M.F.; Baker, T.; Abecassis, M.; Miller, F.H.; et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 151, 1155–1163e1152. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, K.; Kitrou, P.; Spiliopoulos, S.; Maroulis, I.; Petsas, T.; Karnabatidis, D. Comparative effectiveness of different transarterial embolization therapies alone or in combination with local ablative or adjuvant systemic treatments for unresectable hepatocellular carcinoma: A network meta-analysis of randomized controlled trials. PLoS ONE 2017, 12, e0184597. [Google Scholar] [CrossRef] [PubMed]
- Op den Winkel, M.; Nagel, D.; Op den Winkel, P.; Trojan, J.; Paprottka, P.M.; Steib, C.J.; Schmidt, L.; Goller, M.; Stieber, P.; Gohring, P.; et al. Transarterial chemoembolization for hepatocellular carcinoma: development and external validation of the Munich-TACE score. Eur. J. Gastroenterol. Hepatol. 2018, 30, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Kadalayil, L.; Benini, R.; Pallan, L.; O’Beirne, J.; Marelli, L.; Yu, D.; Hackshaw, A.; Fox, R.; Johnson, P.; Burroughs, A.K.; et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann. Oncol. 2013, 24, 2565–2570. [Google Scholar] [CrossRef] [PubMed]
- Hucke, F.; Pinter, M.; Graziadei, I.; Bota, S.; Vogel, W.; Muller, C.; Heinzl, H.; Waneck, F.; Trauner, M.; Peck-Radosavljevic, M.; et al. How to STATE suitability and START transarterial chemoembolization in patients with intermediate stage hepatocellular carcinoma. J. Hepatol. 2014, 61, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Golfieri, R.; Bargellini, I.; Spreafico, C.; Trevisani, F. Patients with Barcelona Clinic Liver Cancer Stages B and C Hepatocellular Carcinoma: Time for a Subclassification. Liver Cancer 2019, 8, 78–91. [Google Scholar] [CrossRef]
- Tsochatzis, E.; Garcovich, M.; Marelli, L.; Papastergiou, V.; Fatourou, E.; Rodriguez-Peralvarez, M.L.; Germani, G.; Davies, N.; Yu, D.; Luong, T.V.; et al. Transarterial embolization as neo-adjuvant therapy pretransplantation in patients with hepatocellular carcinoma. Liver Int. 2013, 33, 944–949. [Google Scholar] [CrossRef]
- Werner, J.D.; Frangakis, C.; Ruck, J.M.; Hong, K.; Philosophe, B.; Cameron, A.M.; Saberi, B.; Gurakar, A.; Georgiades, C. Neoadjuvant Transarterial Chemoembolization Improves Survival After Liver Transplant in Patients With Hepatocellular Carcinoma. Exp. Clin. Transplant. 2018. [Google Scholar] [CrossRef]
- Melstrom, L.G.; Eng, O.S.; Raoof, M.; Singh, G.; Fong, Y.; Latorre, K.; Choi, G.H.; Salem, R.; Bentrem, D.J.; Lewandowski, R.; et al. Is hepatectomy safe following Yttrium-90 therapy? A multi-institutional international experience. HPB (Oxford) 2019. [Google Scholar] [CrossRef]
- Lewandowski, R.J.; Donahue, L.; Chokechanachaisakul, A.; Kulik, L.; Mouli, S.; Caicedo, J.; Abecassis, M.; Fryer, J.; Salem, R.; Baker, T. (90) Y radiation lobectomy: Outcomes following surgical resection in patients with hepatic tumors and small future liver remnant volumes. J. Surg. Oncol. 2016, 114, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Piardi, T.; Memeo, R.; Renard, Y.; Ammendola, M.; Bruno, O.; Habersetzer, F.; Baumert, T.; Pessaux, P.; Sommacale, D. Management of large hepatocellular carcinoma by sequential transarterial chemoembolization and portal vein embolization: a systematic review of the literature. Minerva Chir. 2016, 71, 192–200. [Google Scholar]
