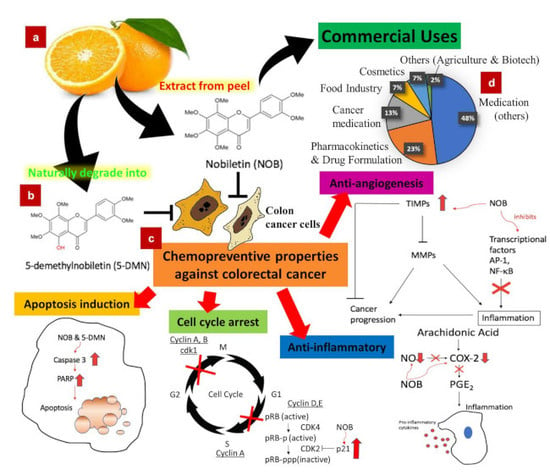
Nobiletin and Derivatives: Functional Compounds from Citrus Fruit Peel for Colon Cancer Chemoprevention
Abstract
1. Introduction
2. Research Methodology
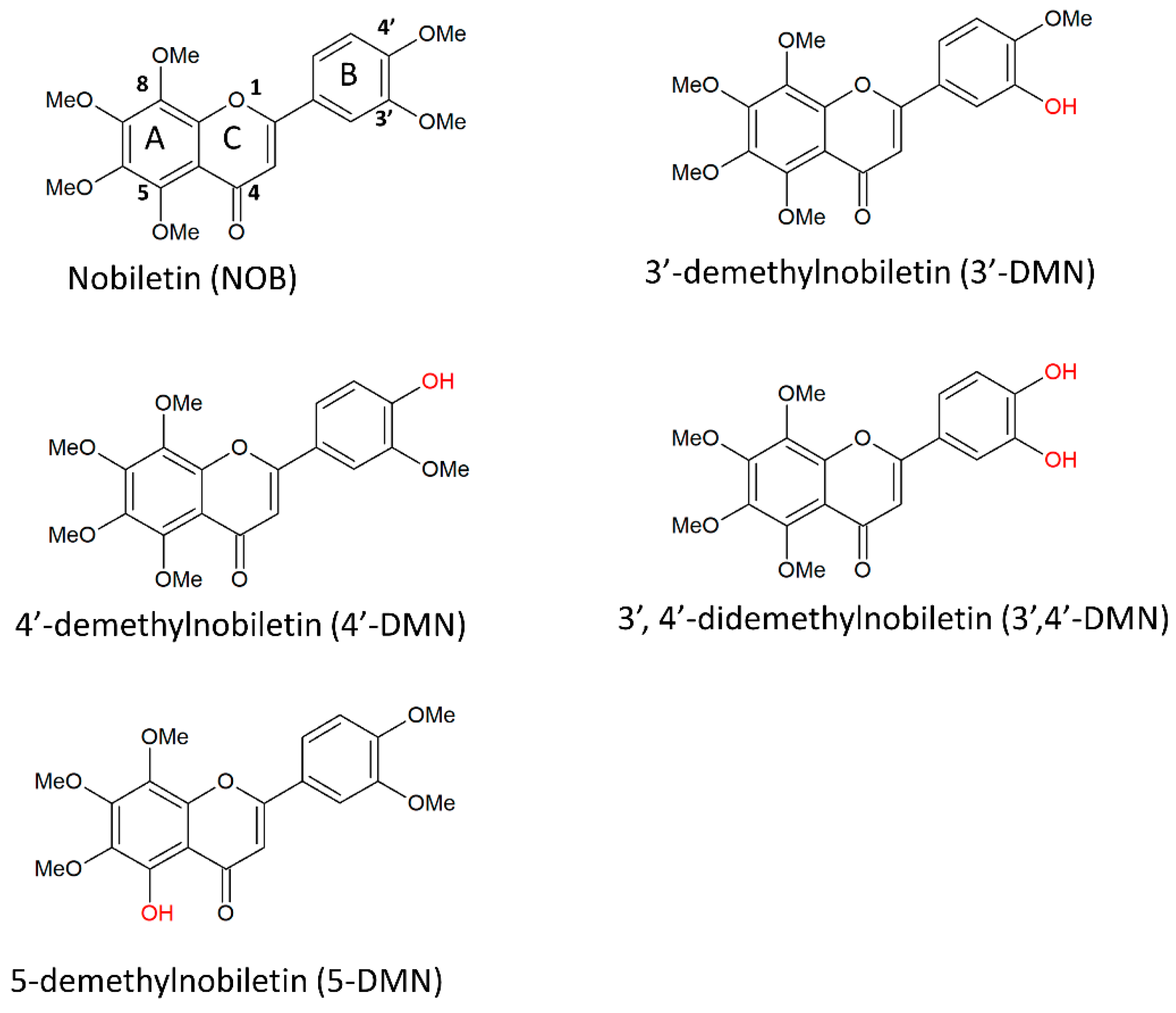
3. Nobiletin and Its Derivatives
4. Pathogenesis of Colorectal Cancer
5. Chemopreventive Effects of Nobiletin, 5-DMN and NOB-Metabolites
5.1. Cell Cycle Arrest
5.1.1. Action of NOB and Its Metabolites Inducing Cell Arrest
5.1.2. Action of 5-DMN Inducing Cell Cycle Arrest
5.2. Programmed Cell Death
Action of NOB and Metabolites Inducing Programmed Cell Death
5.3. Anti-Inflammation
Anti-Inflammation Effect of NOB and Its Metabolites
5.4. Anti-Angiogenesis
Anti-Angiogenesis Effect of NOB
6. Pharmacokinetics, Bioavailability and Delivery Systems of NOB
7. Toxicity
8. Commercial Uses
9. Future Directions
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Blanchard, J.F.; Kliewer, E.; Wajda, A. Cancer risk in patients with inflammatory bowel disease: A population-based study. Cancer 2001, 91, 854–862. [Google Scholar] [CrossRef]
- Rubin, D.C.; Shaker, A.; Levin, M.S. Chronic intestinal inflammation: Inflammatory bowel disease and colitis-associated colon cancer. Front. Immunol. 2012, 3, 107. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Simon, K. Colorectal cancer development and advances in screening. Clin. Interv. Aging 2016, 11, 967–976. [Google Scholar] [PubMed]
- Tan, L.T.H.; Lee, L.H.; Yin, W.F.; Chan, C.K.; Abdul Kadir, H.; Chan, K.G.; Goh, B.H. Traditional Uses, Phytochemistry, and Bioactivities of Cananga odorata (Ylang-Ylang). Evid. Based Complement. Altern. Med. 2015, 2015, 30. [Google Scholar] [CrossRef]
- Chan, W.-K.; Tan, L.T.-H.; Chan, K.-G.; Lee, L.-H.; Goh, B.-H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef]
- Tan, H.-L.; Chan, K.-G.; Pusparajah, P.; Saokaew, S.; Duangjai, A.; Lee, L.-H.; Goh, B.-H. Anti-Cancer Properties of the Naturally Occurring Aphrodisiacs: Icariin and Its Derivatives. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef]
- Steward, W.P.; Brown, K. Cancer chemoprevention: A rapidly evolving field. Br. J. Cancer 2013, 109, 1. [Google Scholar] [CrossRef]
- Tang, C.; Hoo, P.C.-X.; Tan, L.T.-H.; Pusparajah, P.; Khan, T.M.; Lee, L.-H.; Goh, B.-H.; Chan, K.-G. Golden Needle Mushroom: A Culinary Medicine with Evidenced-Based Biological Activities and Health Promoting Properties. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef]
- Alam, M.N.; Almoyad, M.; Huq, F. Polyphenols in Colorectal Cancer: Current State of Knowledge including Clinical Trials and Molecular Mechanism of Action. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cai, D.; Pearce, K.; Sun, P.Y.; Roberts, A.C.; Glanzman, D.L. Reinstatement of long-term memory following erasure of its behavioral and synaptic expression in Aplysia. eLife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Surichan, S.; Arroo, R.R.; Ruparelia, K.; Tsatsakis, A.M.; Androutsopoulos, V.P. Nobiletin bioactivation in MDA-MB-468 breast cancer cells by cytochrome P450 CYP1 enzymes. Food Chem. Toxicol. 2018, 113, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Morley, K.L.; Ferguson, P.J.; Koropatnick, J. Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Lett. 2007, 251, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-P.; Guo, H.; Wang, X.-B. Nobiletin (NOB) suppresses autophagic degradation via over-expressing AKT pathway and enhances apoptosis in multidrug-resistant SKOV3/TAX ovarian cancer cells. Biomed. Pharmacother. 2018, 103, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Cho, M.; Ahn, K.S.; Cho, S.K. Nobiletin induces apoptosis and potentiates the effects of the anticancer drug 5-fluorouracil in p53-mutated SNU-16 human gastric cancer cells. Nutr. Cancer 2013, 65, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Cho, S.K. Nobiletin induces protective autophagy accompanied by ER-stress mediated apoptosis in human gastric cancer SNU-16 cells. Molecules 2016, 21, 914. [Google Scholar] [CrossRef] [PubMed]
- Uesato, S.; Yamashita, H.; Maeda, R.; Hirata, Y.; Yamamoto, M.; Matsue, S.; Nagaoka, Y.; Shibano, M.; Taniguchi, M.; Baba, K. Synergistic antitumor effect of a combination of paclitaxel and carboplatin with nobiletin from Citrus depressa on non-small-cell lung cancer cell lines. Planta Med. 2014, 80, 452–457. [Google Scholar] [CrossRef]
- Song, M.; Wu, X.; Charoensinphon, N.; Wang, M.; Zheng, J.; Gao, Z.; Xu, F.; Li, Z.; Li, F.; Zhou, J. Dietary 5-demethylnobiletin inhibits cigarette carcinogen NNK-induced lung tumorigenesis in mice. Food Funct. 2017, 8, 954–963. [Google Scholar] [CrossRef]
- Ma, X.; Jin, S.; Zhang, Y.; Wan, L.; Zhao, Y.; Zhou, L. Inhibitory effects of nobiletin on hepatocellular carcinoma in vitro and in vivo. Phytother. Res. 2014, 28, 560–567. [Google Scholar] [CrossRef]
- Cheng, H.-L.; Hsieh, M.-J.; Yang, J.-S.; Lin, C.-W.; Lue, K.-H.; Lu, K.-H.; Yang, S.-F. Nobiletin inhibits human osteosarcoma cells metastasis by blocking ERK and JNK-mediated MMPs expression. Oncotarget 2016, 7, 35208–35223. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Behzad, S.; Habtemariam, S.; Ahmed, T.; Daglia, M.; Mohammad Nabavi, S.; Sobarzo-Sanchez, E.; Fazel Nabavi, S. Neuroprotective effects of citrus fruit-derived flavonoids, nobiletin and tangeretin in Alzheimer’s and Parkinson’s disease. CNS Neurol. Disord. Drug Targets (Former. Curr. Drug Targets CNS Neurol. Disord.) 2017, 16, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Guo, R.; Tian, H.; Li, L.; Liu, H.; Mi, Y.; Liu, X. Nobiletin protects against insulin resistance and disorders of lipid metabolism by reprogramming of circadian clock in hepatocytes. Biochim. Biophys. Acta Mol. Cell Biolo. Lipids 2018, 1863, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Veeranjaneyulu, A. Complications of Diabetes and Role of a Citrus Flavonoid Nobiletin in Its Treatment. In Herbs for Diabetes and Neurological Disease Management; Apple Academic Press: Oakville, ON, Canada, 2018; pp. 197–224. [Google Scholar]
- Morrow, N.M.; Telford, D.E.; Sutherland, B.G.; Edwards, J.Y.; Huff, M.W. Nobiletin Corrects Intestinal Lipid Metabolism in Ldlr-/-Mice Fed a High-Fat Diet. Atheroscler. Suppl. 2018, 32, 28. [Google Scholar] [CrossRef]
- Yuk, T.; Kim, Y.; Yang, J.; Sung, J.; Jeong, H.S.; Lee, J. Nobiletin Inhibits Hepatic Lipogenesis via Activation of AMP-Activated Protein Kinase. Evid. Based Complement. Altern. Med. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.-C.; Li, S.; Huang, Q.; Hung, W.-L.; Ho, C.-T.; Wei, G.-J.; Pan, M.-H. 5-Demethylnobiletin and 5-Acetoxy-6, 7, 8, 3′, 4′-pentamethoxyflavone Suppress Lipid Accumulation by Activating the LKB1-AMPK Pathway in 3T3-L1 Preadipocytes and High Fat Diet-Fed C57BL/6 Mice. J. Agric. Food Chem. 2016, 64, 3196–3205. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhu, X.; Pan, S.; Fang, Y.; Jiang, F.; Phillips, G.O.; Xu, X. Antimicrobial activity of nobiletin and tangeretin against Pseudomonas. Food Chem. 2012, 132, 1883–1890. [Google Scholar] [CrossRef]
- Onishi, S.; Nishi, K.; Yasunaga, S.; Muranaka, A.; Maeyama, K.; Kadota, A.; Sugahara, T. Nobiletin, a polymethoxy flavonoid, exerts anti-allergic effect by suppressing activation of phosphoinositide 3-kinase. J. Funct. Foods 2014, 6, 606–614. [Google Scholar] [CrossRef]
- Lai, C.-S.; Li, S.; Chai, C.-Y.; Lo, C.-Y.; Dushenkov, S.; Ho, C.-T.; Pan, M.-H.; Wang, Y.-J. Anti-inflammatory and antitumor promotional effects of a novel urinary metabolite, 3′, 4′-didemethylnobiletin, derived from nobiletin. Carcinogenesis 2008, 29, 2415–2424. [Google Scholar] [CrossRef]
