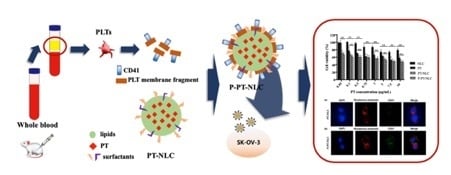
The Delivery Strategy of Paclitaxel Nanostructured Lipid Carrier Coated with Platelet Membrane
Abstract
1. Introduction
2. Results
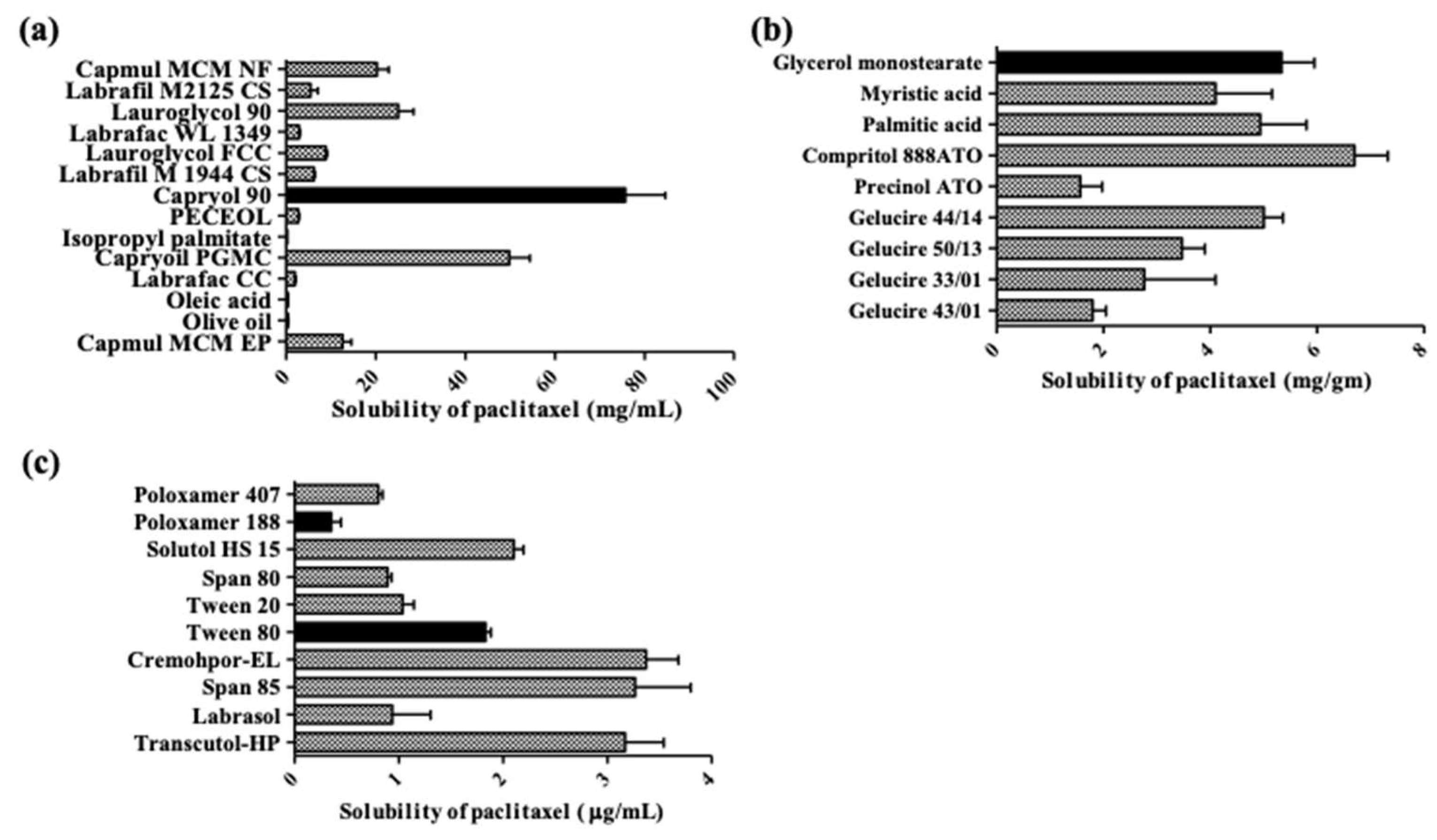
2.1. Screening of Liquid Lipid, Solid Lipid and Surfactant
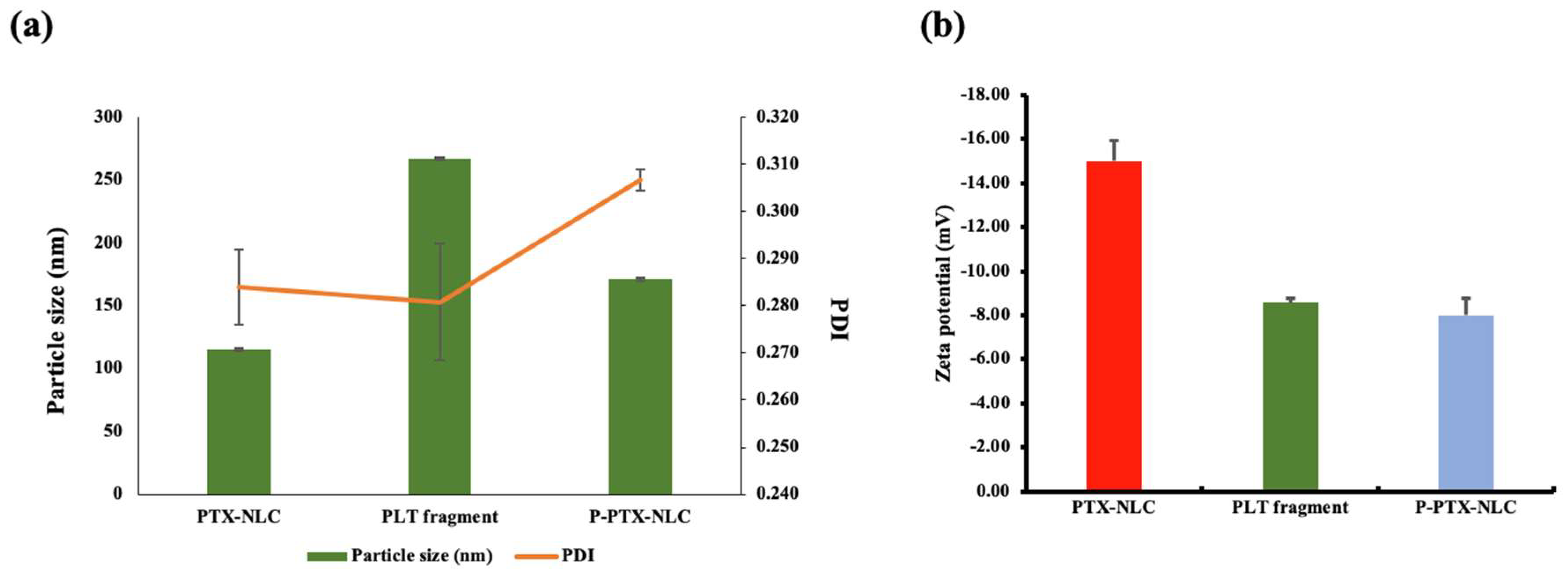
2.2. Physicochemical Properties
2.2.1. Particle Size, Polydispersity Index (PDI), Zeta Potential (ZP), EE and LC
2.2.2. Differential Scanning Calorimetry (DSC) and Powder X-ray Diffraction (PXRD) Analysis
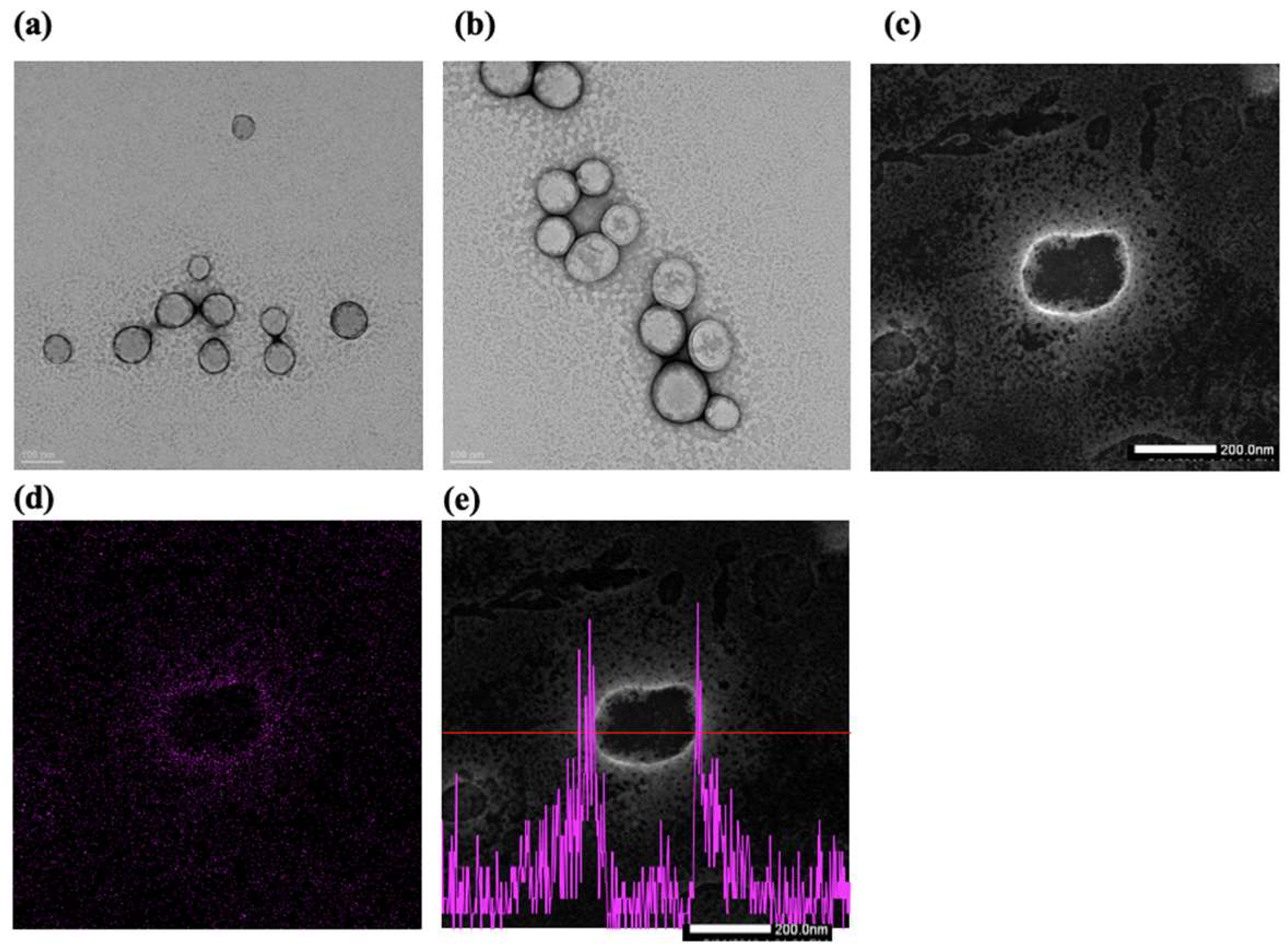
2.2.3. Transmission Electron Microscopy (TEM) Analysis
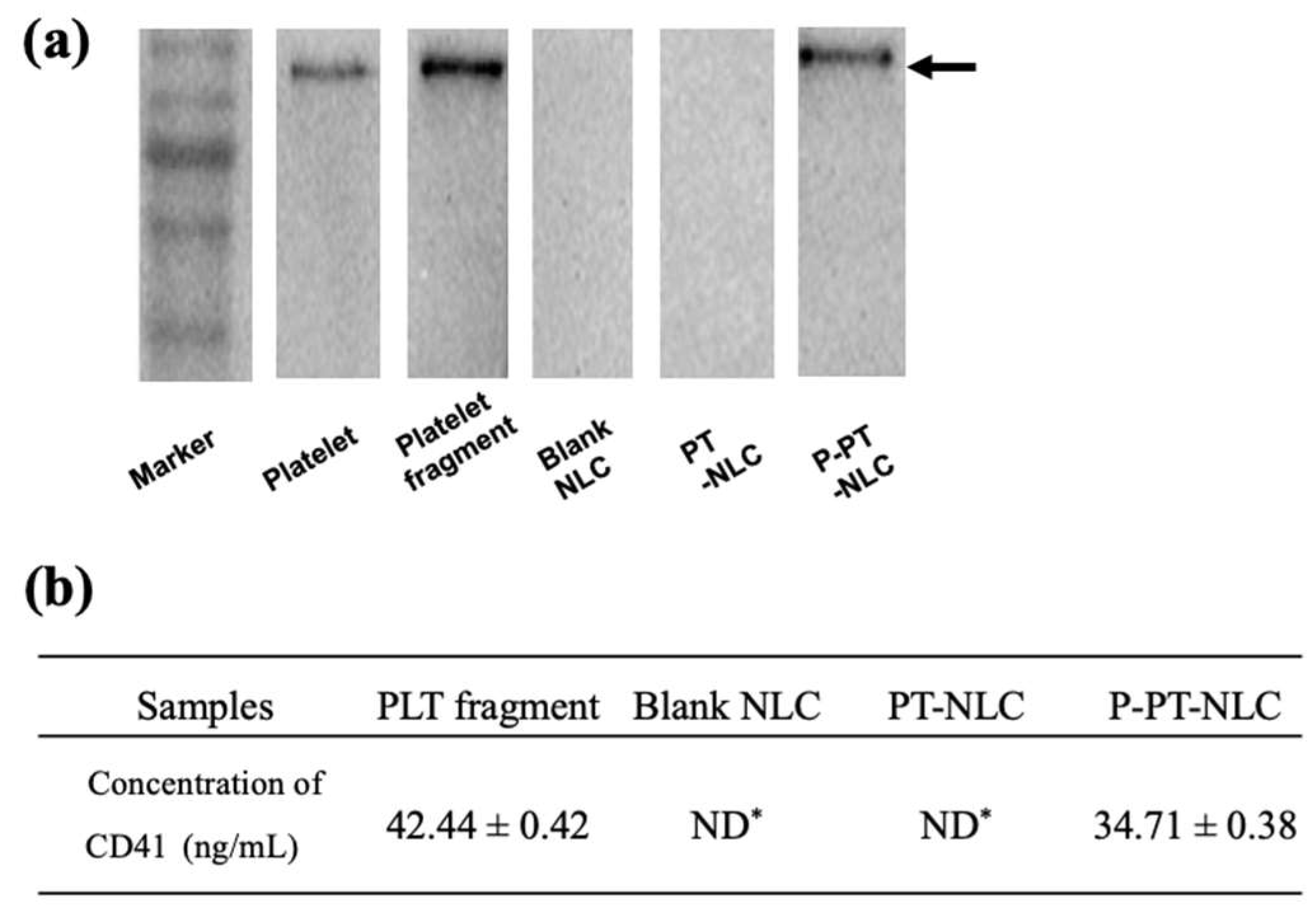
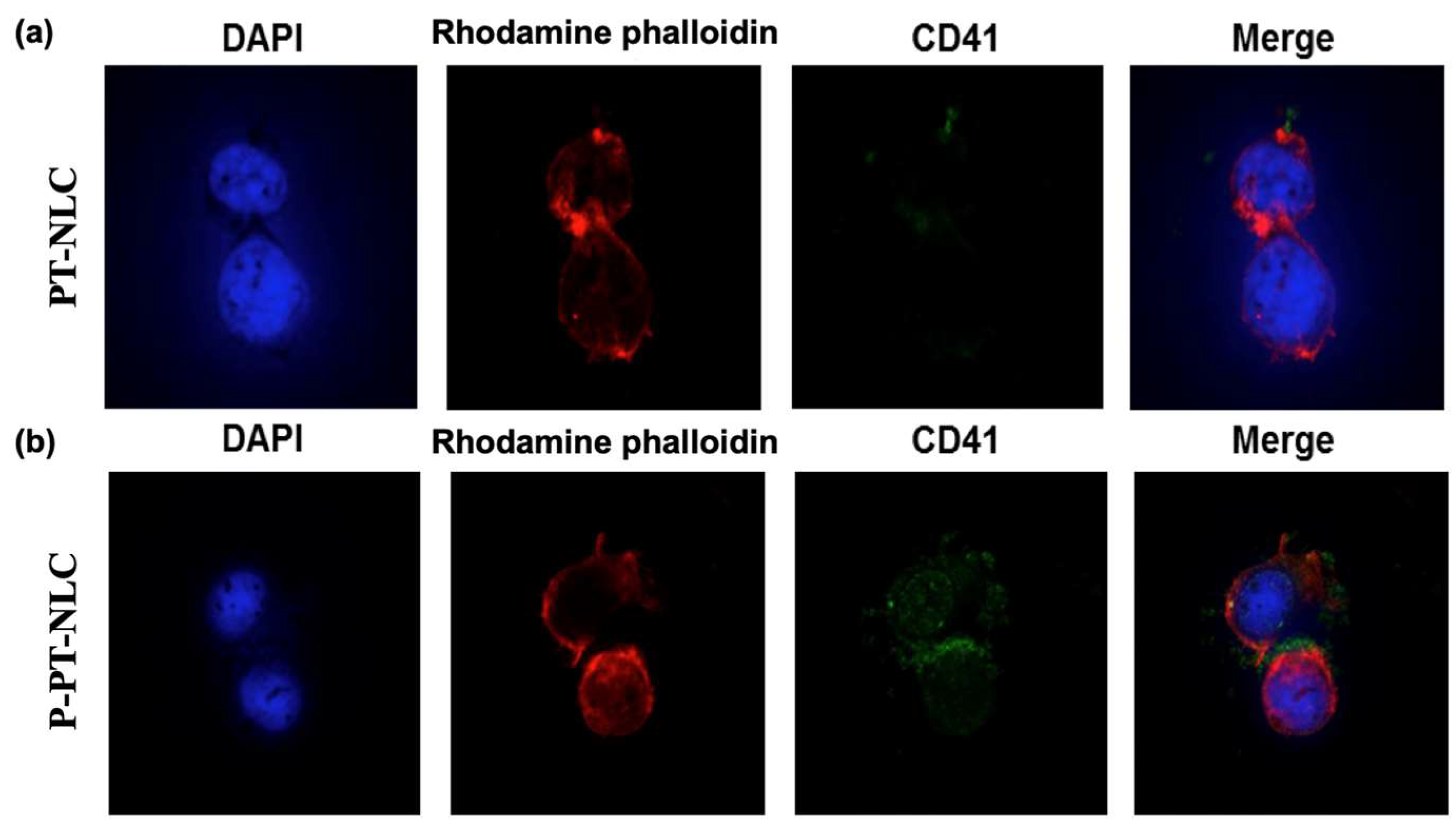
2.3. Western Blot Assay and Enzyme-Linked Immunosorbent Assay (ELISA) of CD41
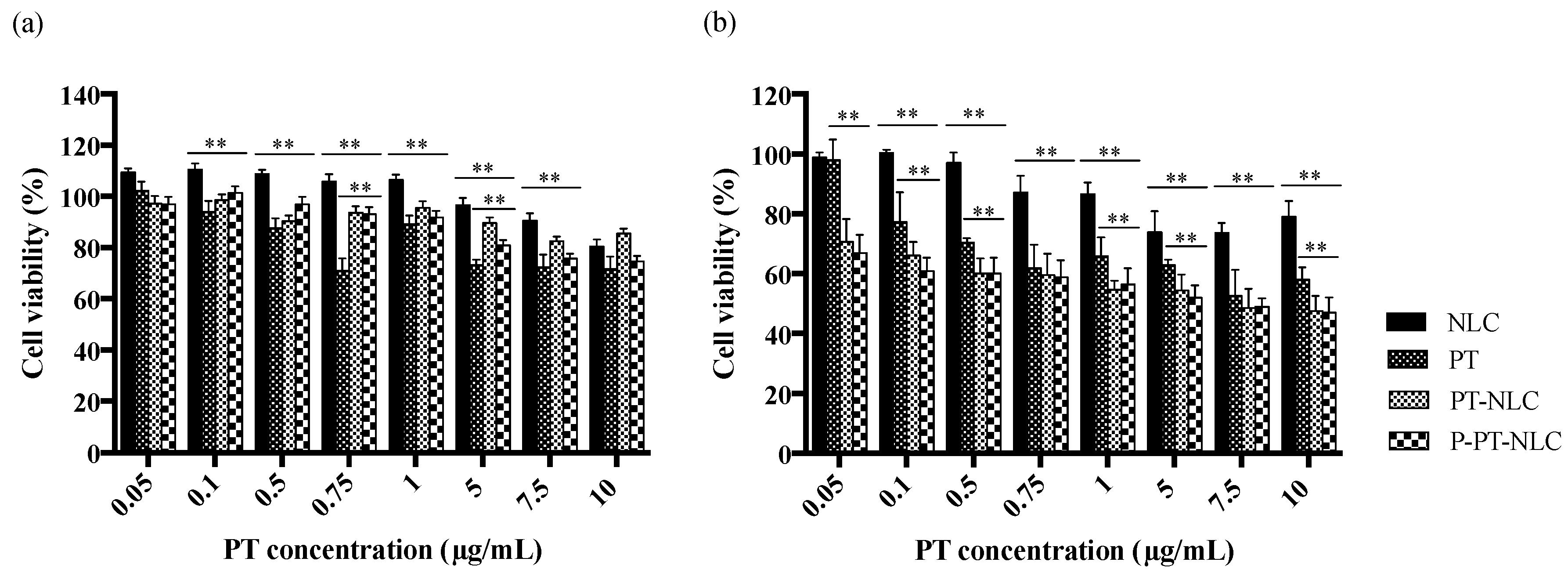
2.4. In Vitro Cell Experiments
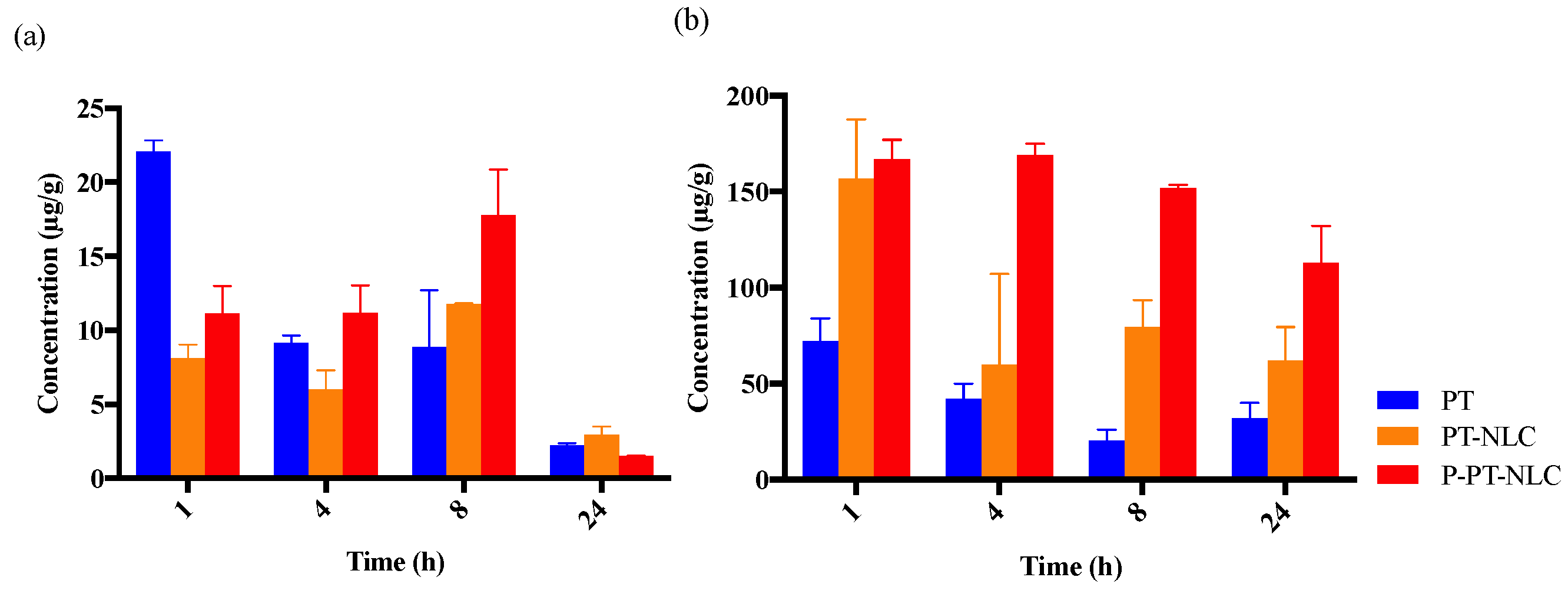
2.5. Biodistribution Study
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Screening of Liquid Lipid, Solid Lipid and Surfactant
4.3. PT-NLC Preparation
4.4. P-PT-NLC Preparation
4.5. Physicochemical Properties of Formulations
4.5.1. Evaluation of Particle Size, PDI and ZP
4.5.2. DSC and PXRD Analysis
4.5.3. TEM Analysis
4.5.4. EE and LC Determination
4.6. Characterization of PLT Membrane Protein Coated NLC (P-PT-NLC)
4.6.1. Western Blot
4.6.2. ELISA
4.6.3. CLSM Analysis
4.7. Cytotoxicity Study
4.8. Biodistribution Study
4.9. HPLC Condition
4.10. Data and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kampan, N.C.; Madondo, M.T.; McNally, O.M.; Quinn, M.; Plebanski, M. Paclitaxel and its evolving role in the management of ovarian cancer. Biomed. Res. Int. 2015, 413076. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mahdi, H.; Bryant, C.; Shah, J.P.; Garg, G.; Munkarah, A. Clinical trials and progress with paclitaxel in ovarian cancer. Int. J. Womens Health 2010, 2, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Li, H.; Du, R.; Meng, J.; Yang, G. Involvement of non-coding RNAs in chemotherapy resistance of ovarian cancer. J. Cancer. 