Gold Nanorod-Assisted Photothermal Therapy Decreases Bleeding during Breast Cancer Surgery in Dogs and Cats
Abstract
1. Introduction
2. Results
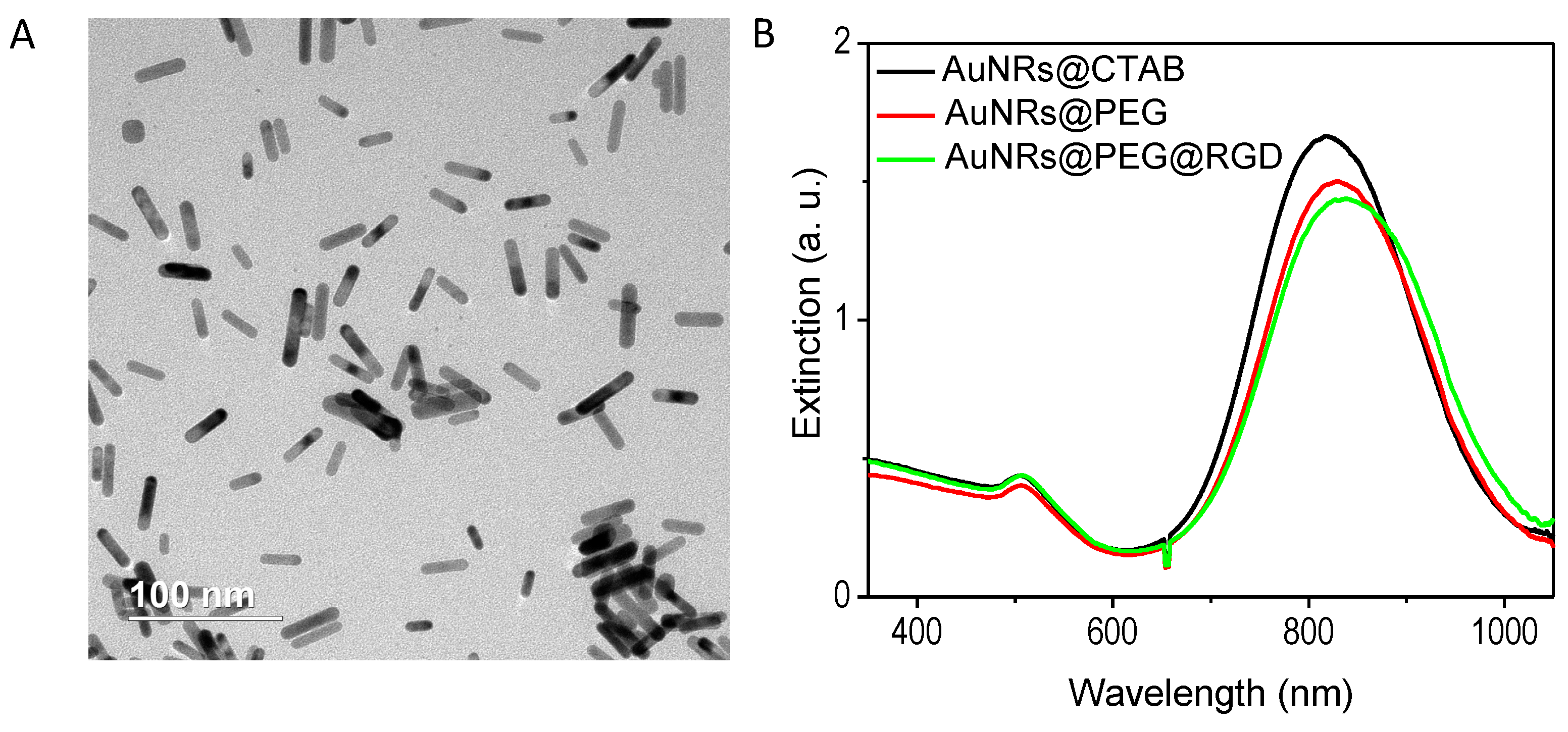
2.1. Preparation and Characterization of the AuNRs
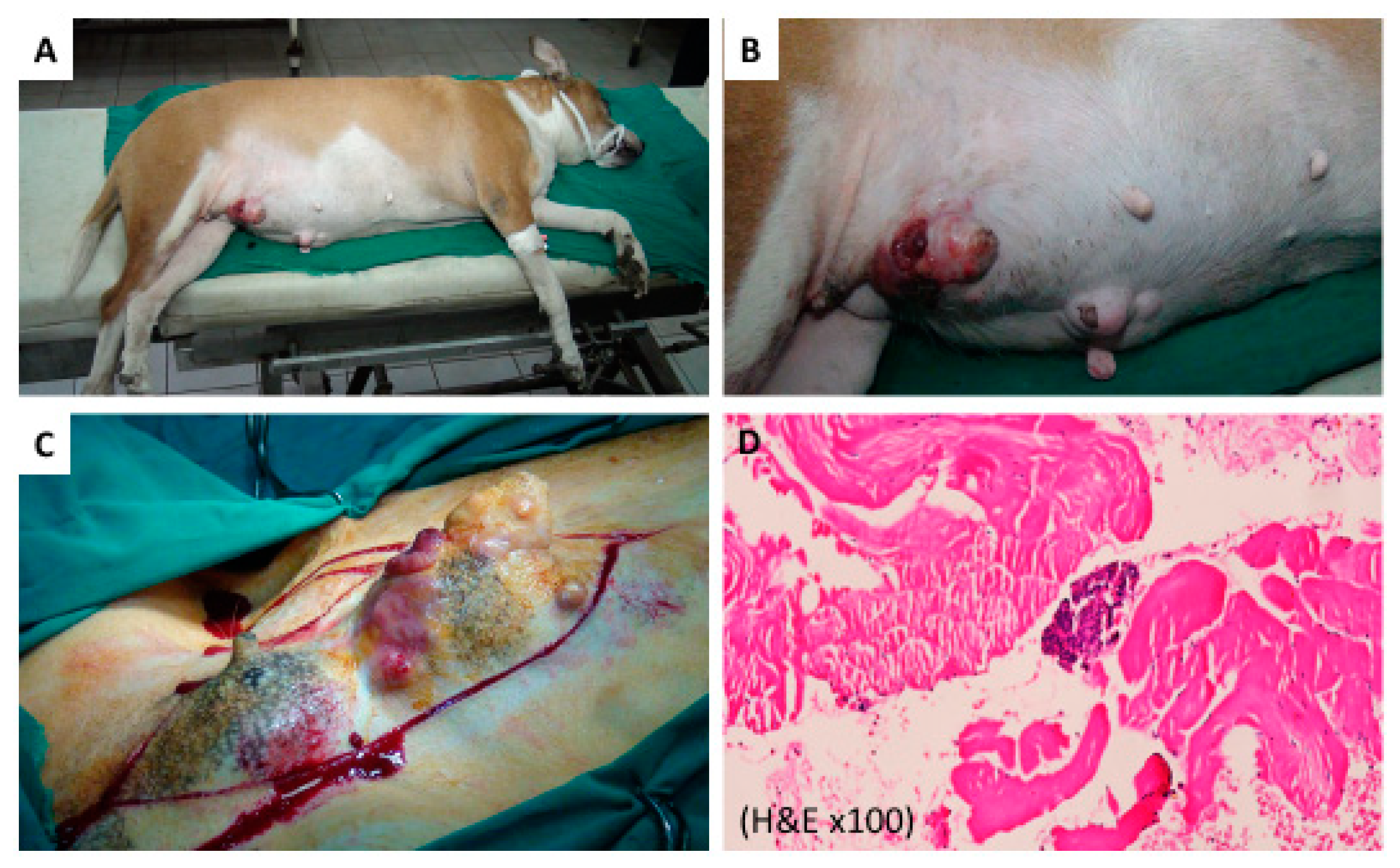
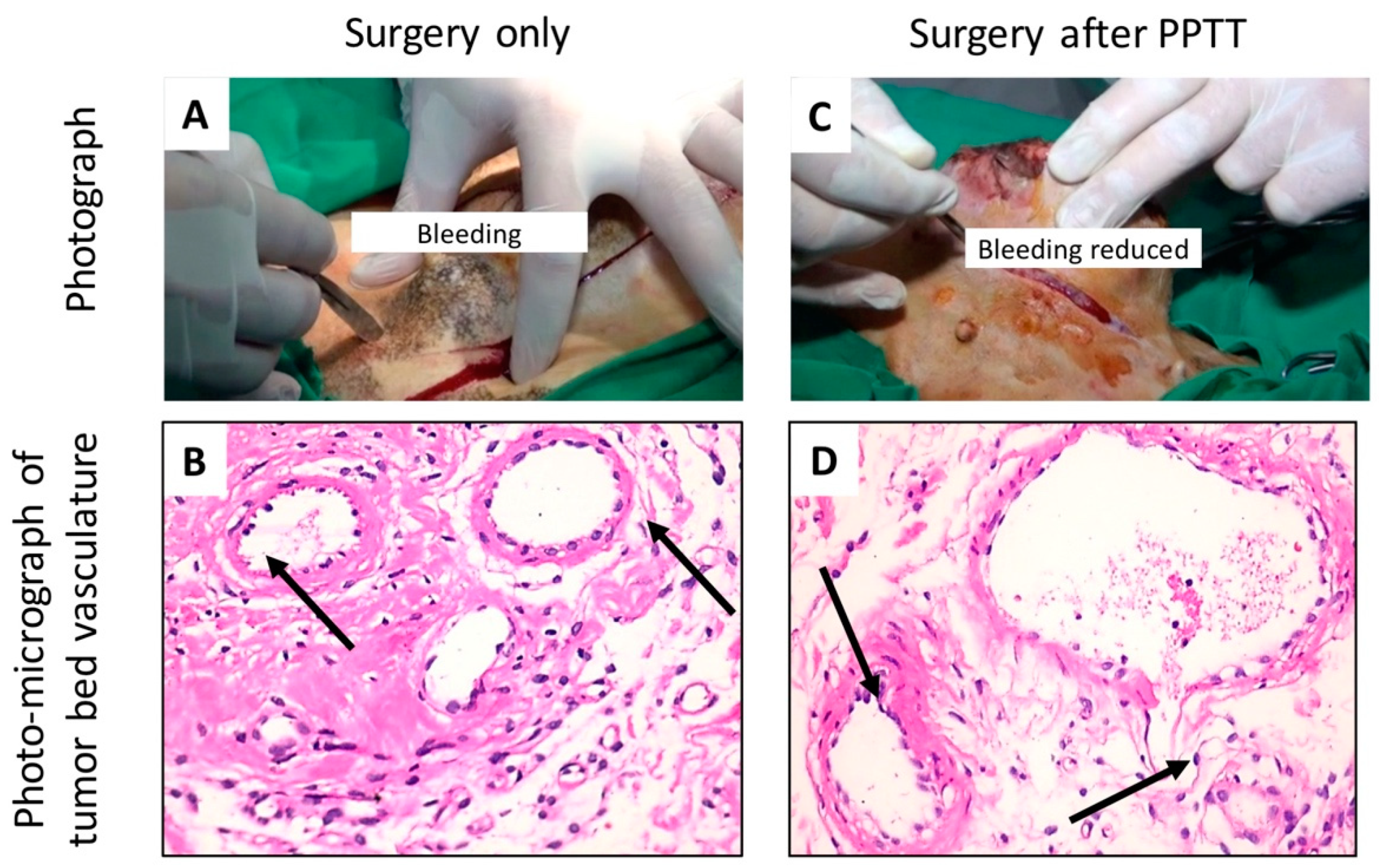
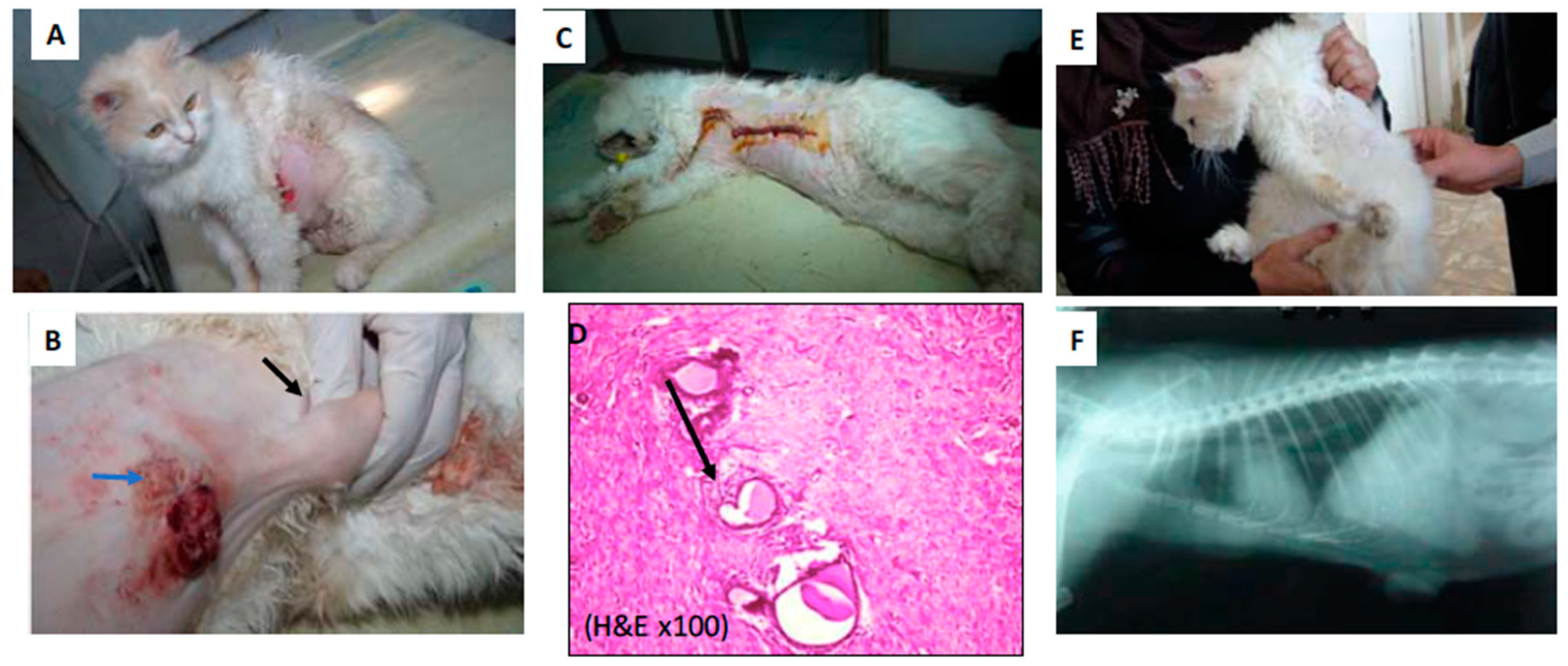
2.2. PPTT Decreases Bleeding during Surgery
3. Discussion
4. Materials and Methods
4.1. Synthesis and Surface Modification of AuNRs
4.2. Characterization of AuNRs
4.3. Animal Diagnosis and X-ray Examination
4.4. Performing PPTT in Animals
4.5. Histopathology Evaluation of the Animal Tumors
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, M.; Del Pino, P.; Mitchell, S.G.; Moros, M.; Stepien, G.; Pelaz, B.; Parak, W.J.; Galvez, E.M.; Pardo, J.; de la Fuente, J.M. Dissecting the molecular mechanism of apoptosis during photothermal therapy using gold nanoprisms. ACS Nano 2015, 9, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.; Ali, H.R.; Rankin, C.R.; El-Sayed, M.A. Targeting heat shock protein 70 using gold nanorods enhances cancer cell apoptosis in low dose plasmonic photothermal therapy. Biomaterials 2016, 102, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.; Ibrahim, I.M.; Ali, H.R.; Selim, S.A.; El-Sayed, M.A. Treatment of natural mammary gland tumors in canines and felines using gold nanorods-assisted plasmonic photothermal therapy to induce tumor apoptosis. Int. J. Nanomed. 2016, 11, 4849–4863. [Google Scholar] [CrossRef]