- Narayana, J.L.; Huang, H.-N.; Wu, C.-J.; Chen, J.-Y. Epinecidin-1 antimicrobial activity: In vitro membrane lysis and In vivo efficacy against Helicobacter pylori infection in a mouse model. Biomaterials 2015, 61, 41–51. [Google Scholar] [CrossRef]
- Eguchi, A.; Murakami, A.; Li, S.; Ho, C.T.; Ohigashi, H. Suppressive effects of demethylated metabolites of nobiletin on phorbol ester-induced expression of scavenger receptor genes in THP-1 human monocytic cells. Biofactors 2007, 31, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, A.; Murakami, A.; Ohigashi, H. Nobiletin, a citrus flavonoid, suppresses phorbol ester-induced expression of multiple scavenger receptor genes in THP-1 human monocytic cells. FEBS Lett. 2006, 580, 3321–3328. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Asai, M.; Choi, S.-S.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.-T.; Cha, B.-Y. Nobiletin prevents body weight gain and bone loss in ovariectomized C57BL/6J mice. Pharmaco. Pharm. 2014, 5, 959–965. [Google Scholar] [CrossRef]
- Tominari, T.; Hirata, M.; Matsumoto, C.; Inada, M.; Miyaura, C. Polymethoxy flavonoids, nobiletin and tangeretin, prevent lipopolysaccharide-induced inflammatory bone loss in an experimental model for periodontitis. J. Pharmacol. Sci. 2012, 119, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Gao, W.; Zeng, S.-L.; Li, P.; Liu, E.-H. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J. Funct. Foods 2018, 40, 498–509. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Chou, Y.-C.; Hung, W.-L.; Cheng, A.-C.; Yu, R.-C.; Ho, C.-T.; Pan, M.-H. Polymethoxyflavones: Chemistry and Molecular Mechanisms for Cancer Prevention and Treatment. Curr. Pharmacol. Rep. 2019, 5, 98–113. [Google Scholar] [CrossRef]
- Uckoo, R.M.; Jayaprakasha, G.; Vikram, A.; Patil, B.S. Polymethoxyflavones isolated from the peel of Miaray Mandarin (Citrus miaray) have biofilm inhibitory activity in Vibrio harveyi. J. Agric. Food Chem. 2015, 63, 7180–7189. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Iwata, C.; Toda, H. Molecular cloning and characterization of a flavonoid-O-methyltransferase with broad substrate specificity and regioselectivity from Citrus depressa. BMC Plant Biol. 2016, 16, 180. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Charles, A.L.; Kung, H.-F.; Ho, C.-T.; Huang, T.-C. Extraction of nobiletin and tangeretin from Citrus depressa Hayata by supercritical carbon dioxide with ethanol as modifier. Ind. Crops Prod. 2010, 31, 59–64. [Google Scholar] [CrossRef]
- Kohno, H.; Yoshitani, S.-I.; Tsukio, Y.; Murakami, A.; Koshimizu, K.; Yano, M.; Tokuda, H.; Nishino, H.; Ohigashi, H.; Tanaka, T. Dietary administration of citrus nobiletin inhibits azoxymethane-induced colonic aberrant crypt foci in rats. Life Sci. 2001, 69, 901–913. [Google Scholar] [CrossRef]
- Murakami, A.; Nakamura, Y.; Torikai, K.; Tanaka, T.; Koshiba, T.; Koshimizu, K.; Kuwahara, S.; Takahashi, Y.; Ogawa, K.; Yano, M. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000, 60, 5059–5066. [Google Scholar] [PubMed]
- Uckoo, R.M.; Jayaprakasha, G.K.; Patil, B.S. Rapid separation method of polymethoxyflavones from citrus using flash chromatography. Sep. Purif. Technol. 2011, 81, 151–158. [Google Scholar] [CrossRef]
- Teruya, T.; Teruya, Y.; Sueyoshi, K.; Yamano, A.; Jitai, Y. Manufacturing method of fermentation treated products containing high-content nobiletin and tangeretin. Patent JP 2015202065, 16 November 2015. [Google Scholar]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. HL-60 differentiating activity and flavonoid content of the readily extractable fraction prepared from Citrus juices. J. Agric. Food Chem. 1999, 47, 128–135. [Google Scholar] [CrossRef]
- Tsukayama, M.; Ichikawa, R.; Yamamoto, K.; Sasaki, T.; Kawamura, Y. Microwave-assisted rapid extraction of polymethoxyflavones from dried peels of Citrus yuko Hort. ex Tanaka. J. Jpn. Soc. Food Sci. Technol. 2009, 56, 359–362. [Google Scholar] [CrossRef][Green Version]
- Silva, I.; Estrada, M.F.; V. Pereira, C.; da Silva, A.B.; Bronze, M.R.; Alves, P.M.; Duarte, C.M.; Brito, C.; Serra, A.T. Polymethoxylated Flavones from Orange Peels Inhibit Cell Proliferation in a 3D Cell Model of Human Colorectal Cancer. Nutr. Cancer 2018, 70, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.W.; Jang, D.R.; Kim, Y.U. Preparation Method of Citrus Peel Extract with Increased Polymethoxyflavone Content by Supercritical Fluid Extraction. Patent KR 1838266, 14 March 2018. [Google Scholar]
- Chiou, Y.-S.; Zheng, Y.-N.; Tsai, M.-L.; Lai, C.-S.; Ho, C.-T.; Pan, M.-H. 5-Demethylnobiletin more potently inhibits colon cancer cell growth than nobiletin in vitro and in vivo. J. Food Bioact. 2018, 2, 91–97. [Google Scholar] [CrossRef]
- Zheng, J.; Bi, J.; Johnson, D.; Sun, Y.; Song, M.; Qiu, P.; Dong, P.; Decker, E.; Xiao, H. Analysis of 10 metabolites of polymethoxyflavones with high sensitivity by electrochemical detection in high-performance liquid chromatography. J. Agric. Food Chem. 2015, 63, 509–516. [Google Scholar] [CrossRef]
- Zheng, J.; Song, M.; Dong, P.; Qiu, P.; Guo, S.; Zhong, Z.; Li, S.; Ho, C.T.; Xiao, H. Identification of novel bioactive metabolites of 5-demethylnobiletin in mice. Mol. Nutr. Food Res. 2013, 57, 1999–2007. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Sang, S.; Huang, M.T.; Ho, C.T. Identification of nobiletin metabolites in mouse urine. Mol. Nutr. Food Res. 2006, 50, 291–299. [Google Scholar] [CrossRef]
- Yasuda, T.; Yoshimura, Y.; Yabuki, H.; Nakazawa, T.; Ohsawa, K.; Mimaki, Y.; Sashida, Y. Urinary metabolites of nobiletin orally administered to rats. Chem. Pharm. Bull. 2003, 51, 1426–1428. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Wang, M.; Zheng, J.; Gao, Z.; Xu, F.; Zhang, G.; Xiao, H. Chemopreventive effects of nobiletin and its colonic metabolites on colon carcinogenesis. Mol. Nutr. Food Res. 2015, 59, 2383–2394. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, S.; Jonca, M.; Lambros, T.; Ferguson, S.; Goodnow, R.; Ho, C.T. Comparison of supercritical fluid chromatography and liquid chromatography for the separation of urinary metabolites of nobiletin with chiral and non-chiral stationary phases. Biomed. Chromatogr. 2006, 20, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Koga, N.; Ohta, C.; Kato, Y.; Haraguchi, K.; Endo, T.; Ogawa, K.; Ohta, H.; Yano, M. In vitro metabolism of nobiletin, a polymethoxy-flavonoid, by human liver microsomes and cytochrome P450. Xenobiotica 2011, 41, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Wang, M. Biotransformation of Polymethoxyflavones and Its Implication on Biological Activities. Ph.D. Thesis, University of Massachusetts, Amherst, MA, USA, 2017. [Google Scholar]
- Xu, L.; He, Y.; Guo, X.; Lu, Y.; Wang, C.; Wang, Z. Identification of metabolites of nobiletin in rats using ultra-performance liquid chromatography coupled with triple-quadrupole mass spectrometry. Yao Xue Xue Bao (Acta Pharm. Sin.) 2011, 46, 1483–1487. [Google Scholar]
- Manthey, J.A.; Bendele, P. Anti-inflammatory activity of an orange peel polymethoxylated flavone, 3′, 4′, 3, 5, 6, 7, 8-heptamethoxyflavone, in the rat carrageenan/paw edema and mouse lipopolysaccharide-challenge assays. J. Agric. Food Chem. 2008, 56, 9399–9403. [Google Scholar] [CrossRef] [PubMed]
- Kemperman, R.A.; Bolca, S.; Roger, L.C.; Vaughan, E.E. Novel approaches for analysing gut microbes and dietary polyphenols: Challenges and opportunities. Microbiology 2010, 156, 3224–3231. [Google Scholar] [CrossRef] [PubMed]
- Ma, C. Biotransformation of Polymethoxyflavones by Gut Microbiome and Molecular Characterization of Polymethoxyflavones by Surface Enhanced Raman Spectroscopy. Ph.D. Thesis, University of Massachusetts, Amherst, MA, USA, 2015. [Google Scholar]