2018, 9, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- Prat, J.; Oncology, F.C.O.G. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2014, 124, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, T.A.; Winter-Roach, B.A.; Heus, P.; Kitchener, H.C. Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer. Cochrane Database Syst. Rev. 2015, CD004706. [Google Scholar] [CrossRef]
- Einzig, A.I.; Wiernik, P.H.; Sasloff, J.; Runowicz, C.D.; Goldberg, G.L. Phase II study and long-term follow-up of patients treated with taxol for advanced ovarian adenocarcinoma. J. Clin. Oncol. 1992, 10, 1748–1753. [Google Scholar] [CrossRef]
- Thigpen, J.T.; Blessing, J.A.; Ball, H.; Hummel, S.J.; Barrett, R.J. Phase II trial of paclitaxel in patients with progressive ovarian carcinoma after platinum-based chemotherapy: A Gynecologic Oncology Group study. J. Clin. Oncol. 1994, 12, 1748–1753. [Google Scholar] [CrossRef]
- Han, S.M.; Baek, J.S.; Kim, M.S.; Hwang, S.J.; Cho, C.W. Surface modification of paclitaxel-loaded liposomes using d-alpha-tocopheryl polyethylene glycol 1000 succinate: Enhanced cellular uptake and cytotoxicity in multidrug resistant breast cancer cells. Chem. Phys. Lipids. 2018, 213, 39–47. [Google Scholar] [CrossRef]
- Zhang, C.; Qu, G.; Sun, Y.; Wu, X.; Yao, Z.; Guo, Q.; Ding, Q.; Yuan, S.; Shen, Z.; Ping, Q.; et al. Pharmacokinetics, biodistribution, efficacy and safety of N-octyl-O-sulfate chitosan micelles loaded with paclitaxel. Biomaterials 2008, 29, 1233–1241. [Google Scholar] [CrossRef]
- Patel, K.; Patil, A.; Mehta, M.; Gota, V.; Vavia, P. Oral delivery of paclitaxel nanocrystal (PNC) with a dual Pgp-CYP3A4 inhibitor: Preparation, characterization and antitumor activity. Int. J. Pharm. 2014, 472, 214–223. [Google Scholar] [CrossRef]
- Gligorov, J.; Lotz, J.P. Preclinical pharmacology of the taxanes: Implications of the differences. Oncologist 2004, 9 (Suppl. 2), 3–8. [Google Scholar] [CrossRef]