- Orosz, P.; Echtenacher, B.; Falk, W.; Ruschoff, J.; Weber, D.; Mannel, D.N. Enhancement of experimental metastasis by tumor necrosis factor. J. Exp. Med. 1993, 177, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Melamed, J.R.; Edelstein, R.S.; Day, E.S. Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS Nano 2015, 9, 6–11. [Google Scholar] [CrossRef]
- Ali, M.R.; Rahman, M.A.; Wu, Y.; Han, T.; Peng, X.; Mackey, M.A.; Wang, D.; Shin, H.J.; Chen, Z.G.; Xiao, H.; et al. Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice. Proc. Natl. Acad. Sci. USA 2017, 114, E3110–E3118. [Google Scholar] [CrossRef]
- Antuofermo, E.; Miller, M.A.; Pirino, S.; Xie, J.; Badve, S.; Mohammed, S.I. Spontaneous mammary intraepithelial lesions in dogs—A model of breast cancer. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2247–2256. [Google Scholar] [CrossRef]
- Adega, F.; Borges, A.; Chaves, R. Cat mammary tumors: Genetic models for the human counterpart. Vet. Sci. 2016, 3, 17. [Google Scholar] [CrossRef]
- Dickerson, E.B.; Dreaden, E.C.; Huang, X.; El-Sayed, I.H.; Chu, H.; Pushpanketh, S.; McDonald, J.F.; El-Sayed, M.A. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008, 269, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.K.; Wu, Y.; Tang, Y.; Xiao, H.; Chen, K.; Han, T.; Fang, N.; Wu, R.; El-Sayed, M.A. Targeting cancer cell integrins using gold nanorods in photothermal therapy inhibits migration through affecting cytoskeletal proteins. Proc. Natl. Acad. Sci. USA 2017, 114, E5655–E5663. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ali, M.R.K.; Dong, B.; Han, T.; Chen, K.; Chen, J.; Tang, Y.; Fang, N.; Wang, F.; El-Sayed, M.A. Gold nanorod photothermal therapy alters cell junctions and actin network in inhibiting cancer cell collective migration. ACS Nano 2018, 12, 9279–9290. [Google Scholar] [CrossRef]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics 2016, 6, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for cancer: A trigger for metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kitahara, T.; Takagi, R.; Kato, R. Does surgery for breast cancer induce angiogenesis and thus promote metastasis? Oncology 2011, 81, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Mackey, M.A.; Ali, M.R.; Austin, L.A.; Near, R.D.; El-Sayed, M.A. The most effective gold nanorod size for plasmonic photothermal therapy: Theory and in vitro experiments. J. Phys. Chem. B 2014, 118, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.; Snyder, B.; El-Sayed, M.A. Synthesis and optical properties of small Au nanorods using a seedless growth technique. Langmuir 2012, 28, 9807–9815. [Google Scholar] [CrossRef]
- Felding-Habermann, B.; O’Toole, T.E.; Smith, J.W.; Fransvea, E.; Ruggeri, Z.M.; Ginsberg, M.H.; Hughes, P.E.; Pampori, N.; Shattil, S.J.; Saven, A.; et al. Integrin activation controls metastasis in human breast cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 1853–1858. [Google Scholar] [CrossRef]
- Ali, M.; Wu, Y.; Ghosh, D.; Do, B.; Chen, K.; Dawson, M.; Fang, N.; Sulchek, T.; El-Sayed, M. Nuclear membrane-targeted gold nanoparticles inhibit cancer cell migration and invasion. Acs Nano 2017, 11, 3716–3726. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.A.; Petryk, A.A.; Giustini, A.J.; Hoopes, P.J. In vivo biodistribution of iron oxide nanoparticles: An overview. Proc. SPIE Int. Soc. Opt. Eng. 2011, 7901, 790117. [Google Scholar] [CrossRef] [PubMed]