- Li, S.; Sang, S.; Pan, M.-H.; Lai, C.-S.; Lo, C.-Y.; Yang, C.S.; Ho, C.-T. Anti-inflammatory property of the urinary metabolites of nobiletin in mouse. Bioorg. Med. Chem. Lett. 2007, 17, 5177–5181. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Dong, P.; Guan, H.; Li, S.; Ho, C.T.; Pan, M.H.; McClements, D.J.; Xiao, H. Inhibitory effects of 5-hydroxy polymethoxyflavones on colon cancer cells. Mol. Nutr. Food Res. 2010, 54, S244–S252. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Ciardiello, F.; Tortora, G. EGFR antagonists in cancer treatment. N. Engl. J. Med. 2008, 358, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massagué, J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Neuzillet, C.; Tijeras-Raballand, A.; Cohen, R.; Cros, J.; Faivre, S.; Raymond, E.; de Gramont, A. Targeting the TGFβ pathway for cancer therapy. Pharmacol. Ther. 2015, 147, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Said, A.H.; Raufman, J.-P.; Xie, G. The role of matrix metalloproteinases in colorectal cancer. Cancers 2014, 6, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Kang, S.-M.; Sawada, T.; Nishiguchi, Y.; Yashiro, M.; Ogawa, Y.; Ohira, M.; Ishikawa, T.; Hirakawa-YS Chung, K. Expression of intercellular adhesion molecule-1 and prognosis in colorectal cancer. Oncol. Rep. 2002, 9, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.A.; Guthrie, N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agric. Food Chem. 2002, 50, 5837–5843. [Google Scholar] [CrossRef] [PubMed]
- Parang, B.; Barrett, C.W.; Williams, C.S. AOM/DSS Model of Colitis-Associated Cancer. In Gastrointestinal Physiology and Diseases; Springer: Berlin/Heidelberg, Germany, 2016; pp. 297–307. [Google Scholar]
- Ito, N.; Hasegawa, R.; Sano, M.; Tamano, S.; Esumi, H.; Takayama, S.; Sugimura, T. A new colon and mammary carcinogen in cooked food, 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP). Carcinogenesis 1991, 12, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Nakagama, H.; Nakanishi, M.; Ochiai, M. Modeling human colon cancer in rodents using a food-borne carcinogen, PhIP. Cancer Sci. 2005, 96, 627–636. [Google Scholar] [CrossRef]
- Suzuki, R.; Kohno, H.; Murakami, A.; Koshimizu, K.; Ohigashi, H.; Yano, M.; Tokuda, H.; Nishino, H.; Tanaka, T. Citrus nobiletin inhibits azoxymethane-induced large bowel carcinogenesis in rats. Biofactors 2004, 21, 111–114. [Google Scholar] [CrossRef]
- Tang, M.X.; Ogawa, K.; Asamoto, M.; Chewonarin, T.; Suzuki, S.; Tanaka, T.; Shirai, T. Effects of nobiletin on PhIP-induced prostate and colon carcinogenesis in F344 rats. Nutr. Cancer 2011, 63, 227–233. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Gao, Z.; Sun, Y.; Wang, M.; Li, F.; Zheng, J.; Xiao, H. Nobiletin and its colonic metabolites suppress colitis-associated colon carcinogenesis by down-regulating iNOS, inducing antioxidative enzymes and arresting cell cycle progression. J. Nutr. Biochem. 2017, 42, 17–25. [Google Scholar] [CrossRef]
- Kawabata, K.; Murakami, A.; Ohigashi, H. Nobiletin, a citrus flavonoid, down-regulates matrix metalloproteinase-7 (matrilysin) expression in HT-29 human colorectal cancer cells. Biosci Biotechnol. Biochem. 2005, 69, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wu, X.; Zheng, J.; Xiao, H. 5-Demethylnobiletin inhibits colon carcinogenesis in azoxymethane/dextran sulfate sodium-treated mice (123.3). FASEB J. 2014, 28, 123.3. [Google Scholar]
- Zheng, Q.; Hirose, Y.; Yoshimi, N.; Murakami, A.; Koshimizu, K.; Ohigashi, H.; Sakata, K.; Matsumoto, Y.; Sayama, Y.; Mori, H. Further investigation of the modifying effect of various chemopreventive agents on apoptosis and cell proliferation in human colon cancer cells. J. Cancer Res. Clin. Oncol. 2002, 128, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Guan, H.; Dong, P.; Li, S.; Ho, C.T.; Pan, M.H.; McClements, D.J.; Xiao, H. The p53-, Bax-and p21-dependent inhibition of colon cancer cell growth by 5-hydroxy polymethoxyflavones. Mol. Nutr. Food Res. 2011, 55, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Sato, T.; Takayama, Y.; Mimaki, Y.; Sashida, Y.; Yano, M.; Ito, A. Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochem. Pharmacol. 2003, 65, 2065–2071. [Google Scholar] [CrossRef]
- Yasunaga, S.; Domen, M.; Nishi, K.; Kadota, A.; Sugahara, T. Nobiletin suppresses monocyte chemoattractant protein-1 (MCP-1) expression by regulating MAPK signaling in 3T3-L1 cells. J. Funct. Foods 2016, 27, 406–415. [Google Scholar] [CrossRef]
- Miyamoto, S.; Yasui, Y.; Ohigashi, H.; Tanaka, T.; Murakami, A. Dietary flavonoids suppress azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Chem. Biol. Interact. 2010, 183, 276–283. [Google Scholar] [CrossRef]
- Miyamoto, S.; Yasui, Y.; Tanaka, T.; Ohigashi, H.; Murakami, A. Suppressive effects of nobiletin on hyperleptinemia and colitis-related colon carcinogenesis in male ICR mice. Carcinogenesis 2008, 29, 1057–1063. [Google Scholar] [CrossRef]
- Armaghany, T.; Wilson, J.D.; Chu, Q.; Mills, G. Genetic alterations in colorectal cancer. Gastrointest. Cancer Res. 2012, 5, 19. [Google Scholar]
- Owa, T.; Yoshino, H.; Yoshimatsu, K.; Nagasu, T. Cell cycle regulation in the G1 phase: A promising target for the development of new chemotherapeutic anticancer agents. Curr. Med. Chem. 2001, 8, 1487–1503. [Google Scholar] [CrossRef]
- Johnson, D.; Walker, C. Cyclins and cell cycle checkpoints. Ann. Rev. Pharmacol. Toxicol. 1999, 39, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. Cancer cell cycles. Science 1996, 274, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Kurki, P.; Vanderlaan, M.; Dolbeare, F.; Gray, J.; Tan, E. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp. Cell Res. 1986, 166, 209–219. [Google Scholar] [CrossRef]
- McKay, J.A.; Douglas, J.J.; Ross, V.G.; Curran, S.; Loane, J.F.; Ahmed, F.Y.; Cassidy, J.; McLeod, H.L.; Murray, G.I. Analysis of key cell-cycle checkpoint proteins in colorectal tumours. J. Pathol. J. Pathol. Soc. Great Br. Irel. 2002, 196, 386–393. [Google Scholar] [CrossRef]
- Kroker, A.J.; Bruning, J.B. p21 exploits residue Tyr151 as a tether for high-affinity PCNA binding. Biochemistry 2015, 54, 3483–3493. [Google Scholar] [CrossRef] [PubMed]
- Soria, G.; Gottifredi, V. PCNA-coupled p21 degradation after DNA damage: The exception that confirms the rule? DNA Repair 2010, 9, 358–364. [Google Scholar] [CrossRef]
- Morgan, D.O. Cyclin-dependent kinases: Engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 1997, 13, 261–291. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef]
- Bertoli, C.; Skotheim, J.M.; De Bruin, R.A. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef]
- Taylor, W.R.; Stark, G.R. Regulation of the G2/M transition by p53. Oncogene 2001, 20, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Borgne, A.; Meijer, L. Sequential dephosphorylation of p34cdc2 on Thr-14 and Tyr-15 at the prophase/metaphase transition. J. Biol. Chem. 1996, 271, 27847–27854. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.-X.; Ditsworth, D.; Bauer, D.E.; Wang, Z.-Q.; Thompson, C.B. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004, 18, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Sayers, T.J. Targeting the extrinsic apoptosis signaling pathway for cancer therapy. Cancer Immunol. Immunother 2011, 60, 1173–1180. [Google Scholar] [CrossRef]
- Wang, X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001, 15, 2922–2933. [Google Scholar] [PubMed]
- Llambi, F.; Green, D.R. Apoptosis and oncogenesis: Give and take in the BCL-2 family. Curr. Opin. Genet. Dev. 2011, 21, 12–20. [Google Scholar] [CrossRef]
