A Multifunctional Graphene Oxide Platform for Targeting Cancer
Abstract
1. Introduction
2. Results
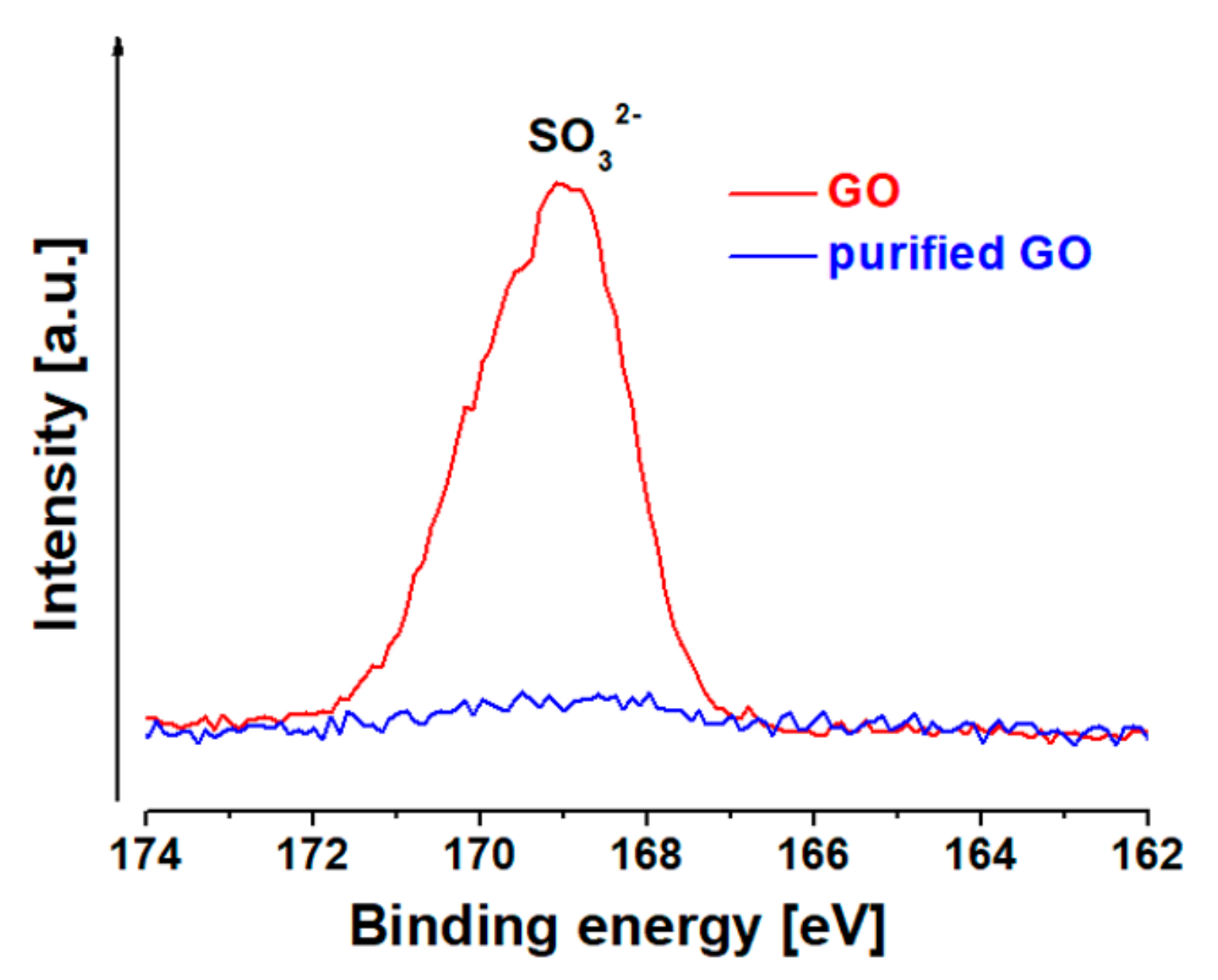
