Nomogram for Predicting Survival in Patients Treated with Liposomal Irinotecan Plus Fluorouracil and Leucovorin in Metastatic Pancreatic Cancer
Abstract
1. Introduction
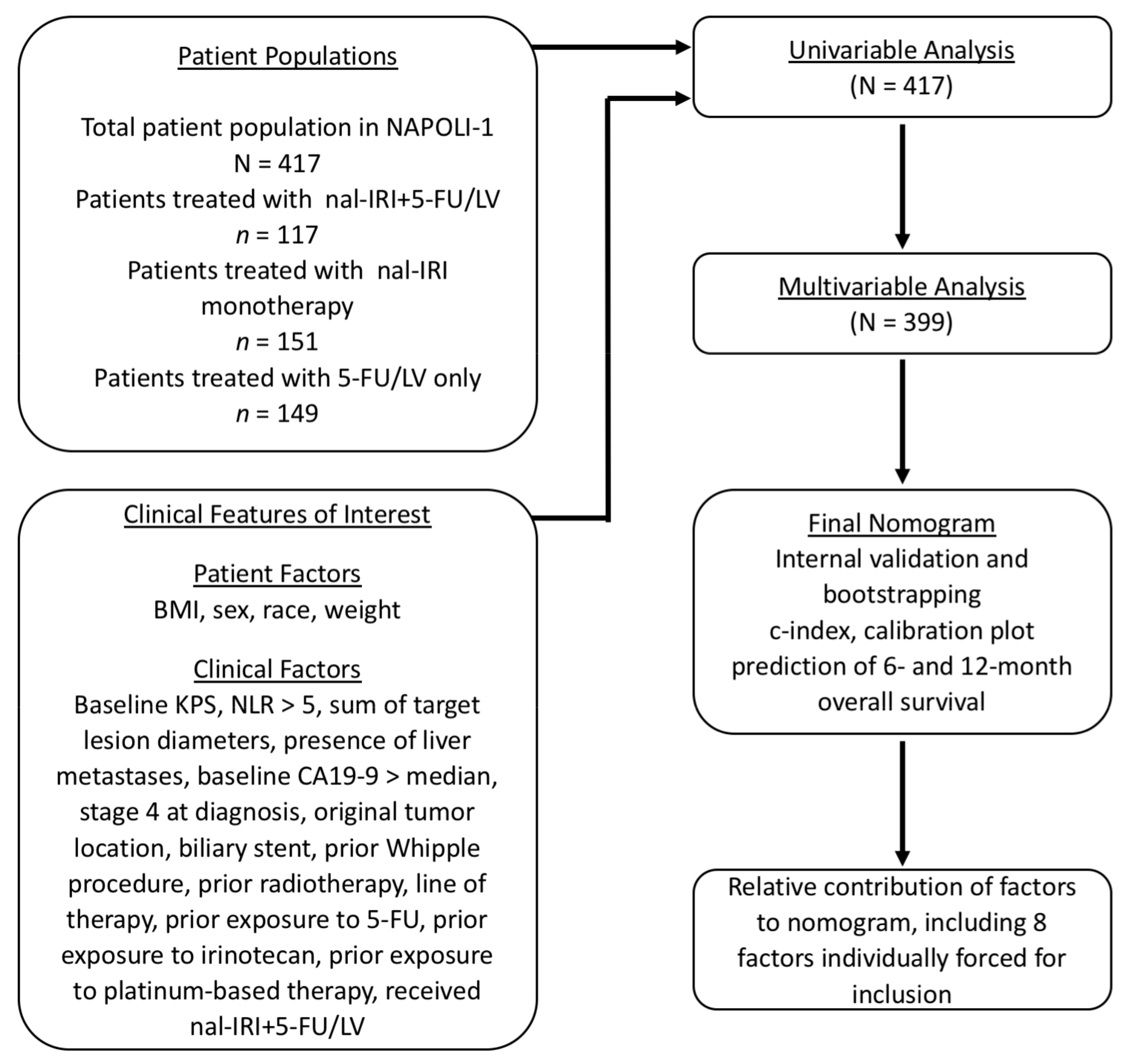
2. Materials and Methods
2.1. Patients
2.2. Statistical Analyses
3. Results
3.1. Patient Distribution and Demographics
3.2. Prognostic Factors of Overall Survival