- Engel, T.; Henshall, D.C. Apoptosis, Bcl-2 family proteins and caspases: The ABCs of seizure-damage and epileptogenesis? Int. J. Physiol. Pathophysiol. Pharmacol. 2009, 1, 97–115. [Google Scholar]
- Chan, C.K.; Supriady, H.; Goh, B.H.; Kadir, H.A. Elephantopus scaber induces apoptosis through ROS-dependent mitochondrial signaling pathway in HCT116 human colorectal carcinoma cells. J. Ethnopharmacol. 2015, 168, 291–304. [Google Scholar] [CrossRef]
- Nuñez, G.; Benedict, M.A.; Hu, Y.; Inohara, N. Caspases: The proteases of the apoptotic pathway. Oncogene 1998, 17, 3237–3245. [Google Scholar] [CrossRef]
- Fernandes-Alnemri, T.; Litwack, G.; Alnemri, E.S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J. Biol. Chem. 1994, 269, 30761–30764. [Google Scholar] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wideranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239. [Google Scholar] [CrossRef] [PubMed]
- Oliver, F.J.; de la Rubia, G.; Rolli, V.; Ruiz-Ruiz, M.C.; de Murcia, G.; Ménissier-de Murcia, J. Importance of poly (ADP-ribose) polymerase and its cleavage in apoptosis Lesson from an uncleavable mutant. J. Biol. Chem. 1998, 273, 33533–33539. [Google Scholar] [CrossRef] [PubMed]
- Saraste, A.; Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef]
- Martin, S.J.; Green, D.R. Protease activation during apoptosis: Death by a thousand cuts? Cell 1995, 82, 349–352. [Google Scholar] [CrossRef]
- Lee, W.-R.; Shen, S.-C.; Lin, H.-Y.; Hou, W.-C.; Yang, L.-L.; Chen, Y.-C. Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca2+-dependent endonuclease. Biochem. Pharmacol. 2002, 63, 225–236. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Huang, D.C. Controlling the cell death mediators Bax and Bak: Puzzles and conundrums. Cell Cycle 2008, 7, 39–44. [Google Scholar] [CrossRef]
- Wei, M.C.; Zong, W.-X.; Cheng, E.H.-Y.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 2001, 292, 727–730. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Yu, Q.-J.; Zhang, R.-D.; Liu, B. Core signaling pathways of survival/death in autophagy-related cancer networks. Int. J. Biochem. Cell Biol. 2011, 43, 1263–1266. [Google Scholar] [CrossRef]
- Kundu, M.; Thompson, C.B. Autophagy: Basic principles and relevance to disease. Annu. Rev. Pathmechdis Mech. Dis. 2008, 3, 427–455. [Google Scholar] [CrossRef]
- Eum, K.-H.; Lee, M. Crosstalk between autophagy and apoptosis in the regulation of paclitaxel-induced cell death in v-Ha-ras-transformed fibroblasts. Mol. Cell. Biochem. 2011, 348, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Amelio, I.; Melino, G.; Knight, R.A. Cell death pathology: Cross-talk with autophagy and its clinical implications. Biochem. Biophys. Res. Commun. 2011, 414, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114.e5. [Google Scholar] [CrossRef] [PubMed]
- Klampfer, L. Cytokines, inflammation and colon cancer. Curr. Cancer Drug Targets 2011, 11, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Meira, L.B.; Bugni, J.M.; Green, S.L.; Lee, C.-W.; Pang, B.; Borenshtein, D.; Rickman, B.H.; Rogers, A.B.; Moroski-Erkul, C.A.; McFaline, J.L. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Investig. 2008, 118, 2516–2525. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, A.M.; Wei, B.; Braun, J.; Schiestl, R.H. Intestinal mucosal inflammation leads to systemic genotoxicity in mice. Cancer Res. 2009, 69, 4827–4834. [Google Scholar] [CrossRef]
- Kraus, S.; Arber, N. Inflammation and colorectal cancer. Curr. Opin. Pharmacol. 2009, 9, 405–410. [Google Scholar] [CrossRef]
- Itzkowitz, S.H.; Yio, X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: The role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G7–G17. [Google Scholar] [CrossRef]
- Takahashi, M.; Wakabayashi, K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004, 95, 475–480. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, D.; Yu, C.; Lv, B.; Peng, J.; Wang, J.; Lin, Y. Citrus nobiletin ameliorates experimental colitis by reducing inflammation and restoring impaired intestinal barrier function. Mol. Nutr. Food Res. 2015, 59, 829–842. [Google Scholar] [CrossRef]
- Kaidi, A.; Qualtrough, D.; Williams, A.C.; Paraskeva, C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006, 66, 6683–6691. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J. Are prostaglandins proinflammatory, antiinflammatory, both or neither? J. Rheumatol. Suppl. 1991, 28, 26–29. [Google Scholar] [PubMed]
- Surh, Y.-J.; Chun, K.-S.; Cha, H.-H.; Han, S.S.; Keum, Y.-S.; Park, K.-K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2001, 480, 243–268. [Google Scholar] [CrossRef]
- Rao, C.V. Nitric oxide signaling in colon cancer chemoprevention. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2004, 555, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, S.A.; MacEwan, D.J.; O’Connell, M.A. Lipopolysaccharide-induced expression of NAD (P) H: Quinone oxidoreductase 1 and heme oxygenase-1 protects against excessive inflammatory responses in human monocytes. J. Immunol. 2008, 181, 6730–6737. [Google Scholar] [CrossRef]
- Khor, T.O.; Huang, M.-T.; Kwon, K.H.; Chan, J.Y.; Reddy, B.S.; Kong, A.-N. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium–induced colitis. Cancer Res. 2006, 66, 11580–11584. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef]
- Kwak, M.-K.; Kensler, T.W. Targeting NRF2 signaling for cancer chemoprevention. Toxicol. Appl. Pharmacol. 2010, 244, 66–76. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Prager, G.; Poettler, M. Angiogenesis in cancer. Hämostaseologie 2012, 32, 105–114. [Google Scholar]
- Park, M.H.; Hong, J.T. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Berra, E.; Pagès, G.; Pouysségur, J. MAP kinases and hypoxia in the control of VEGF expression. Cancer Metastasis Rev. 2000, 19, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Saxena, N.K.; Taliaferro-Smith, L.; Knight, B.B.; Merlin, D.; Anania, F.A.; O’Regan, R.M.; Sharma, D. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res. 2008, 68, 9712–9722. [Google Scholar] [CrossRef] [PubMed]
- Fenton, J.I.; Hord, N.G.; Lavigne, J.A.; Perkins, S.N.; Hursting, S.D. Leptin, insulin-like growth factor-1, and insulin-like growth factor-2 are mitogens in ApcMin/+ but not Apc+/+ colonic epithelial cell lines. Cancer Epidemiol. Prev. Biomark. 2005, 14, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Rouet-Benzineb, P.; Aparicio, T.; Guilmeau, S.; Pouzet, C.; Descatoire, V.; Buyse, M.; Bado, A. Leptin counteracts sodium butyrate-induced apoptosis in human colon cancer HT-29 cells via NF-κB signaling. J. Biol. Chem. 2004, 279, 16495–16502. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Sato, T.; Yano, M.; Ito, A. Activation of protein kinase C βII/ε-c-Jun NH2-terminal kinase pathway and inhibition of mitogen-activated protein/extracellular signal-regulated kinase 1/2 phosphorylation in antitumor invasive activity induced by the polymethoxy flavonoid, nobiletin. Mol. Cancer Ther. 2004, 3, 839–847. [Google Scholar] [PubMed]
- Miyata, Y.; Sato, T.; Imada, K.; Dobashi, A.; Yano, M.; Ito, A. A citrus polymethoxyflavonoid, nobiletin, is a novel MEK inhibitor that exhibits antitumor metastasis in human fibrosarcoma HT-1080 cells. Biochem. Biophys. Res. Commun. 2008, 366, 168–173. [Google Scholar] [CrossRef]