- Salvalaggio, P.R.; Felga, G.; Axelrod, D.A.; Della Guardia, B.; Almeida, M.D.; Rezende, M.B. List and liver transplant survival according to waiting time in patients with hepatocellular carcinoma. Am. J. Transplant. 2015, 15, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Agopian, V.G.; Harlander-Locke, M.P.; Ruiz, R.M.; Klintmalm, G.B.; Senguttuvan, S.; Florman, S.S.; Haydel, B.; Hoteit, M.; Levine, M.H.; Lee, D.D.; et al. Impact of Pretransplant Bridging Locoregional Therapy for Patients With Hepatocellular Carcinoma Within Milan Criteria Undergoing Liver Transplantation: Analysis of 3601 Patients From the US Multicenter HCC Transplant Consortium. Ann. Surg. 2017, 266, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Frenette, C.T.; Osorio, R.C.; Stark, J.; Fok, B.; Boktour, M.R.; Guy, J.; Rhee, J.; Osorio, R.W. Conventional TACE and drug-eluting bead TACE as locoregional therapy before orthotopic liver transplantation: comparison of explant pathologic response. Transplantation 2014, 98, 781–787. [Google Scholar] [CrossRef]
- Dorcaratto, D.; Udupa, V.; Hogan, N.M.; Brophy, D.P.; McCann, J.W.; Maguire, D.; Geoghegan, J.; Cantwell, C.P.; Hoti, E. Does neoadjuvant doxorubicin drug-eluting bead transarterial chemoembolization improve survival in patients undergoing liver transplant for hepatocellular carcinoma? Diagn. Interv. Radiol. 2017, 23, 441–447. [Google Scholar] [CrossRef]
- Sapisochin, G.; Barry, A.; Doherty, M.; Fischer, S.; Goldaracena, N.; Rosales, R.; Russo, M.; Beecroft, R.; Ghanekar, A.; Bhat, M.; et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J. Hepatol. 2017, 67, 92–99. [Google Scholar] [CrossRef]
- Lewandowski, R.J.; Kulik, L.M.; Riaz, A.; Senthilnathan, S.; Mulcahy, M.F.; Ryu, R.K.; Ibrahim, S.M.; Sato, K.T.; Baker, T.; Miller, F.H.; et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am. J. Transplant. 2009, 9, 1920–1928. [Google Scholar] [CrossRef]
- Gabr, A.; Abouchaleh, N.; Ali, R.; Vouche, M.; Atassi, R.; Memon, K.; Asadi, A.A.; Baker, T.; Caicedo, J.C.; Desai, K.; et al. Comparative study of post-transplant outcomes in hepatocellular carcinoma patients treated with chemoembolization or radioembolization. Eur. J. Radiol. 2017, 93, 100–106. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Bhoori, S.; Romito, R.; Chiesa, C.; Morosi, C.; Maccauro, M.; Marchiano, A.; Bongini, M.; Lanocita, R.; et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology 2013, 57, 1826–1837. [Google Scholar] [CrossRef]
- Ali, R.; Gabr, A.; Abouchaleh, N.; Al Asadi, A.; Mora, R.A.; Kulik, L.; Abecassis, M.; Riaz, A.; Salem, R.; Lewandowski, R.J. Survival Analysis of Advanced HCC Treated with Radioembolization: Comparing Impact of Clinical Performance Status Versus Vascular Invasion/Metastases. Cardiovasc. Intervent. Radiol. 2018, 41, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Levi Sandri, G.B.; Ettorre, G.M.; Colasanti, M.; De Werra, E.; Masciana, G.; Ferraro, D.; Tortorelli, G.; Sciuto, R.; Lucatelli, P.; Pizzi, G.; et al. Hepatocellular carcinoma with macrovascular invasion treated with yttrium-90 radioembolization prior to transplantation. Hepatobiliary Surg. Nutr. 2017, 6, 44–48. [Google Scholar] [CrossRef]