- Sharma, S.; Verma, A.; Teja, B.V.; Shukla, P.; Mishra, P.R. Development of stabilized Paclitaxel nanocrystals: In-vitro and in-vivo efficacy studies. Eur. J. Pharm. Sci. 2015, 69, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.S.; Cho, C.W. Controlled release and reversal of multidrug resistance by co-encapsulation of paclitaxel and verapamil in solid lipid nanoparticles. Int. J. Pharm. 2015, 478, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Sofias, A.M.; Dunne, M.; Storm, G.; Allen, C. The battle of “nano“ paclitaxel. Adv. Drug Deliv. Rev. 2017, 122, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Lee, S.G.; Kang, M.J.; Lee, S.; Choi, Y.W. Surface modification of lipid-based nanocarriers for cancer cell-specific drug targeting. J. Pharm. Investig. 2017, 47, 203–227. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Muller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.D.; Muller, R.H. Lipid nanoparticles for parenteral delivery of actives. Eur. J. Pharm. Biopharm. 2009, 71, 161–172. [Google Scholar] [CrossRef]
- Gupta, B.; Yong, C.S.; Kim, J.O. Solid matrix-based lipid nanoplatforms as carriers for combinational therapeutics in cancer. J. Pharm. Investig. 2017, 47, 461–473. [Google Scholar] [CrossRef]
- Emami, J.; Rezazadeh, M.; Varshosaz, J.; Tabbakhian, M.; Aslani, A. Formulation of LDL targeted nanostructured lipid carriers loaded with paclitaxel: A detailed study of preparation, freeze drying condition, and in vitro cytotoxicity. J. Nanomater. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Zolnik, B.S.; Gonzalez-Fernandez, A.; Sadrieh, N.; Dobrovolskaia, M.A. Nanoparticles and the immune system. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Le, Q.-V.; Choi, J.; Oh, Y.-K. Nano delivery systems and cancer immunotherapy. J. Pharm. Investig. 2018, 48, 527–539. [Google Scholar] [CrossRef]