- Fong, Y. Surgical therapy of hepatic colorectal metastasis. CA Cancer J. Clin. 1999, 49, 231–255. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, S.-H.; Kang, J.-G.; Ko, J.-H. Expression level and glycan dynamics determine the net effects of TIMP-1 on cancer progression. BMB Rep. 2012, 45, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.F.; Matrisian, L.M. Changing views of the role of matrix metalloproteinases in metastasis. J. Natl. Cancer Inst. 1997, 89, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Waas, E.; Wobbes, T.; Lomme, R.; DeGroot, J.; Ruers, T.; Hendriks, T. Matrix metalloproteinase 2 and 9 activity in patients with colorectal cancer liver metastasis. Br. J. Surg. 2003, 90, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Zucker, S.; Vacirca, J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004, 23, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Jung, A.; Dag, S.; Hlubek, F.; Kirchner, T. β-Catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am. J. Pathol. 1999, 155, 1033–1038. [Google Scholar] [CrossRef]
- Crawford, H.C.; Fingleton, B.M.; Rudolph-Owen, L.A.; Goss, K.J.H.; Rubinfeld, B.; Polakis, P.; Matrisian, L.M. The metalloproteinase matrilysin is a target of β-catenin transactivation in intestinal tumors. Oncogene 1999, 18, 2883–2891. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Bingle, á.; Brown, N.; Lewis, C. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. J. Pathol. J. Pathol. Soc. Great Br. Irel. 2002, 196, 254–265. [Google Scholar] [CrossRef]
- Kerkelä, E.; Ala-aho, R.; Klemi, P.; Grénman, S.; Shapiro, S.D.; Kähäri, V.M.; Saarialho-Kere, U. Metalloelastase (MMP-12) expression by tumour cells in squamous cell carcinoma of the vulva correlates with invasiveness, while that by macrophages predicts better outcome. J. Pathol. 2002, 198, 258–269. [Google Scholar] [CrossRef]
- Li, S.; Pan, M.-H.; Lo, C.-Y.; Tan, D.; Wang, Y.; Shahidi, F.; Ho, C.-T. Chemistry and health effects of polymethoxyflavones and hydroxylated polymethoxyflavones. J. Funct. Foods 2009, 1, 2–12. [Google Scholar] [CrossRef]
- Scholz; Williamson. Interactions affecting the bioavailability of dietary polyphenols in vivo. Int. J. Vitam. Nutr. Res. 2007, 77, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Kuwahara, S.; Takahashi, Y.; Ito, C.; Furukawa, H.; Ju-ichi, M.; Koshimizu, K.; OHIGASHI, H. In vitro absorption and metabolism of nobiletin, a chemopreventive polymethoxyflavonoid in citrus fruits. Biosci. Biotechnol. Biochem. 2001, 65, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Kansy, M.; Senner, F.; Gubernator, K. Physicochemical high throughput screening: Parallel artificial membrane permeation assay in the description of passive absorption processes. J. Med. Chem. 1998, 41, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Walle, T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab. Dispos. 2006, 34, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Van de Waterbeemd, H. Physico-Chemical Approaches to Drug Absorption. In Drug Bioavailability: Estimation of Solubility, Permeability, Absorption and Bioavailability; Wiley: Hoboken, NJ, USA, 2003; pp. 3–20. [Google Scholar]
- Murakami, A.; Koshimizu, K.; Ohigashi, H.; Kuwahara, S.; Kuki, W.; Takahashi, Y.; Hosotani, K.; Kawahara, S.; Matsuoka, Y. Characteristic rat tissue accumulation of nobiletin, a chemopreventive polymethoxyflavonoid, in comparison with luteolin. Biofactors 2002, 16, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, J.; Zhong, Z.; Song, M.; Wu, X. Tissue Distribution of Nobiletin and Its Metabolites in Mice after Oral Administration of Nobiletin; Federation of American Societies for Experimental Biology: Bethesda, MD, USA, 2013. [Google Scholar]
- Wu, X.; Song, M.; Qiu, P.; Rakariyatham, K.; Li, F.; Gao, Z.; Cai, X.; Wang, M.; Xu, F.; Zheng, J. Synergistic chemopreventive effects of nobiletin and atorvastatin on colon carcinogenesis. Carcinogenesis 2017, 38, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Devaraj, V.; Giri, K.C.; Giri, S.; Rajagopal, S.; Mullangi, R. Development and validation of a highly sensitive LC-MS/MS-ESI method for the determination of nobiletin in rat plasma: Application to a pharmacokinetic study. Biomed. Chromatogr. 2012, 26, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Tewari, D.; Patel, K.; Jain, G.K. Permeability determination and pharmacokinetic study of nobiletin in rat plasma and brain by validated high-performance liquid chromatography method. Fitoterapia 2011, 82, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.A.; Cesar, T.B.; Jackson, E.; Mertens-Talcott, S. Pharmacokinetic Study of Nobiletin and Tangeretin in Rat Serum by High-Performance Liquid Chromatography—Electrospray Ionization—Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 145–151. [Google Scholar] [CrossRef]
- McClements, D.J. Emulsion design to improve the delivery of functional lipophilic components. Annu. Rev. Food Sci. Technol. 2010, 1, 241–269. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, C.; Chen, J.; Tian, G.; McClements, D.J.; Xiao, H.; Zheng, J. Encapsulation of polymethoxyflavones in citrus oil emulsion-based delivery systems. J. Agric. Food Chem. 2017, 65, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Lu, Y.; Zhou, J.P. Preparation of nobiletin in self-microemulsifying systems and its intestinal permeability in rats. J. Pharm. Pharm. Sci. 2008, 11, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Yao, J.; Zhou, J. Preparation of self-assemble nobiletin proliposomes and its pharmacokinetics in rats. Yao Xue Xue Bao (Acta Pharm. Sin.) 2009, 44, 192–196. [Google Scholar]
- Chen, H.; An, Y.; Yan, X.; McClements, D.J.; Li, B.; Li, Y. Designing self-nanoemulsifying delivery systems to enhance bioaccessibility of hydrophobic bioactives (nobiletin): Influence of hydroxypropyl methylcellulose and thermal processing. Food Hydrocoll. 2015, 51, 395–404. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, Y.; He, L.; Wu, S.; Li, B.; Li, Y. Fabrication of nanoemulsion-filled alginate hydrogel to control the digestion behavior of hydrophobic nobiletin. LWT Food Sci. Technol. 2017, 82, 260–267. [Google Scholar] [CrossRef]
- Onoue, S.; Uchida, A.; Takahashi, H.; Seto, Y.; Kawabata, Y.; Ogawa, K.; Yuminoki, K.; Hashimoto, N.; Yamada, S. Development of high-energy amorphous solid dispersion of nanosized nobiletin, a citrus polymethoxylated flavone, with improved oral bioavailability. J. Pharm. Sci. 2011, 100, 3793–3801. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.; Murty, M.; Pilon, K. Products containing bitter orange or synephrine: Suspected cardiovascular adverse reactions. Can. Med. Assoc. J. 2004, 171, 993–994. [Google Scholar]
- Yang, G.; Li, S.; Long, T.; Yang, Y.; Li, Y. Application of Polymethoxylflavone in Preparation of Prevention Drug for Cardiovascular Inflammation. Patent CN 107281179, 24 October 2017. [Google Scholar]
- Wu, X.; Zheng, D.; Qin, Y.; Liu, Z.; Zhu, X. Application of Nobiletin in Medicine for Preventing or Treating Heart Failure. Patent CN 106924241, 7 July 2017. [Google Scholar]
- Morimoto, T.; Hasegawa, K.; Murakami, A.; Fukuda, H.; Takahashi, K. Cardiac Disease Treatment Agents Containing Nobiletin. Patent JP 2011037798, 24 February 2011. [Google Scholar]
- Caramelli, G. Product with Blood Lipid-Lowering Activity. Patent IT 2008RM0232, 2 August 2008. [Google Scholar]
- Ohizumi, Y.; Kajima, K.; Maruyama, K.; Ishibashi, M. Pharmaceutical Composition and Food Containing Citrus Butanol Extract for Preventing and/or Treating Central Nervous System Disease. Patent WO 2017208869, 7 December 2017. [Google Scholar]
- Ohizumi, Y.; Kajima, K.; Maruyama, K. Pharmaceutical and food composition containing Anredera cordifolia and nobiletin. Patent JP 6238089, 29 November 2017. [Google Scholar]
- Jeon, M.R.; L, S.A.; Yoon, G.J.; Park, J.H. Composition for Preventing or Treating Neurodegenerative Disease Comprising Nobiletin as Active Ingredient. Patent KR 2017090073, 25 September 2017. [Google Scholar]