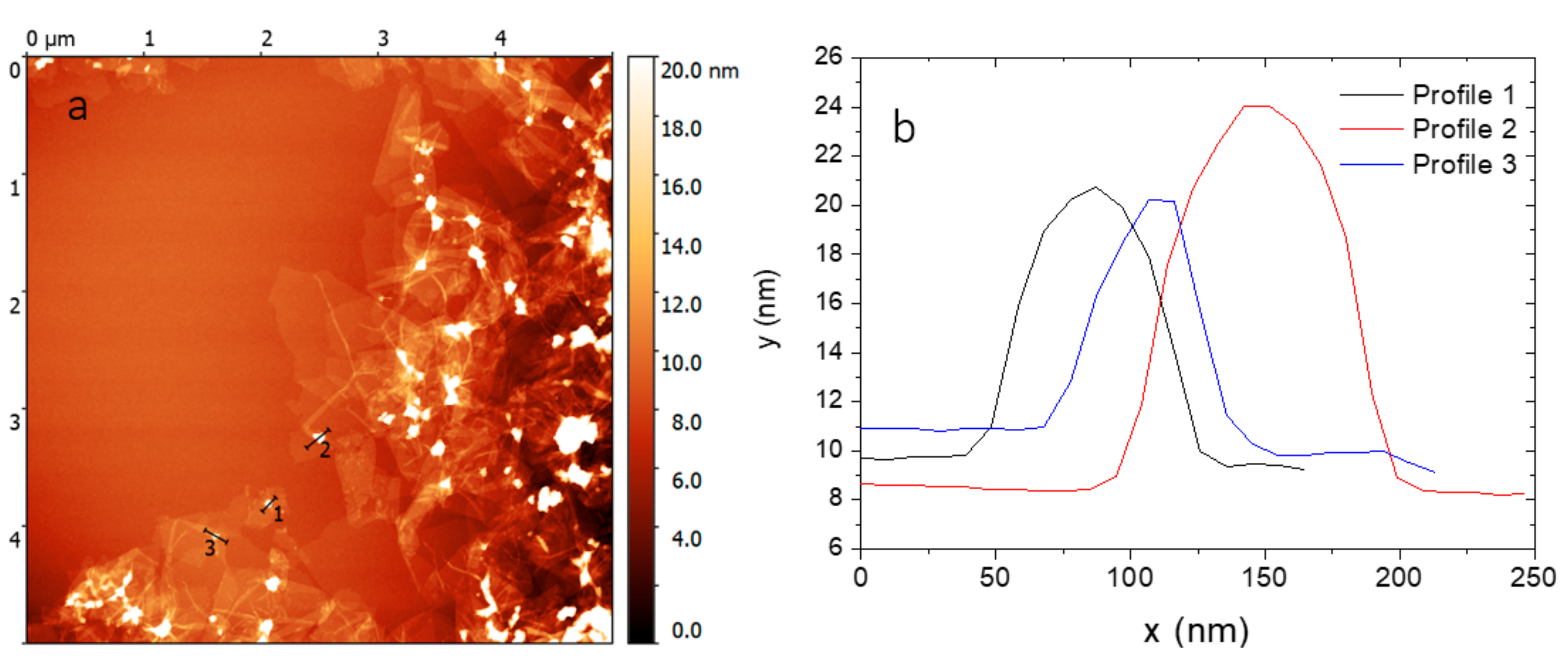
2.1. Preparation and Functionalization of Graphene Oxide