- Rao, L.; Bu, L.-L.; Meng, Q.-F.; Cai, B.; Deng, W.-W.; Li, A.; Li, K.; Guo, S.-S.; Zhang, W.-F.; Liu, W.; et al. Antitumor Platelet-Mimicking Magnetic Nanoparticles. Adv. Funct. Mater. 2017, 27, 1604774. [Google Scholar] [CrossRef]
- Hu, H.; Liu, D.; Zhao, X.; Qiao, M.; Chen, D. Preparation, characterization, cellular uptake and evaluation in vivo of solid lipid nanoparticles loaded with cucurbitacin B. Drug Dev. Ind. Pharm. 2013, 39, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Borsig, L.; Wong, R.; Feramisco, J.; Nadeau, D.R.; Varki, N.M.; Varki, A. Heparin and cancer revisited: Mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc. Natl. Acad. Sci. USA 2001, 98, 3352–3357. [Google Scholar] [CrossRef] [PubMed]
- Labelle, M.; Begum, S.; Hynes, R.O. Platelets guide the formation of early metastatic niches. Proc. Natl. Acad. Sci. USA 2014, 111, E3053–3061. [Google Scholar] [CrossRef] [PubMed]
- Goubran, H.A.; Stakiw, J.; Radosevic, M.; Burnouf, T. Platelet-cancer interactions. Semin. Thromb. Hemost. 2014, 40, 296–305. [Google Scholar] [PubMed]
- Hu, C.M.; Fang, R.H.; Wang, K.C.; Luk, B.T.; Thamphiwatana, S.; Dehaini, D.; Nguyen, P.; Angsantikul, P.; Wen, C.H.; Kroll, A.V.; et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526, 118–121. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Qian, C.; Wang, C.; Bomba, H.N.; Gu, Z. Anticancer Platelet-Mimicking Nanovehicles. Adv. Mater. 2015, 27, 7043–7050. [Google Scholar] [CrossRef]
- Shete, H.; Patravale, V. Long chain lipid based tamoxifen NLC. Part I: Preformulation studies, formulation development and physicochemical characterization. Int. J. Pharm. 2013, 454, 573–583. [Google Scholar] [CrossRef]
- Donovan, L.E.; Dammer, E.B.; Duong, D.M.; Hanfelt, J.J.; Levey, A.I.; Seyfried, N.T.; Lah, J.J. Exploring the potential of the platelet membrane proteome as a source of peripheral biomarkers for alzheimer’s disease. Alzheimer Res. Ther. 2013, 5, 32. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar]