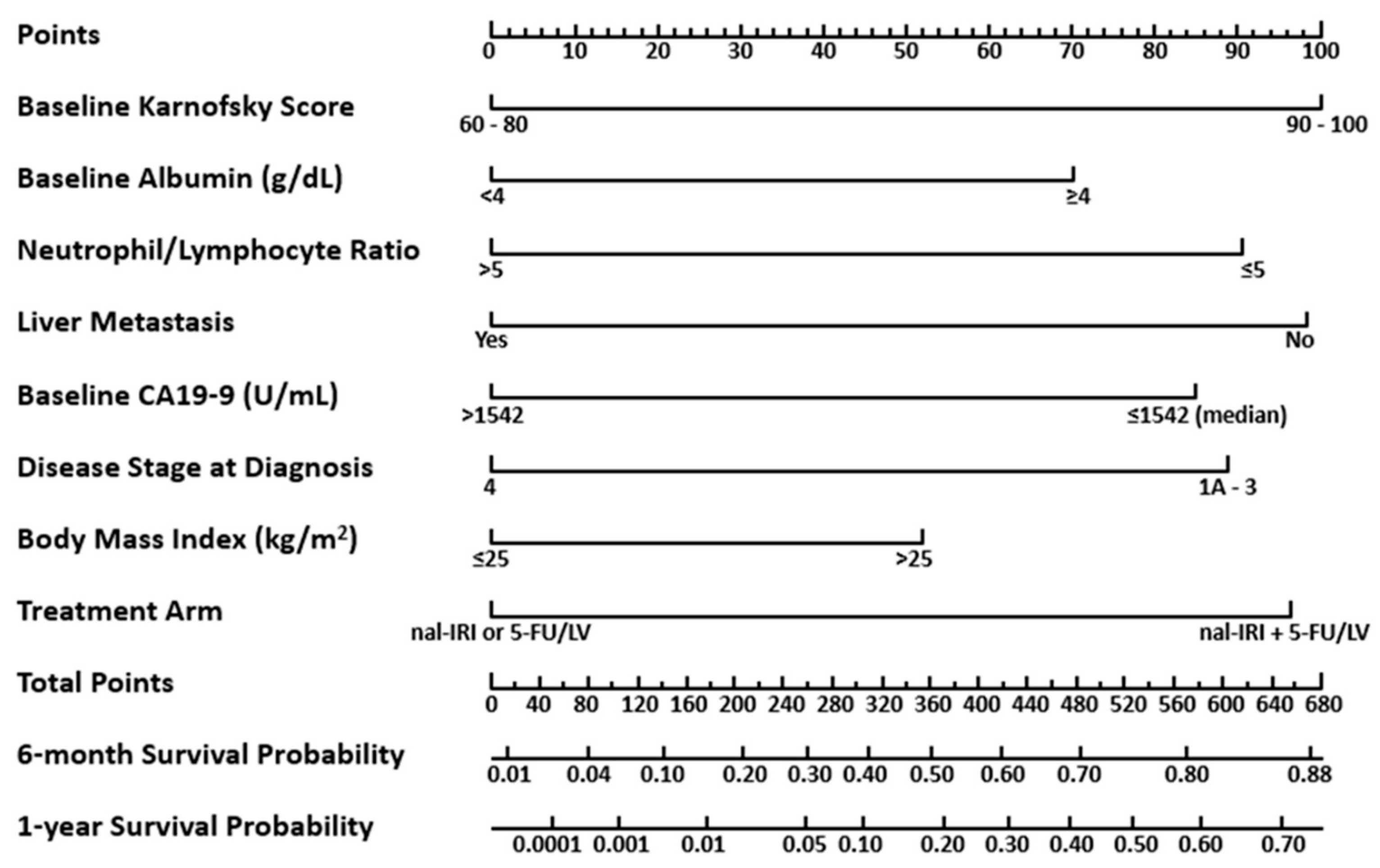
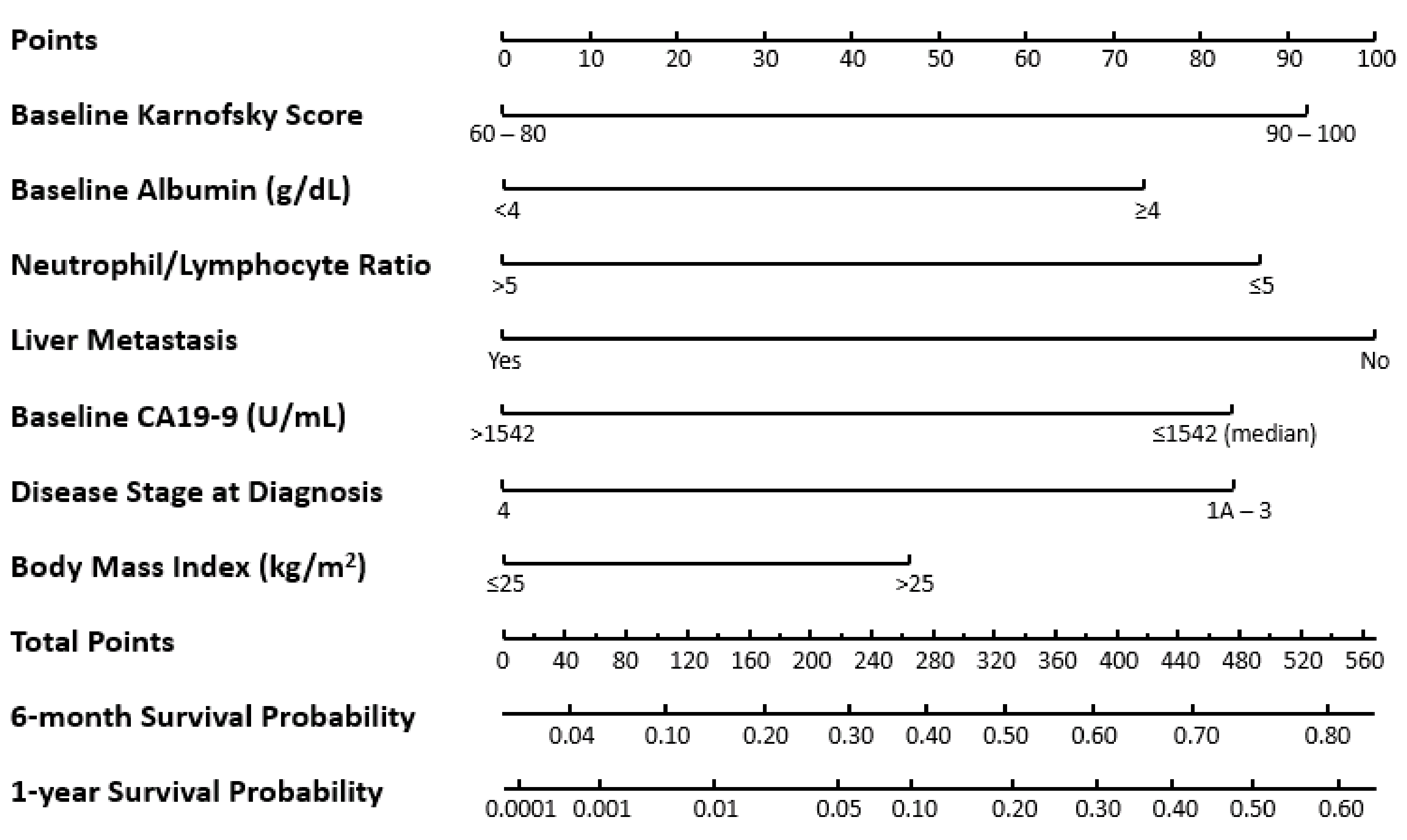
3.3. Nomogram for OS
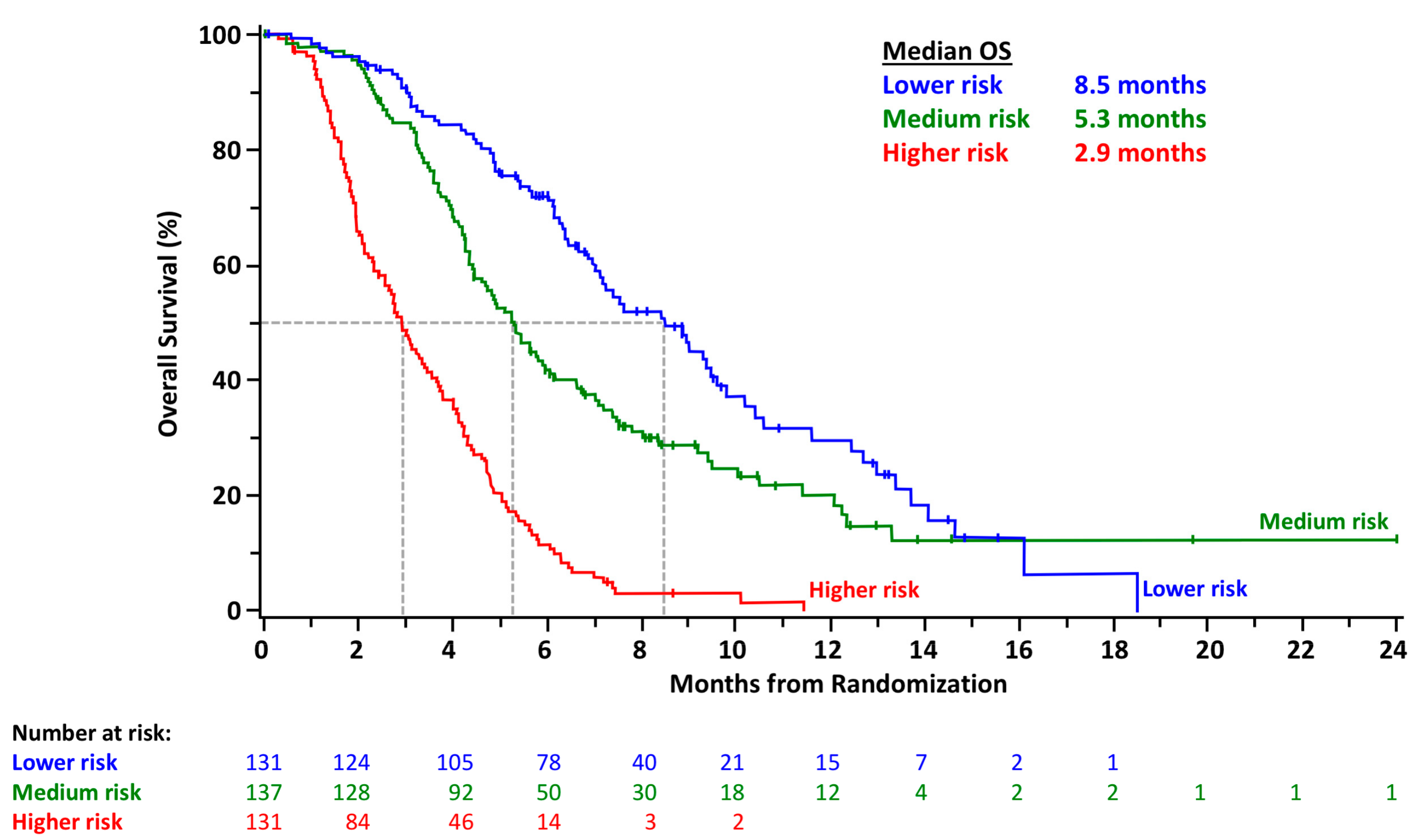
3.4. Utilizing the Nomogram for OS
3.5. Model Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization-International Agency for Research on Cancer (IARC). GLOBOCAN 2018: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2018. Available online: http://gco.iarc.fr/today/home (accessed on 10 October 2018).
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, M.; Bertuccio, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2014. Ann. Oncol. 2014, 25, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- SEER Cancer Statistics Factsheets: Pancreatic Cancer. National Cancer Institute. Available online: http://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 12 October 2018).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, 56–68. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Guidelines Version 2.2018 Pancreatic Adenocarcinoma. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 9 October 2018).