- Wu, X.; Mei, Z.; Zheng, D.; Liu, Z.; Zhu, X.; Zhou, Y.; Zeng, L.; Liang, Z. Application of Nobiletin in Preparation or Screening of Diabetic Cardiomyopathy Drug. Patent CN 108403684, 17 August 2018. [Google Scholar]
- Guthrie, N. Compositions Comprising at Least one Polymethoxyflavone, Flavonoid, Liminoid, and/or Tocotrienol Useful in Combination Therapies for Treating Diabetes. Patent WO 2014203059, 24 December 2014. [Google Scholar]
- Kim, T.J.; Kim, H.G.; Kwon, Y.I.; Lee, J.U. Obesity inhibiting Composition Comprising Powder of Citrus Grandis Cultivated by Eco Friendly Method as Active Ingredient. Patent KR 2016111554, 27 September 2016. [Google Scholar]
- Miyaura, C.; Inada, M. Preventive or Therapeutic Compositions Containing Heptamethoxyflavone for Bone Diseases. Patent JP 2012232916, 22 October 2012. [Google Scholar]
- Liao, X. Manufacture Method of Chinese Medicine Composition for Treatment of Halitosis. Patent CN 105434729, 30 March 2016. [Google Scholar]
- Wang, L.; Tian, A.; Li, S.; Chen, J.; Li, B. Mouth Smell-Improving Agent and Its Preparation Method. Patent CN 103893334, 2 July 2014. [Google Scholar]
- Huang, R.L.; Hsu, S.W. Polymethoxylated Flavone for Manufacturing Drugs Against Hepatitis-B with Drug Resistance. Patent TW I535439, 1 June 2016. [Google Scholar]
- Kim, D.H.; Han, M.J.; Cho, E.H.; Kim, Y.R. Natural Products for Treating Cancer and HIV-Related diseases. Patent KR 2012011169, 7 February 2012. [Google Scholar]
- Zhang, T.; Liao, M.; Gong, S.; Xie, X.; Sun, W.; Wang, L.; Zheng, Y. Application of Total Flavonoid Extract from Citrus Aurantium in Manufacturing Medicines for Treating Asthma. Patent CN 102935131, 20 February 2013. [Google Scholar]
- Li, K. Application of Nobiletin in Medicine for Treating Allergic Asthma. Patent CN 102552242, 11 July 2012. [Google Scholar]
- Sugawara, T.; Kadota, A.; Kikuchi, T. Antiallergic Oral Composition Containing β-Lactoglobulin and Nobiletin. Patent JP 2015036369, 23 February 2015. [Google Scholar]
- Seo, J.W.; Choi, B.G.; Cheng, J.H.; Cho, M.J. Citrus Pericarp Extracts for Preventing Hair Loss and Promoting Hair Growth. Patent KR 1651833, 19 September 2016. [Google Scholar]
- Ito, Y.; Hikiyama, E.; Yamada, S.; Woo, J.-T.; Teruya, Y.; Sugaya, K.; Nishijima, S.; Wakuda, H.; Shinozuka, K. Medicinal Composition for Preventing or Improving Dysuria, Antagonist Against Dysuria-Related Receptor, and Method for Preventing or Improving Dysuria Using Medicinal Composition or Antagonist. Patent WO 2016075960, 19 May 2016. [Google Scholar]
- Sakata, Y.; Nakamura, H.; Oshio, K. Muscular Atrophy Preventing Agent Containing Citrus Depressa Extract. Patent WO 2013099982, 4 July 2013. [Google Scholar]
- Li, S.; Yang, G.; Long, T. Application of (demethyl) polymethoxyflavone and taxol medicine in producing the medicine for treating non-small cell lung cancer. Patent CN 106562954, 19 April 2017. [Google Scholar]
- Nakano, S.; Ono, M.; Hayashi, C. Agent and Method for Inhibiting Breast Cancer Cell Proliferation Comprising Nobiletin. Patent JP 2016017042, 4 February 2016. [Google Scholar]
- Chen, G.; Wang, H. Application of Nobiletin in the Preparation of Health Products or Medicines for Preventing and/or Treating Oral Cancer. Patent CN 105030559, 11 November 2015. [Google Scholar]
- Ma, W.-Z.; Feng, S.-L.; Yao, X.-J.; Yuan, Z.-W.; Liu, L.; Xie, Y. Use of Nobiletin in Cancer Treatment. Patent AU 2015101287, 22 October 2015. [Google Scholar]
- Zhang, Z. Chinese Medicinal Composition Containing Extracts from Citrus and Scutellaria for Treating Cancer Chemotherapy Related Diarrhea. Patent CN 103655835, 26 March 2014. [Google Scholar]
- Li, M.; Jin, H.; Yang, Z.; Xu, G.; Lin, Y.; Lin, Q.; Zhang, Z. Medical Application of Flavonoids of Citrus Reticulata Pericarp as Angiogenesis Inhibitor. Patent CN 101947215, 19 January 2011. [Google Scholar]
- Zhou, H.; Xie, B.; Zang, X.; Cheng, L.; Liang, G. A Multiple Index Component content Determination, Fingerprint Construction and Preparation Method for Liver-Tonifying Eyesight-Improving Oral Liquid [Machine Translation]. Patent CN 105510452, 20 April 2016. [Google Scholar]
- Guo, J.; Liang, L.; Song, J.; Li, H.; Yang, J.; Chen, B.; Wang, S. Method for Extracting Nobiletin and Hesperetin from Citrus. Patent CN 106632196, 10 May 2017. [Google Scholar]
- Cao, J.; Hu, S.; Liu, X.; Cao, W.; Pang, X.; Dai, H.; Da, J. A method of Extracting Flavonoids active Ingredients in Citrus Reticulata Pericarp. Patent CN 104297026, 21 January 2015. [Google Scholar]
- Yamaguchi, K.; Mogami, K.; Yamaguchi, Y.; Hitomi, N.; Murata, K.; Tani, Y. Manufacture of Nobiletin by Solvent Extraction and Nobiletin-Containing Extract. Patent JP 2012056938, 22 March 2012. [Google Scholar]
- Sun, C.; Wang, Y.; Chen, K.; Li, X.; Cao, J. Process Forextn. And Purifn. of Polymethoxylated Flavonoids Compound from Fruit of Citrus Reticulate. Patent CN 107011308, 4 August 2017. [Google Scholar]
- Li, X.; Zhang, J.; Sun, C.; Chen, K. Method for Isolating and Purifying Seven Flavonoids from Citrus Tangerina oil Cell Layer. Patent CN 103610800, 5 March 2014. [Google Scholar]
- Liang, H.; Wu, D.; Li, B.; Li, Y.; Li, J. Stable Nobiletin liquid Preparation and Preparation Method Thereof. Patent CN 107998073, 8 May 2018. [Google Scholar]
- Yang, W.; Song, Y.; Chen, H.; Luo, X.; Yuan, J. A Technique Based on Multi-Solvents for Preparing Nobiletin. Patent CN 105669626, 15 June 2016. [Google Scholar]
- Iwashita, M.; Umehara, M.; Onishi, S.; Yamamoto, M.; Yamagami, K.; Ishigami, T. Method for Manufacturing Nobiletin-Containing Solid Dispersion. Patent WO 2018025871, 8 February 2018. [Google Scholar]
- Woo, J.T.; Komaki, M. Polymethoxyflavonoid Dissolved Composition and its Manufacturing Method. Patent JP 2015221761, 10 December 2015. [Google Scholar]
- Chen, Y.; Yu, Y.; Yang, D.; Wei, W.; He, Z.; Lin, X.; Xie, H. Measurement Method for Seventeen Kinds of Phenol Substances in Grape and Citrus Fruit Using High Performance Liquid Chromatography (HPLC). Patent CN 102706980, 3 October 2012. [Google Scholar]
- Kusano, S.; Tamasu, S. Composition Containing 4’-Demethylnobiletin for skin Whitening Cosmetics, Medicines, Foods and Drinks. Patent JP 2017226612, 28 December 2017. [Google Scholar]
- Choi, B.G.; Lee, D.R. Skin Moisturizers Containing Citrus Peel Extracts. Patent KR 2017000068, 6 January 2017. [Google Scholar]
- Karabey, F. Nobiletin Molecules in Cosmetic Preparationsuse. Patent TR 2014000324, 2015. [Google Scholar]
- Zhang, X.; Chen, S.; Wang, X.; Xie, F.; Liu, X.; Wang, J.; Yan, A.; Gao, N.; Li, F. A Snap Bean Preservative [Machine Translation]. Patent CN 106172719, 7 December 2016. [Google Scholar]
- Krohn, M.; Seibert, S.; Kleber, A.; Wonschik, J. Sweetener and/or Sweetness Enhancer, Sweetener Composition, Methods of Making the Same and Consumables Containing the Same. Patent WO 2012107203, 16 August 2012. [Google Scholar]
- Zhang, L.; Zhu, W.; Yang, C.; Guo, H.; Yu, A.; Ji, J.; Gao, Y.; Sun, M.; Zhai, G. A novel folate-modified self-microemulsifying drug delivery system of curcumin for colon targeting. Int. J. Nanomed. 2012, 7, 151–162. [Google Scholar] [CrossRef]
- Bansode, S.T.; Kshirsagar, S.J.; Madgulkar, A.R.; Bhalekar, M.R.; Bandivadekar, M.M. Design and development of SMEDDS for colon-specific drug delivery. Drug Dev. Ind. Pharm. 2016, 42, 611–623. [Google Scholar] [CrossRef]