- Olerile, L.D.; Liu, Y.; Zhang, B.; Wang, T.; Mu, S.; Zhang, J.; Selotlegeng, L.; Zhang, N. Near-infrared mediated quantum dots and paclitaxel co-loaded nanostructured lipid carriers for cancer theragnostic. Colloids Surf. B Biointerfaces 2017, 150, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ying, M.; Dehaini, D.; Su, Y.; Kroll, A.V.; Zhou, J.; Gao, W.; Fang, R.H.; Chien, S.; Zhang, L. Nanoparticle functionalization with platelet membrane enables multifactored biological targeting and detection of atherosclerosis. ACS Nano 2018, 12, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Demetzos, C. Differential Scanning Calorimetry (DSC): A tool to study the thermal behavior of lipid bilayers and liposomal stability. J. Liposome Res. 2008, 18, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Bi, J.; Tian, H.; Jin, Y.; Wang, Y.; Yang, X.; Yang, Z.; Kou, J.; Li, F. Preparation and evaluation of a self-nanoemulsifying drug delivery system loaded with Akebia saponin D-phospholipid complex. Int. J. Nanomed. 2016, 11, 4919–4929. [Google Scholar]
- Kassem, A.A.; Abd El-Alim, S.H.; Basha, M.; Salama, A. Phospholipid complex enriched micelles: A novel drug delivery approach for promoting the antidiabetic effect of repaglinide. Eur. J. Pharm. Sci. 2017, 99, 75–84. [Google Scholar] [CrossRef]
- Tao, L.; Jiang, J.; Gao, Y.; Wu, C.; Liu, Y. Biodegradable Alginate-Chitosan Hollow Nanospheres for Codelivery of Doxorubicin and Paclitaxel for the Effect of Human Lung Cancer A549 Cells. Biomed. Res. Int. 2018, 4607945. [Google Scholar] [CrossRef]
- Xu, J.; He, F.; Gai, S.; Zhang, S.; Li, L.; Yang, P. Nitrogen-enriched, double-shelled carbon/layered double hydroxide hollow microspheres for excellent electrochemical performance. Nanoscale 2014, 6, 10887–10895. [Google Scholar] [CrossRef]
- Bao, H.; Wang, L.; Li, C.; Luo, J. Structural Characterization and Identification of Graphdiyne and Graphdiyne-Based Materials. ACS Appl. Mater. Interfaces 2018, 11, 2717–2729. [Google Scholar] [CrossRef]