- Chow, P.K.H.; Gandhi, M.; Tan, S.B.; Khin, M.W.; Khasbazar, A.; Ong, J.; Choo, S.P.; Cheow, P.C.; Chotipanich, C.; Lim, K.; et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J. Clin. Oncol. 2018, 36, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1624–1636. [Google Scholar] [CrossRef]
- Niu, G.; Chen, X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr. Drug Targets 2010, 11, 1000–1017. [Google Scholar] [CrossRef] [PubMed]
- Kaseb, A.O.; Morris, J.S.; Hassan, M.M.; Siddiqui, A.M.; Lin, E.; Xiao, L.; Abdalla, E.K.; Vauthey, J.N.; Aloia, T.A.; Krishnan, S.; et al. Clinical and prognostic implications of plasma insulin-like growth factor-1 and vascular endothelial growth factor in patients with hepatocellular carcinoma. J. Clin. Oncol. 2011, 29, 3892–3899. [Google Scholar] [CrossRef]
- Zhang, W.; Kim, R.; Quintini, C.; Hashimoto, K.; Fujiki, M.; Diago, T.; Eghtesad, B.; Miller, C.; Fung, J.; Tan, A.; et al. Prognostic role of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma undergoing liver transplantation. Liver Transpl. 2015, 21, 101–111. [Google Scholar] [CrossRef]
- Campagnolo, L.; Telesca, C.; Massimiani, M.; Stuhlmann, H.; Angelico, M.; Lenci, I.; Manzia, T.M.; Tariciotti, L.; Lehmann, G.; Baiocchi, L. Different expression of VEGF and EGFL7 in human hepatocellular carcinoma. Dig. Liver Dis. 2016, 48, 76–80. [Google Scholar] [CrossRef]
- Zhan, P.; Qian, Q.; Yu, L.K. Serum VEGF level is associated with the outcome of patients with hepatocellular carcinoma: a meta-analysis. Hepatobiliary Surg. Nutr. 2013, 2, 209–215. [Google Scholar] [CrossRef]
- Nahm, J.H.; Rhee, H.; Kim, H.; Yoo, J.E.; San Lee, J.; Jeon, Y.; Choi, G.H.; Park, Y.N. Increased expression of stemness markers and altered tumor stroma in hepatocellular carcinoma under TACE-induced hypoxia: A biopsy and resection matched study. Oncotarget 2017, 8, 99359–99371. [Google Scholar] [CrossRef]
- Tong, Y.; Yang, H.; Xu, X.; Ruan, J.; Liang, M.; Wu, J.; Luo, B. Effect of a hypoxic microenvironment after radiofrequency ablation on residual hepatocellular cell migration and invasion. Cancer Sci. 2017, 108, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Marech, I.; Lorusso, V.; Goffredo, V.; Paradiso, A.; Ribatti, D.; Gadaleta, C.D. Molecular targeting agents associated with transarterial chemoembolization or radiofrequency ablation in hepatocarcinoma treatment. World J. Gastroenterol. 2014, 20, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.M.; Rodrigues, P.D.; Alvares-da-Silva, M.R.; Scaffaro, L.A.; Farenzena, M.; Teixeira, U.F.; Waechter, F.L. Treatment strategies for locally advanced hepatocellular carcinoma. Transl. Gastroenterol. Hepatol. 2019, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.W.; Jayant, K.; Lee, P.H.; Yang, P.C.; Hsiao, C.Y.; Habib, N.; Sodergren, M.H. Positive Immuno-Modulation Following Radiofrequency Assisted Liver Resection in Hepatocellular Carcinoma. J. Clin. Med. 2019, 8, 385. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Song, G.W.; Park, S.; Jung, M.K.; Kim, M.H.; Kang, H.J.; Yoo, C.; Yi, K.; Kim, K.H.; Eo, S.; et al. Association Between Expression Level of PD1 by Tumor-Infiltrating CD8(+) T Cells and Features of Hepatocellular Carcinoma. Gastroenterology 2018, 155, 1936–1950e1917. [Google Scholar] [CrossRef] [PubMed]