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouche, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; De La Fouchardiere, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Iasonos, A.; Schrag, D.; Raj, G.V.; Panageas, K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 2008, 26, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Touijer, K.; Scardino, P.T. Nomograms for staging, prognosis, and predicting treatment outcomes. Cancer 2009, 115, 3107–3111. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology–more than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef]
- Lee, C.K.; Simes, R.J.; Brown, C.; Lord, S.J.; Wagner, U.; Plante, M.; Vergote, I.; Pisano, C.; Parma, G.; Burges, A.; et al. Prognostic nomogram to predict progression-free survival in patients with platinum-sensitive recurrent ovarian cancer. Br. J. Cancer 2011, 105, 1144–1150. [Google Scholar] [CrossRef]
- Delpech, Y.; I Bashour, S.; Lousquy, R.; Rouzier, R.; Hess, K.; Coutant, C.; Barranger, E.; Esteva, F.J.; Ueno, N.T.; Pusztai, L.; et al. Clinical nomogram to predict bone-only metastasis in patients with early breast carcinoma. Br. J. Cancer 2015, 113, 1003–1009. [Google Scholar] [CrossRef]
- Niu, X.K.; Li, J.; Das, S.K.; Xiong, Y.; Yang, C.B.; Peng, T. Developing a nomogram based on multiparametric magnetic resonance imaging for forecasting high-grade prostate cancer to reduce unnecessary biopsies within the prostate-specific antigen gray zone. BMC Med. Imaging 2017, 17, 277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Luo, Q.F.; Yin, X.W.; Dai, Z.L.; Basnet, S.; Ge, H.Y. Nomograms to predict survival after colorectal cancer resection without preoperative therapy. BMC Cancer 2016, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.C.; Shiah, H.S.; Yang, C.H.; Yeh, K.H.; Cheng, A.L.; Shen, B.N.; Wang, Y.W.; Yeh, C.G.; Chiang, N.J.; Chang, J.Y.; et al. Phase I study of nanoliposomal irinotecan (PEP02) in advanced solid tumor patients. Cancer Chemother. Pharmacol. 2015, 75, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.V.; Kim, J.; Klinz, S.G.; Paz, N.; Cain, J.; Drummond, D.C.; Nielsen, U.B.; Fitzgerald, J.B. Preclinical activity of anoliposomal irinotecan is governed by tumor deposition and intratumor prodrug conversion. Cancer Res. 2014, 74, 7003–7013. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.K.; Korn, R.L.; Raghunand, N.; Sachdev, J.C.; Newbold, R.G.; Jameson, G.; Fetterly, G.J.; Prey, J.; Klinz, S.G.; Kim, J.; et al. Correlation between Ferumoxytol uptake in tumor lesions by MRI and response to nanoliposomal irinotecan in patients with advanced solid tumors: a pilot study. Clin. Cancer Res. 2017, 23, 3638–3648. [Google Scholar] [CrossRef]
- Roy, A.C.; Park, S.R.; Cunningham, D.; Kang, Y.K.; Chao, Y.; Chen, L.T.; Rees, C.; Lim, H.Y.; Tabernero, J.; Ramos, F.J.; et al. A randomized phase II study of PEP02 (MM-398), irinotecan or docetaxel as a second-line therapy in patients with locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma. Ann. Oncol. 2013, 24, 1567–1573. [Google Scholar] [CrossRef]
- Ma, W.W.; Chung, I.; Lang, I.; Csõszi, T.; Wenczl, M.W.; Cubillo, A.; Chen, J.; Wong, M.; Park, J.O.; Kim, J.S.; et al. Nanoliposomal irinotecan (MM-398, nal-IRI) population pharmacokinetics (PK) and its association with efficacy and safety in patients with solid tumors based on the phase 3 study NAPOLI-1 and five phase 1 and 2 studies [Poster 327]. In Proceedings of the European Cancer Congress, Vienna, Austria, 25–29 September 2015. [Google Scholar]