- Dehaini, D.; Wei, X.; Fang, R.H.; Masson, S.; Angsantikul, P.; Luk, B.T.; Zhang, Y.; Ying, M.; Jiang, Y.; Kroll, A.V.; et al. Erythrocyte-platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv. Mater. 2017, 29, 1606209. [Google Scholar] [CrossRef]
- Danhier, F.; Vroman, B.; Lecouturier, N.; Crokart, N.; Pourcelle, V.; Freichels, H.; Jerome, C.; Marchand-Brynaert, J.; Feron, O.; Preat, V. Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with paclitaxel. J. Control. Release 2009, 140, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chen, Y.; Thompson, D.H.; Park, K.; Li, T. Impact of surfactant treatment of paclitaxel nanocrystals on biodistribution and tumor accumulation in tumor-bearing mice. J. Control. Release 2016, 237, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ji, J.; Wang, F.; Shi, L.; Yu, J.; Wang, D. Formulation, characterization, and in vitro/vivo studies of aclacinomycin A-loaded solid lipid nanoparticles. Drug Deliv. 2016, 23, 1317–1325. [Google Scholar] [PubMed]
- Grozovsky, R.; Hoffmeister, K.M.; Falet, H. Novel clearance mechanisms of platelets. Curr. Opin. Hematol. 2010, 17, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) Effects; What is the appropriate target? Theranostics 2014, 4, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Z.H.; Li, T.; McNally, H.; Park, K.; Sturek, M. Development and evaluation of transferrin-stabilized paclitaxel nanocrystal formulation. J. Control. Release 2014, 176, 76–85. [Google Scholar] [CrossRef]
- Son, G.H.; Na, Y.G.; Huh, H.W.; Wang, M.; Kim, M.K.; Han, M.G.; Byeon, J.J.; Lee, H.K.; Cho, C.W. Systemic design and evaluation of ticagrelor-loaded nanostructured lipid carriers for enhancing bioavailability and antiplatelet activity. Pharmaceutics 2019, 11. [Google Scholar] [CrossRef]
- Patel, K.; Padhye, S.; Nagarsenker, M. Duloxetine HCl lipid nanoparticles: Preparation, characterization, and dosage form design. AAPS PharmSciTech 2012, 13, 125–133. [Google Scholar] [CrossRef]