- Chew, V.; Lee, Y.H.; Pan, L.; Nasir, N.J.M.; Lim, C.J.; Chua, C.; Lai, L.; Hazirah, S.N.; Lim, T.K.H.; Goh, B.K.P.; et al. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut 2019, 68, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.; Thadi, A.; Shailubhai, K. Hepatocellular Carcinoma: Etiology and Current and Future Drugs. J. Clin. Exp. Hepatol. 2019, 9, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Labeur, T.A.; Ten Cate, D.W.G.; Bart Takkenberg, R.; Azahaf, H.; van Oijen, M.G.H.; van Delden, O.M.; de Man, R.A.; van Vugt, J.L.A.; JNM, I.J.; Eskens, F.; et al. Are we SHARP enough? The importance of adequate patient selection in sorafenib treatment for hepatocellular carcinoma. Acta Oncol. 2018, 57, 1467–1474. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 2017, 169, 1327–1341e1323. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Smits, R.; Hao, H.; He, C. Wnt/beta-Catenin Signaling in Liver Cancers. Cancers (Basel) 2019, 11, 926. [Google Scholar] [CrossRef] [PubMed]
- Adebayo Michael, A.O.; Ko, S.; Tao, J.; Moghe, A.; Yang, H.; Xu, M.; Russell, J.O.; Pradhan-Sundd, T.; Liu, S.; Singh, S.; et al. Inhibiting Glutamine-Dependent mTORC1 Activation Ameliorates Liver Cancers Driven by beta-Catenin Mutations. Cell Metab. 2019, 29, 1135–1150e1136. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Imanaka, K.; Chida, N.; Nakachi, K.; Tak, W.Y.; Takayama, T.; Yoon, J.H.; Hori, T.; Kumada, H.; Hayashi, N.; et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur. J. Cancer 2011, 47, 2117–2127. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M.; Han, G.; Tak, W.Y.; Yang, J.; Guglielmi, A.; Paik, S.W.; Reig, M.; Kim, D.Y.; Chau, G.Y.; et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J. Hepatol. 2016, 64, 1090–1098. [Google Scholar] [CrossRef]
- Meyer, T.; Fox, R.; Ma, Y.T.; Ross, P.J.; James, M.W.; Sturgess, R.; Stubbs, C.; Stocken, D.D.; Wall, L.; Watkinson, A.; et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol. 2017, 2, 565–575. [Google Scholar] [CrossRef]
- Kudo, M. Proposal of Primary Endpoints for TACE Combination Trials with Systemic Therapy: Lessons Learned from 5 Negative Trials and the Positive TACTICS Trial. Liver Cancer 2018, 7, 225–234. [Google Scholar] [CrossRef]
- Pokuri, V.K.; Tomaszewski, G.M.; Ait-Oudhia, S.; Groman, A.; Khushalani, N.I.; Lugade, A.A.; Thanavala, Y.; Ashton, E.A.; Grande, C.; Fetterly, G.J.; et al. Efficacy, Safety, and Potential Biomarkers of Sunitinib and Transarterial Chemoembolization (TACE) Combination in Advanced Hepatocellular Carcinoma (HCC): Phase II Trial. Am. J. Clin. Oncol. 