- Elgrabli, D.; Beaudouin, R.; Jbilou, N.; Floriani, M.; Pery, A.; Rogerieux, F.; Lacroix, G. Biodistribution and clearance of TiO2 nanoparticles in rats after intravenous injection. PLoS ONE 2015, 10, e0124490. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shi, J.; Fu, X.; Yang, Y.; Zhang, H. Long-term in vivo biodistribution and toxicity study of functionalized near-infrared persistent luminescence nanoparticles. Sci. Rep. 2018, 8, 10595. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Wei, H.; Xu, H. Comparisons of the biodistribution and toxicological examinations after repeated intravenous administration of silver and gold nanoparticles in mice. Sci. Rep. 2017, 7, 3303. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Heuer-Jungemann, A.; Fernandes, A.R.; Kanaras, A.G.; Baptista, P.V. Peptide-coated gold nanoparticles for modulation of angiogenesis in vivo. Int. J. Nanomed. 2016, 11, 2633–2639. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Rana, S.; Miranda, O.R.; Bhattacharya, R.; Rotello, V.M.; Mukherjee, P. Mechanism of anti-angiogenic property of gold nanoparticles: Role of nanoparticle size and surface charge. Nanomedicine 2011, 7, 580–587. [Google Scholar] [CrossRef]
- Wu, M.Z.Y.; Zhang, Y.; Wu, M.; Wu, H.; Cao, L.; Li, L.; Li, X.; Zhang, X. Tumor angiogenesis targeting and imaging using gold nanoparticle probe with directly conjugated cyclic NGR. RSC Adv. 2018, 8, 1706–1716. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Z.; Yu, H.; Chen, X.; Feng, B.; Cui, Z.; Lin, B.; Yin, Q.; Zhang, Z.; Chen, C.; et al. Treatment of metastatic breast cancer by combination of chemotherapy and photothermal ablation using doxorubicin-loaded DNA wrapped gold nanorods. Biomaterials 2014, 35, 8374–8384. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Tay, C.Y.; Bay, B.H.; Leong, D.T. Gold nanoparticles induced endothelial leakiness depends on particle size and endothelial cell origin. ACS Nano 2017, 11, 5020–5030. [Google Scholar] [CrossRef]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol. Biol. 2010, 624, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Farghali, H.A.; AbdElKader, N.A.; Khattab, M.S.; AbuBakr, H.O. Novel approach to gastric mucosal defect repair using fresh amniotic membrane allograft in dogs (experimental study). Stem Cell Res. Ther. 2017, 8, 235. [Google Scholar] [CrossRef]
- Farghali, H.A.; AbdElKader, N.A.; Khattab, M.S.; AbuBakr, H.O. Evaluation of subcutaneous infiltration of autologous platelet-rich plasma on skin-wound healing in dogs. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Krall, J.A.; Reinhardt, F.; Mercury, O.A.; Pattabiraman, D.R.; Brooks, M.W.; Dougan, M.; Lambert, A.W.; Bierie, B.; Ploegh, H.L.; Dougan, S.K.; et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
| No. # | Species and Age | Site of Tumors | Size of Tumors (cm) | Grade | Therapy |
|---|---|---|---|---|---|