- Wei, X.; Gao, J.; Fang, R.H.; Luk, B.T.; Kroll, A.V.; Dehaini, D.; Zhou, J.; Kim, H.W.; Gao, W.; Lu, W.; et al. Nanoparticles camouflaged in platelet membrane coating as an antibody decoy for the treatment of immune thrombocytopenia. Biomaterials 2016, 111, 116–123. [Google Scholar] [CrossRef]
- He, Y.; Li, R.; Liang, J.; Zhu, Y.; Zhang, S.; Zheng, Z.; Qin, J.; Pang, Z.; Wang, J. Drug targeting through platelet membrane-coated nanoparticles for the treatment of rheumatoid arthritis. Nano Res. 2018, 11, 6086–6101. [Google Scholar] [CrossRef]
- Asadi, J.; Ferguson, S.; Raja, H.; Hacker, C.; Marius, P.; Ward, R.; Pliotas, C.; Naismith, J.; Lucocq, J. Enhanced imaging of lipid rich nanoparticles embedded in methylcellulose films for transmission electron microscopy using mixtures of heavy metals. Micron 2017, 99, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Sato, S.; Oh-hara, T.; Takami, M.; Koike, S.; Fujita, N. Platelets promote tumor growth and metastasis via direct interaction between Aggrus/podoplanin and CLEC-2. PLoS ONE 2013, 8, e73609. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.L.; Sun, J.; Gong, S.Q.; Yu, X.F.; Gong, R.; Deng, H. Interaction between circulating cancer cells and platelets: Clinical implication. Chinese J. Cancer Res. 2015, 27, 450–460. [Google Scholar]
- Bobek, V.; Kovarik, J. Antitumor and antimetastatic effect of warfarin and heparins. Biomed. Pharmacother. 2004, 58, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Smorenburg, S.M.; Van Noorden, C.J. The complex effects of heparins on cancer progression and metastasis in experimental studies. Pharmacol. Rev. 2001, 53, 93–105. [Google Scholar] [PubMed]

| (a) Various Compositions for PT-NLCs | |||||
| Code | GMS (mg) | Capryol 90 (mg) | PT (mg) | % of Poloxamer 188 in 10 mL | % of Tween 80 in 10 mL |
| 1 | 70 | 70 | 5 | 0.5 | 1 |
| 2 | 120 | 60 | 5 | 0.5 | 1 |
| 3 | 140 | 70 | 5 | 1 | 0.5 |
| 4 | 140 | 70 | 5 | 0.5 | 1 |
| 5 | 160 | 80 | 5 | 0.5 | 1 |