- Wang-Gillam, A.; Li, C.P.; Bodoky, G.; Dean, A.; Shan, Y.S.; Jameson, G.; Macarulla, T.; Lee, K.H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef]
- Vienot, A.; Beinse, G.; Louvet, C.; Fein, F.; Heyd, B.; Cleau, D.; D’Engremont, C.; Dupont-Gossart, A.C.; Lakkis, Z.; Tournigand, C.; et al. Overall survival prediction and usefulness of second-line chemotherapy in advanced pancreatic adenocarcinoma. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Goldstein, D.; Hoff, D.D.V.; Chiorean, E.G.; Reni, M.; Tabernero, J.; Ramanathan, R.K.; Aly, A.; Botteman, M.; Wilkersen, J.; Margunato-Debay, S.; et al. Nomogram for predicting overall survival (OS) in patients (pts) treated with nab-paclitaxel (nab-P) plus gemcitabine (Gem) or Gem alone for metastatic pancreatic cancer (MPC). J. Clin. Oncol. 2017, 35, 4109. [Google Scholar] [CrossRef]
- Hamada, T.; Nakai, Y.; Yasunaga, H.; Isayama, H.; Matsui, H.; Takahara, N.; Sasaki, T.; Takagi, K.; Watanabe, T.; Yagioka, H.; et al. Prognostic nomogram for nonresectable pancreatic cancer treated with gemcitabine-based chemotherapy. Br. J. Cancer 2014, 110, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Miao, D.L.; Chen, L. Nomogram for predicting survival in patients with pancreatic cancer. OncoTargets Ther. 2018, 11, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.H.; Chung, M.J.; Park, J.Y.; Bang, S.; Park, S.W.; Chung, J.B.; Song, S.Y. Clinical characteristics of long-term survivors of inoperable pancreatic cancer: An 8-year cohort analysis in Korea. Pancreas 2014, 43, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Ter Veer, E.; Van Rijssen, L.B.; Besselink, M.G.; A Mali, R.M.; Berlin, J.D.; Boeck, S.; Bonnetain, F.; Chau, I.; Conroy, T.; Van Cutsem, E.; et al. Consensus statement on mandatory measurements in pancreatic cancer trials (COMM-PACT) for systemic treatment of unresectable disease. Lancet Oncol. 2018, 19, e151–e160. [Google Scholar] [CrossRef]
| Parameter, M (SD) | n | Wald p-Value | Hazard Ratio (95% CI) |
|---|---|---|---|
| Baseline Karnofsky Performance Score 1, ≥90 vs. <90 | 232, 185 | <0.0001 | 0.527 (0.421, 0.660) |
| Baseline albumin, ≥4 g/dL vs. <4 g/dL | 227, 190 | <0.0001 | 0.643 (0.515, 0.802) |
| Neutrophil/lymphocyte ratio, ≤5 vs. >5 | 292, 123 | <0.0001 | 0.582 (0.458, 0.741) |
| Sum of longest diameter of target lesions (mm), 71.4 (46.31) | 417 | <0.0001 | 1.005 (1.003, 1.007) |
| Presence of liver metastases, yes vs. no | 285, 132 | <0.0001 | 1.688 (1.314, 2.168) |
| Baseline CA19-9, >median (1542 U/mL) vs. ≤median | 202, 202 | <0.0001 | 1.620 (1.291, 2.032) |
| Stage 4 disease at time of diagnosis 2, yes vs. no | 213, 200 | <0.0001 | 1.774 (1.413, 2.226) |
| Primary tumor location: Head of pancreas vs. other | 256, 161 | 0.19 | 0.860 (0.685, 1.079) |
| Prior biliary stent, yes vs. no | 37, 380 | 0.90 | 0.973 (0.651, 1.455) |
| Prior Whipple procedure, yes vs. no | 113, 304 | 0.021 | 0.739 (0.573, 0.955) |
| Prior radiotherapy, yes vs. no | 97, 320 | 0.0046 | 0.668 (0.506, 0.883) |
| Prior line of therapy in metastatic setting, (0, 1, 2+) | 51, 234, 132 | 0.71 | 1.033 (0.870, 1.227) |
| Prior exposure to 5-FU, yes vs. no | 183, 234 | 0.43 | 1.095 (0.874, 1.370) |