2018, 41, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Mahvash, A.; Murthy, R.; Odisio, B.C.; Raghav, K.P.; Girard, L.; Cheung, S.; Nguyen, V.; Ensor, J.; Gadani, S.; Elsayes, K.M.; et al. Yttrium-90 resin microspheres as an adjunct to sorafenib in patients with unresectable hepatocellular carcinoma. J. Hepatocell Carcinoma 2016, 3, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- van Beek, A.A.; Zhou, G.; Doukas, M.; Boor, P.P.C.; Noordam, L.; Mancham, S.; Campos Carrascosa, L.; van der Heide-Mulder, M.; Polak, W.G.; Ijzermans, J.N.M.; et al. GITR ligation enhances functionality of tumor-infiltrating T cells in hepatocellular carcinoma. Int. J. Cancer 2019, 145, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Wehrenberg-Klee, E.; Goyal, L.; Dugan, M.; Zhu, A.X.; Ganguli, S. Y-90 Radioembolization Combined with a PD-1 Inhibitor for Advanced Hepatocellular Carcinoma. Cardiovasc. Intervent. Radiol. 2018, 41, 1799–1802. [Google Scholar] [CrossRef] [PubMed]
- Adcock, C.S.; Puneky, L.V.; Campbell, G.S. Favorable Response of Metastatic Hepatocellular Carcinoma to Treatment with Trans-arterial Radioembolization Followed by Sorafenib and Nivolumab. Cureus 2019, 11, e4083. [Google Scholar] [CrossRef] [PubMed]
| Study | Inclusion Criteria | Regimens | Primary Endpoint | Duration of Sorafenib | Reference |
|---|---|---|---|---|---|
| SPACE trial (n = 307) Phase 2 | Unresectable, multinodular HCC | DEB-TACE + sorafenib vs. DEB-TACE | TTP mRECIST 169 vs. 166 days HR 0.80 p > 0.05 | 21 weeks | Lencioni et al. [98] |
| TACE 2 (n = 313) Phase 3 | Unresectable | DEB-TACE + sorafenib vs. DEB-TACE | PFS RECIST 1.1 238 vs. 235 days HR 0.99 p > 0.05 | 17 weeks | Meyer et al. [99] |
| TACTICS (n = 156), 2018 Phase3 | Unresectable Child-A 10 lesions max 10 cm max | cTACE + sorafenib vs. cTACE | UnTACEable PFS 25.2 vs. 13.5, HR 0.59 p < 0.01 | 39 weeks | Kudo et al. [100] |
| Study | Inclusion Criteria | Regimens | Primary Endpoint | Reference |
|---|---|---|---|---|
| SARAH (n = 467), 2017 Phase3 | Unresectable or refractory to TACE or BCLC C | Y-90 vs. sorafenib | OS 8.0 vs. 9.9, p < 0.18 | Vilgrain et al. [74] |
| SIRveNIB (n = 360) Phase 2 | Locally advanced | Y-90 vs. sorafenib | OS 8.8 vs. 10, p < 0.36 | Chow et al. [73] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viveiros, P.; Riaz, A.; Lewandowski, R.J.; Mahalingam, D. Current State of Liver-Directed Therapies and Combinatory Approaches with Systemic Therapy in Hepatocellular Carcinoma (HCC). Cancers 2019, 11, 1085. https://doi.org/10.3390/cancers11081085
Viveiros P, Riaz A, Lewandowski RJ, Mahalingam D. Current State of Liver-Directed Therapies and Combinatory Approaches with Systemic Therapy in Hepatocellular Carcinoma (HCC). Cancers. 2019; 11(8):1085. https://doi.org/10.3390/cancers11081085
Chicago/Turabian StyleViveiros, Pedro, Ahsun Riaz, Robert J. Lewandowski, and Devalingam Mahalingam. 2019. "Current State of Liver-Directed Therapies and Combinatory Approaches with Systemic Therapy in Hepatocellular Carcinoma (HCC)" Cancers 11, no. 8: 1085. https://doi.org/10.3390/cancers11081085
APA StyleViveiros, P., Riaz, A., Lewandowski, R. J., & Mahalingam, D. (2019). Current State of Liver-Directed Therapies and Combinatory Approaches with Systemic Therapy in Hepatocellular Carcinoma (HCC). Cancers, 11(8), 1085. https://doi.org/10.3390/cancers11081085