| 6 | 180 | 90 | 5 | 0.5 | 1 |
| 7 | 210 | 70 | 5 | 0.5 | 1 |
| 8 | 280 | 70 | 5 | 0.5 | 1 |
| (b) Physicochemical Properties | |||||
| Code | Particle Size (nm) | PDI | EE (%) | LC (%) | ZP (mV) |
| 1 | 279.2 ± 10.4 | 0.308 ± 0.012 | 99.79 ± 0.18 | 3.44 ± 0.01 | 2.24 ± 0.51 |
| 2 | 266.5 ± 46.8 | 0.360 ± 0.011 | 99.94 ± 0.03 | 2.70 ± 0.03 | −22.70 ± 1.46 |
| 3 | 161.3 ± 0.9 | 0.311 ± 0.301 | 99.62 ± 0.05 | 2.25 ± 0.01 | −16.40 ± 0.82 |
| 4 | 115.2 ± 3.9 | 0.284 ± 0.015 | 99.98 ± 0.01 | 2.33 ± 0.01 | −15.00 ± 0.93 |
| 5 | 123.0 ± 0.9 | 0.352 ± 0.022 | 99.95 ± 0.02 | 2.04 ± 0.01 | −30.60 ± 0.31 |
| 6 | 164.4 ± 12.2 | 0.321 ± 0.017 | 99.94 ± 0.05 | 1.82 ± 0.02 | −29.40 ± 1.59 |
| 7 | 158.6 ± 9.8 | 0.297 ± 0.012 | 99.96 ± 0.06 | 1.75 ± 0.01 | −25.33 ± 0.62 |
| 8 | 2599.4 ± 392.7 | 0.220 ± 0.150 | 99.51 ± 0.03 | 1.40 ± 0.01 | −31.43 ± 0.54 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bang, K.-H.; Na, Y.-G.; Huh, H.W.; Hwang, S.-J.; Kim, M.-S.; Kim, M.; Lee, H.-K.; Cho, C.-W. The Delivery Strategy of Paclitaxel Nanostructured Lipid Carrier Coated with Platelet Membrane. Cancers 2019, 11, 807. https://doi.org/10.3390/cancers11060807
Bang K-H, Na Y-G, Huh HW, Hwang S-J, Kim M-S, Kim M, Lee H-K, Cho C-W. The Delivery Strategy of Paclitaxel Nanostructured Lipid Carrier Coated with Platelet Membrane. Cancers. 2019; 11(6):807. https://doi.org/10.3390/cancers11060807
Chicago/Turabian StyleBang, Ki-Hyun, Young-Guk Na, Hyun Wook Huh, Sung-Joo Hwang, Min-Soo Kim, Minki Kim, Hong-Ki Lee, and Cheong-Weon Cho. 2019. "The Delivery Strategy of Paclitaxel Nanostructured Lipid Carrier Coated with Platelet Membrane" Cancers 11, no. 6: 807. https://doi.org/10.3390/cancers11060807
APA StyleBang, K.-H., Na, Y.-G., Huh, H. W., Hwang, S.-J., Kim, M.-S., Kim, M., Lee, H.-K., & Cho, C.-W. (2019). The Delivery Strategy of Paclitaxel Nanostructured Lipid Carrier Coated with Platelet Membrane. Cancers, 11(6), 807. https://doi.org/10.3390/cancers11060807