| Prior exposure to irinotecan, yes vs. no | 46, 371 | 0.13 | 1.324 (0.922, 1.902) |
| Prior exposure to platinum-based therapy, yes vs. no | 137, 280 | 0.44 | 1.099 (0.867, 1.394) |
| Received nal-IRI+5-FU/LV, yes vs. no | 117, 300 | 0.0008 | 0.640 (0.493, 0.830) |
| Age (years), 62.8 (9.68) | 417 | 0.13 | 1.009 (0.997, 1.020) |
| Body mass index >25 kg/m2 vs. ≤25 kg/m2 | 122, 295 | 0.020 | 0.746 (0.584, 0.954) |
| Race: White vs. non-white | 253, 164 | 0.30 | 1.129 (0.900, 1.416) |
| Race: Asian vs. non-Asian | 136, 281 | 0.20 | 0.857 (0.677, 1.085) |
| Sex: Female vs. male | 180, 237 | 0.86 | 0.979 (0.782, 1.227) |
| Weight (kg), 65.3 (15.66) | 417 | 0.065 | 0.993 (0.987, 1.000) |
| Parameter | Eight-Parameter Model | Seven-Parameter Model | |||||
|---|---|---|---|---|---|---|---|
| Number of Patients | Parameter Estimate (β) | Wald p-Value | Hazard Ratio (95% CI) | Parameter Estimate (β) | Wald p-Value | Hazard Ratio (95% CI) | |
| Baseline Karnofsky score ≥90 vs. 60–80 | 219/180 | –0.545 | <0.0001 | 0.58 (0.46, 0.74) | –0.502 | <0.0001 | 0.61 (0.478, 0.768) |
| Baseline albumin ≥4 vs. <4 g/dL | 221/178 | –0.382 | 0.0013 | 0.68 (0.54, 0.86) | –0.398 | 0.0008 | 0.67 (0.532, 0.848) |
| Neutrophil/lymph- ocyte ratio ≤5 vs. >5 | 284/115 | –0.493 | 0.0001 | 0.61 (0.47, 0.79) | –0.471 | 0.0002 | 0.63 (0.486, 0.802) |
| No liver metastasis vs. liver metastases | 124/275 | –0.534 | <0.0001 | 0.59 (0.45, 0.76) | –0.541 | <0.0001 | 0.58 (0.449, 0.754) |
| Baseline CA19-9 ≤1542 vs. >1542 U/mL | 199/200 | –0.462 | <0.0001 | 0.63 (0.50, 0.79) | –0.454 | 0.0001 | 0.63 (0.504, 0.801) |
| Stage <4 vs. Stage 4 at diagnosis 2 | 190/209 | –0.483 | <0.0001 | 0.62 (0.49, 0.78) | –0.454 | 0.0002 | 0.63 (0.501, 0.805) |
| Body mass index >25 vs. ≤25 kg/m2 | 121/278 | –0.283 | 0.030 | 0.75 (0.58, 0.97) | –0.252 | 0.052 | 0.78 (0.603, 1.002) |
| nal-IRI+5-FU/LV vs. 5-FU/LV or nal-IRI | 112/287 | –0.523 | 0.0001 | 0.59 (0.45, 0.77) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-T.; Macarulla, T.; Blanc, J.-F.; Mirakhur, B.; de Jong, F.A.; Belanger, B.; Bekaii-Saab, T.; Siveke, J.T. Nomogram for Predicting Survival in Patients Treated with Liposomal Irinotecan Plus Fluorouracil and Leucovorin in Metastatic Pancreatic Cancer. Cancers 2019, 11, 1068. https://doi.org/10.3390/cancers11081068
Chen L-T, Macarulla T, Blanc J-F, Mirakhur B, de Jong FA, Belanger B, Bekaii-Saab T, Siveke JT. Nomogram for Predicting Survival in Patients Treated with Liposomal Irinotecan Plus Fluorouracil and Leucovorin in Metastatic Pancreatic Cancer. Cancers. 2019; 11(8):1068. https://doi.org/10.3390/cancers11081068
Chicago/Turabian StyleChen, Li-Tzong, Teresa Macarulla, Jean-Frédéric Blanc, Beloo Mirakhur, Floris A. de Jong, Bruce Belanger, Tanios Bekaii-Saab, and Jens T. Siveke. 2019. "Nomogram for Predicting Survival in Patients Treated with Liposomal Irinotecan Plus Fluorouracil and Leucovorin in Metastatic Pancreatic Cancer" Cancers 11, no. 8: 1068. https://doi.org/10.3390/cancers11081068
APA StyleChen, L.-T., Macarulla, T., Blanc, J.-F., Mirakhur, B., de Jong, F. A., Belanger, B., Bekaii-Saab, T., & Siveke, J. T. (2019). Nomogram for Predicting Survival in Patients Treated with Liposomal Irinotecan Plus Fluorouracil and Leucovorin in Metastatic Pancreatic Cancer. Cancers, 11(8), 1068. https://doi.org/10.3390/cancers11081068