- Low, L.E.; Tan, L.T.-H.; Goh, B.-H.; Tey, B.T.; Ong, B.H.; Tang, S.Y. Magnetic cellulose nanocrystal stabilized Pickering emulsions for enhanced bioactive release and human colon cancer therapy. Int. J. Biol. Macromol. 2019, 127, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Low, L.E.; Tey, B.T.; Ong, B.H.; Chan, E.S.; Tang, S.Y. Palm olein-in-water Pickering emulsion stabilized by Fe3O4-cellulose nanocrystal nanocomposites and their responses to pH. Carbohydr. Polym. 2017, 155, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Exp. Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.T.; Zhao, X.J.; Zhang, Y.D.; Li, Y.F. Fast separation and sensitive quantitation of polymethoxylated flavonoids in the peels of citrus using UPLC-Q-TOF-MS. J. Agric. Food Chem. 2017, 65, 2615–2627. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Rycaj, K.; Liu, Z.-M.; Tang, D.G. Cancer Stem Cells: Constantly Evolving and Functionally Heterogeneous Therapeutic Targets; AACR: Philadelphia, PA, USA, 2014. [Google Scholar]
- Chen, K.; Huang, Y.-H.; Chen, J.-L. Understanding and targeting cancer stem cells: Therapeutic implications and challenges. Acta Pharmacol. Sin. 2013, 34, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.H.; Low, L.E.; Tang, S.Y.; Yap, W.H.; Chuah, L.H.; Chan, C.K.; Lee, L.H.; Goh, B.H. A reliable and affordable 3D tumor spheroid model for natural product drug discovery: A case study of curcumin. Prog. Drug Discov. Biomed. Sci. 2019, 2, 1–5. [Google Scholar]
- DiMarco-Crook, C.; Xiao, H. Diet-based strategies for cancer chemoprevention: The role of combination regimens using dietary bioactive components. Annu. Rev. Food Sci. Technol. 2015, 6, 505–526. [Google Scholar] [CrossRef]
- Funaro, A.; Wu, X.; Song, M.; Zheng, J.; Guo, S.; Rakariyatham, K.; Rodriguez-Estrada, M.T.; Xiao, H. Enhanced Anti-Inflammatory Activities by the Combination of Luteolin and Tangeretin. J. Food Sci. 2016, 81, H1320–H1327. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Qiu, P.; Li, F.; Wang, M.; Zheng, J.; Wang, Q.; Xu, F.; Xiao, H. A metabolite of nobiletin, 4′-demethylnobiletin and atorvastatin synergistically inhibits human colon cancer cell growth by inducing G0/G1 cell cycle arrest and apoptosis. Food Funct. 2018, 9, 87–95. [Google Scholar] [CrossRef]
| Compounds | Activities | Cell lines | Treatment/Assay (Treatment Duration) | Assays/Results/Mechanisms | References |
|---|---|---|---|---|---|
| NOB | Anti-proliferative | HT-29 | H-thymidine uptake assay | - IC50 of NOB = 4.7 μM | [70] |
| - IC90 of NOB = 13.9 μM | |||||
| 5-DMN | - IC50 of 5-DMN = 8.5 μM | ||||
| - IC90 of 5-DMN = 171 μM | |||||
| NOB | Cytotoxicity | COLO320, SW480 and Caco-2 | MTS viability assay (48 h) | - IC50 for COLO320 = 40.4 ± 9.1 μM | [79] |
| - IC50 for SW480 = 245 ± 9.1 μΜ | |||||
| - IC50 for Caco-2 = 305.6 ± 41.9 μΜ | |||||
| Apoptosis-inducing | Apoptosis assays—DNA fragmentation | - DNA ladder pattern | |||
| 200 μΜ—2-fold increase DNA fragmentation in COLO320 | |||||
| - gel electrophoresis (48 h) | |||||
| Anti-proliferative | BrdU labelling index | - 34.7 ± 4.7% BrdU-binding cells at 100 μΜ | |||
| - 44.4 ± 6.4% BrdU-binding cells at 40 μΜ | |||||
| NOB | Anti-metastasis | HT-29 | ELISA | [77] | |
| - proMMP-7 expression | - At 100 μM, no detection of proMMP-7 in media, ~280 pg/mL proMMP-7 in media | ||||
| qPCR and Western blot | - >25 μM, reduced RNA and protein (both intracellular and supernatant) expression of proMMP-7 | ||||
| AP-1 binding activity | - Inhibited binding activity of AP-1 (transcription factor for MMP-7 gene) | ||||
| NOB | Anti-proliferative | HT-29 | Cell counting assay | - IC50 of NOB ≈ 50 μM | [14] |
| - Inhibited cell proliferation in a time- and dose-dependent manner | |||||
| Cell cycle arrest | |||||
| Cell cycle analysis | - Induced G1 phase cell cycle arrest (60 and 200 μM) | ||||
| - Propidium iodide staining | |||||
| Apoptosis-inducing | Apoptosis assay | - No significant apoptosis detected at 60 and 100 μM | |||
| Resumption of proliferation | - Resumed proliferation within 24 h of removal of NOB and achieve the same stage of growth as compared to control after four days of removal of NOB | ||||
| NOB 5-DMN | Cytotoxicity | HCT116, HT-29 | MTT viability assay (48 h) | - IC50 of NOB on HCT116 = 37 μM | [63] |
| - IC50 of 5-DMN on HCT116 = 8.7 μM | |||||
| - IC50 of NOB on HT-29 = 46.2 μM | |||||
| - IC50 of 5-DMN on HT-29 = 22 μM | |||||
| Cell cycle arrest | Cell cycle analysis - Propidium iodide staining (24 h) Western blot | - At 8 μM, 5-DMN induced G2/M phase arrest in HCT116 | |||
| - At 36 μM, 5-DMN induced G2/M phase arrest in HT-29 | |||||
| - At 16 μM, NOB reduced CDK-2 expression | |||||
| - At 4 μM and 8 μM, 5-DMN increased p21 and Rb, while decreased CDK-2 and p-Rb. | |||||
| Apoptosis-inducing | Apoptosis assay | - At 8 μM, 5-DMN increased early apoptosis by 2.2-fold in HCT116 | |||
| Annexin-V/PI (48 h) | - At 36 μM, 5-DMN increased early apoptosis by ~2-fold in HT-29 | ||||
| Western blot | - At 16 μM, NOB did not increase apoptotic cell population in HCT116/HT-29 | ||||
| - At 4 μM and 8 μM, 5-DMN increased expressions of cleaved caspase 8, cleaved caspase 3 and cleaved PARP. | |||||
| 5-DMN | Apoptosis-inducing | HCT116 (p53 +/+) and HCT116 (p53 −/−); HCT116 (Bax +/−) and HCT116 (Bax −/−); HCT116 (p21 −/−) | Apoptosis assay Annexin-V/PI | - At 15 μM, 5-DMN increased late apoptotic/necrotic cell in HCT116 (p53 −/−) > HCT115 (p53 +/+), suggesting the apoptotic inducing action is independent of p53 | [80] |
| - At 15 μM, 5-DMN increased early apoptotic cell in HCT116 (Bax +/−), but not in HCT116 (Bax −/−) | |||||
| Cell cycle arrest | Cell cycle analysis - Propidium iodide staining | - At 15 μM, 5-DMN arrested cells at G2/M and G0/G1 phases in HCT116 (p53 +/+) cells, but only caused G2/M phase arrest in HCT116 (p53 −/−) cells | |||
| - G0/G1 is p53 dependent and G2/M is p53-independent | |||||
| NOB; 3′-DMN; 4′-DMN; 3′,4′-DMN | Cytotoxicity | HCT116, HT-29 | MTT viability assay | - At 2.03 μM and 3.28 μM, NOB and 3′-DMN, respectively showed no significant cytotoxicity against HCT116 and HT-29 | [54] |
| - At 24.13 μM, 4′-DMN inhibited growth of HCT-116 by 45% and HT-29 by 33% | |||||
| - At 12.03 μM, 3’,4’-DMN inhibited growth of HCT116 by 30% and HT-29 by 9% | |||||
| - combination of all three NOB-metabolites inhibited growth of HCT116 by 64% and HT-29 by 62% (no significant difference to three NOB-metabolites + NOB) | |||||
| Cell cycle arrest | Cell cycle analysis - Propidium iodide staining (24 h) | - NOB (40 μM) arrested cells at G0/G1 phase in both HCT-116 and HT-29 | |||
| - 3′-DMN (40 μM) arrested cells at both S phase and G2/M phase in HCT-116; while arrested cells at both G0/G1 and G2/M phase in HT-29 | |||||
| - 4′-DMN (40 μM) induced a stronger effect than NOB in arresting cells at G0/G1 phase in HCT-116 and HT-29 | |||||
| - 3′,4′-DMN (20 μM) arrested cells at both S phase and G2/M phase in HCT-116; while arrested cells at both G0/G1 and G2/M phase in HT-29 | |||||
| Apoptosis inducing | Western blot | - NOB and all three NOB-metabolites cause profound increase in expression of p21Cip1/Waf1 | |||
| Annexin-V/PI (48 h) | - NOB (40 μM) increased early apoptotic cell population by 3.3-fold, increased late apoptotic cell population by 4.2-fold in HCT116 | ||||
| - 3′-DMN (40 μM) increased early apoptotic cell population by 5.0-fold, increased late apoptotic cell population by 3.5-fold in HCT116 | |||||
| - 4′-DMN (40 μM) increased early apoptotic cell population by 4.9-fold, increased late apoptotic cell population by 7.1-fold in HCT116 | |||||
| - 3′,4′-DMN (20 μM) increased early apoptotic cell population by 7.6-fold, increase late apoptotic cell population by 4.5-fold in HCT116 | |||||
| -3′-DMN (40 μM) and 4’-DMN (40 μM) did not cause significant apoptosis in HT-29 | |||||
| - 3′,4′-DMN (20 μM) exhibits stronger apoptosis effect than NOB (40 μM) in HT-29 | |||||
| Western blot | - NOB (40 μM) only increased activation of caspase-9 and did not affect caspase-3 or PARP levels in HCT116 | ||||