| GI-1 | Dog—Mixed Boxer, 14 years | 1-R caudoabdominal ulcerated | (8 × 5) | II | Only mastectomy |
| 2-R cranioabdominal | (18 × 4) | ||||
| 3-R inguinal Lymph node All the three connected to form one chain | |||||
| GI-2 | Dog—Griffon, 7 years | 1-R Cranioinquinal | (4.1 × 2.9) | II | Laser only, followed by mastectomy |
| GI-3 | Cat—15 years | 1-R caudo abdominal | (4 × 4.5). | II | 1 + 2 + 3 tumors form chain; Laser only, followed by mastectomy |
| 2-R inguinal LN | (3 × 2.1). | ||||
| 3-L cranioabdominal | (5 × 2) | ||||
| 4-R inguinal | (1 × 2) | I | 4 + 5 + 6 tumors treated by AuNRs only, followed by mastectomy | ||
| 5-L inguinal | (1 × 1) | ||||
| 6-L caudothoracic | (1 × 1) | ||||
| GII-1 | Cat—9 years | L caudothoracic | (4.5 × 4) | III | Three sessions of plasmonic photothermal therapy (PPTT), followed by surgery |
| R caudothoracic | (4 × 4) | ||||
| GII-2 | Dog—Griffon, 10 years | R Caudoquinal | (3.1 × 3.6) | III | Three session of PPTT, followed by surgery |
| GII-3 | Dog—Griffon, 11 years | 1-L inguinal | (5 × 5) | II | Three session of PPTT, followed by surgery |
| 2-L inguinal | (2 × 1.5) | PPTT only | |||
| 3 small tumor | (1.5 × 1) | PPTT only | |||
| GII-4 | Dog—Griffon, 5 years | 1-R caudo thoracic | (3 × 2.5) (8 × 6.5) | II | Three sessions of PPTT, followed by surgery |
| 2-R cranio abdominal both tumors form chain | |||||
| GII-5 | Dog—Griffon, 8 years | 1-L inguinal large calcified | (5 × 5) (2 × 1.5) (1.5 × 1) | III | Three session of PPTT, followed by surgery |
| 2-small caudal abdomenial | 2 and 3 treated by PPTT only | ||||
| 3-two attached small tumors |