| - NOB-metabolites increased activation of caspase-3, caspase-9 and other downstream proteins like PARP in HCT116 | |||||
| NOB-Met (2.03 μM NOB: 3.28 μM 3′-DMN: 24.13 μM 4′-DMN: 12.03 μM 3′,4′-DMN | Anti-inflammatory | RAW264.7 | Western Blot | - At 0.5× concentration of NOB-Met, supressed LPS-induced iNOS expression by 56.4% | [76] |
| - At 1× and 2× concentration of NOB-Met, completely abrogated LPS-induced iNOS expression | |||||
| - At ×0.5, increased expression of NQO1 by 21% as compared to LPS-treated cells | |||||
| - At ×1, increased expression of HO-1 by 10%, increased expression of NQO1 by 34% as compared to LPS-treated cells | |||||
| - At ×2, increased expression of HO-1 by 37%, increased expression of NQO1 by 50% as compared to LPS-treated cells | |||||
| - Induced translocation of Nrf2 | |||||
| Cell cycle arrest | HCT116 | Cell cycle analysis - Propidium iodide staining Western blot | - At 1×, induced G0/G1 phase arrest; while at 2×, induced G0/G1 and G2/M phases arrest | ||
| - Reduced expressions of CDK-2, CDK-4, CDK-6 and cyclin D, while increased expressions of p53 and p27 | |||||
| NOB, 5-DMN | Cytotoxicity | HCT116, HT-29, COLO205 | MTT viability assay | - At 40 μM, NOB significantly reduced viability of HCT116, HT-29 and COLO205 by ~20–30% | [49] |
| - At >5 μM, 5-DMN significantly reduced viability of HCT116, HT-29 and COLO205 | |||||
| Apoptosis inducing | Cell cycle analysis - SubG1 quantification Western | - At 20 μM, 5-DMN increased apoptosis ratio by ~26%, while no increased in subG1 population in NOB-treated COLO205 | |||
| - At 10 and 20 μM, significantly increased expression of cleaved PARP in COLO205 | |||||
| NOB | Anti-inflammatory | Human synovial fibroblast, mouse macrophage J774A.1 | ELISA | - At >4 μM, NOB inhibited PGE2 induced by IL-1α in human synovial fibroblast | [81] |
| Western blot and qPCR | - At >16 μM, NOB reduced mRNA of COX-2 induced by IL-1α in human synovial fibroblast | ||||
| - At 64 μM, NOB inhibited COX-2 protein expression induced by IL-1α in human synovial fibroblast | |||||
| qPCR | - At 32 μM, NOB reduced mRNA of IL-1α, IL-1β, IL-6, TNF-α induced by LPS in J774A.1 | ||||
| Western blot | - At >16 μM, NOB reduced proMMP-1 and proMMP-3 induced by IL-1α in human synovial fibroblast | ||||
| - At >16 μM, NOB enhanced TIMP-1 expression in response to IL-1α in human synovial fibroblast | |||||
| NOB | Anti-inflammatory | Mouse adipocyte 3T3-L1 | ELISA | - At 50 and 100 μM, NOB suppressed MCP-1 secretion induced by TNF-α IN 3T3-L1 adipocytes | [82] |
| Western blot | - At 50 and 100 μM, NOB reduced ERK phosphorylation in 3T3-L1 adipocytes treated with TNF-α | ||||
| Animal Models | Treatment/Dosage | Mechanisms | Detailed Results | References |
|---|---|---|---|---|
Colitis-associated colon carcinogenesis model
| AIN93G diet containing 0.05% wt NOB (20 weeks) | Cell cycle arrest | Protein expression in colonic mucosa by Western blot - Reduced levels of CDK-2, CDK-4, CDK-6, cyclin D and cyclin E - Increased levels of p21, p27 and p53 | [76] |
| Anti-inflammatory effects | Immunohistochemical analysis - Reduced expression of iNOS reduced by 35% when compared to the positive control Protein expression in colonic mucosa by Western blot - Increased level of HO-1 - Increased level of NQO1 - Induced translocation of level of Nrf2 transcription factor (Nuclear fraction < Cytoplasmic fraction) | |||
Colitis-associated colon carcinogenesis model
| AIN93G diet containing 0.05% wt NOB (20 weeks) | Inhibit AOM/DSS-induced colon carcinogenesis | - Prevented shortening of colon length, reduced the increased colon weight/length ratio - Reduced tumor incidence by 40% and tumor multiplicity by 71% - Maintained histological characteristic of normal mucosa | [54] |
| Anti-proliferative effect | - Reduced PCNA-positive colonocytes by 69% in mucosal crypts | |||
| Apoptosis-inducing effect | - Increased cleaved caspase-3 positive cells by 2.3-fold in colonic tumor | |||
| Anti-inflammatory effects | - Reduced levels of proinflammatory cytokines - ELISA showed reduction of TNF-α by 51%, IL-1ß by 92% and IL-6 by 69% compared - qRT-PCR analysis showed reduction of TNF-α by 65%, IL-1ß by 69% and IL-6 by 45% | |||
Colon carcinogenesis model
| Diet containing 100 ppm NOB (0.1% wt) (10 weeks) | Inhibit AOM induced colon carcinogenesis | - Reduced frequency of preneoplastic lesions (colonic aberrant crypt foci (ACF) and β-catenin-accumulated crypts (BCAC)) - Reduced incidence of ACF by 68-91% and BCAC by 64–71% - Reduced PCNA-labeling index in ACF by 21% and BCAC by 19% | [83] |
Colon carcinogenesis model
| Diet containing 100 ppm NOB (0.1% wt) (for 17 weeks) | Inhibit AOM/DSS-induced colon carcinogenesis | - Suppressed incidence of neoplasms (adenoma and adenocarcinoma), lowered multiplicity of tumor | [84] |
| Inhibit leptin-induced colon carcinogenesis | ||||
| - Suppressed serum levels of leptin by 75–84% | ||||
Colon carcinogenesis model
| Diet containing NOB (0.01% wt and 0.05% wt) (34 weeks) | Inhibit AOM induced colon carcinogenesis | - Reduced incidence and multiplicity of colonic adenocarcinoma | [74] |
| Anti-proliferative effect | ||||
| - Increased apoptosis index of adenocarcinoma | ||||
| Anti-inflammatory effect | ||||
| - Reduced level of PGE2 in colonic adenocarcinoma and surrounding mucosa | ||||
Colon carcinogenesis model
| Diet containing NOB (0.01% wt and 0.05% wt) (5 weeks) | Inhibit AOM-induced colon carcinogenesis | - Reduced the frequency of colonic aberrant crypt foci formation - Reduced number of ACF in proximal, middle and distal colon | [41] |
| Anti-proliferative effect | ||||
| - Reduced MIB-5 labeling index of ACF but not of normal colonic crypts | ||||
| Anti-inflammatory effect | ||||
| - Reduced level of PGE2 in colonic mucosa | ||||
Colon carcinogenesis model
| Diet containing NOB (0.05% wt.) (50 weeks) | Inhibit PhIP-induced ACF in transverse colon | - Reduced the total colonic ACF indices in transverse colon | [75] |
Colorectal cancer xenograft mouse model
| NOB 100 mg/kg i.p. daily for 3 weeks 5-DMN 50 mg/kg and 100 mg/kg i.p. daily for 3 weeks | Anti-tumor effect | - NOB reduced tumor size and weight but not significant as compared to control - 5-DMN reduced tumor size and weight significantly as compared to control | [49] |
| Autophagy induction | - 5-DMN increased LC3 expression | |||
| Anti-inflammatory effect | ||||
| - 5-DMN increased p53 expression - 5-DMN reduced COX-2 expression | ||||
| Anti-angiogenesis | ||||
| - 5-DMN reduced VEGF expression |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, J.X.H.; Tan, L.T.-H.; Goh, J.K.; Chan, K.G.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Nobiletin and Derivatives: Functional Compounds from Citrus Fruit Peel for Colon Cancer Chemoprevention. Cancers 2019, 11, 867. https://doi.org/10.3390/cancers11060867
Goh JXH, Tan LT-H, Goh JK, Chan KG, Pusparajah P, Lee L-H, Goh B-H. Nobiletin and Derivatives: Functional Compounds from Citrus Fruit Peel for Colon Cancer Chemoprevention. Cancers. 2019; 11(6):867. https://doi.org/10.3390/cancers11060867
Chicago/Turabian StyleGoh, Joanna Xuan Hui, Loh Teng-Hern Tan, Joo Kheng Goh, Kok Gan Chan, Priyia Pusparajah, Learn-Han Lee, and Bey-Hing Goh. 2019. "Nobiletin and Derivatives: Functional Compounds from Citrus Fruit Peel for Colon Cancer Chemoprevention" Cancers 11, no. 6: 867. https://doi.org/10.3390/cancers11060867
APA StyleGoh, J. X. H., Tan, L. T.-H., Goh, J. K., Chan, K. G., Pusparajah, P., Lee, L.-H., & Goh, B.-H. (2019). Nobiletin and Derivatives: Functional Compounds from Citrus Fruit Peel for Colon Cancer Chemoprevention. Cancers, 11(6), 867. https://doi.org/10.3390/cancers11060867