| No. # | EBL (g) | LR | DM (Time Mo) | OSS (mo) | Status |
|---|---|---|---|---|---|
| GI-1 | >100 | +1 month | +(CS, LN) | 3 | DOD |
| GI-2 | 66.5 | - | +(CS, LN) | 1 | DOC |
| GI-3 | 47.5 | - | +(LN) | 1 | DOC |
| GII-1 | <1 | - | - | 24 | ADF |
| GII-2 | <1 | - | - | 48 | ADF |
| GII-3 | <1 | - | - | 3 | DOC (pneumonia) |
| GII-4 | <1 | - | +(LN, CS) | 1 | DOD |
| GII-5 | <1 | - | - | 6 | DOC (pneumonia) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.R.K.; Farghali, H.A.M.; Wu, Y.; El-Sayed, I.; Osman, A.H.; Selim, S.A.; El-Sayed, M.A. Gold Nanorod-Assisted Photothermal Therapy Decreases Bleeding during Breast Cancer Surgery in Dogs and Cats. Cancers 2019, 11, 851. https://doi.org/10.3390/cancers11060851
Ali MRK, Farghali HAM, Wu Y, El-Sayed I, Osman AH, Selim SA, El-Sayed MA. Gold Nanorod-Assisted Photothermal Therapy Decreases Bleeding during Breast Cancer Surgery in Dogs and Cats. Cancers. 2019; 11(6):851. https://doi.org/10.3390/cancers11060851
Chicago/Turabian StyleAli, Moustafa R. K., Haithem A. M. Farghali, Yue Wu, Ivan El-Sayed, Ahmed H. Osman, Salah A. Selim, and Mostafa A. El-Sayed. 2019. "Gold Nanorod-Assisted Photothermal Therapy Decreases Bleeding during Breast Cancer Surgery in Dogs and Cats" Cancers 11, no. 6: 851. https://doi.org/10.3390/cancers11060851
APA StyleAli, M. R. K., Farghali, H. A. M., Wu, Y., El-Sayed, I., Osman, A. H., Selim, S. A., & El-Sayed, M. A. (2019). Gold Nanorod-Assisted Photothermal Therapy Decreases Bleeding during Breast Cancer Surgery in Dogs and Cats. Cancers, 11(6), 851. https://doi.org/10.3390/cancers11060851