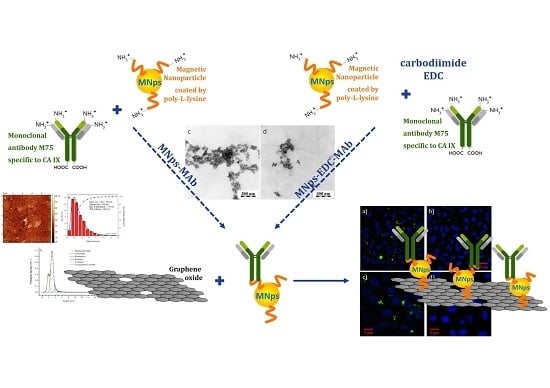
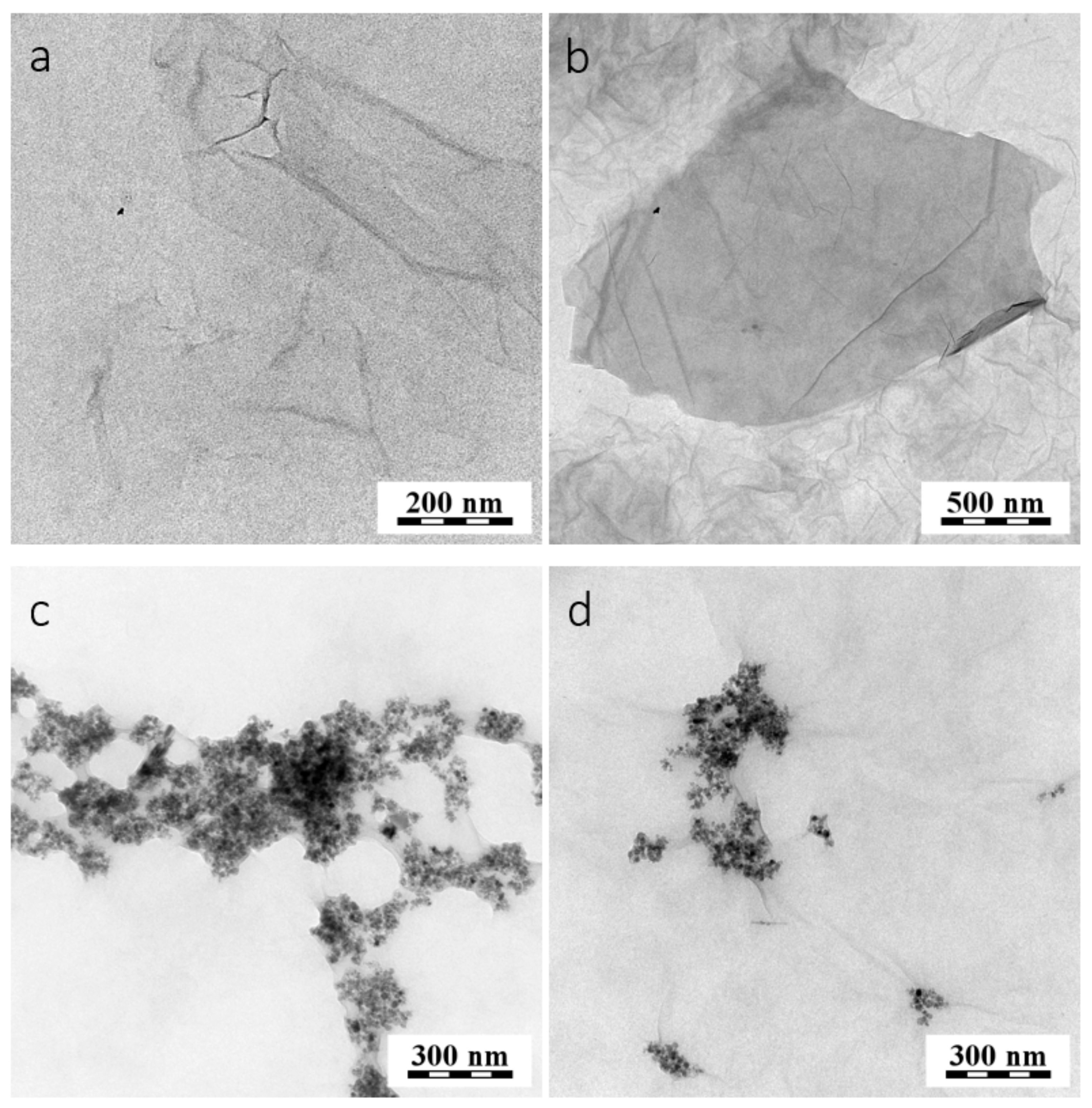
2.2. Synthesis of Modified GO with MNps and MAb
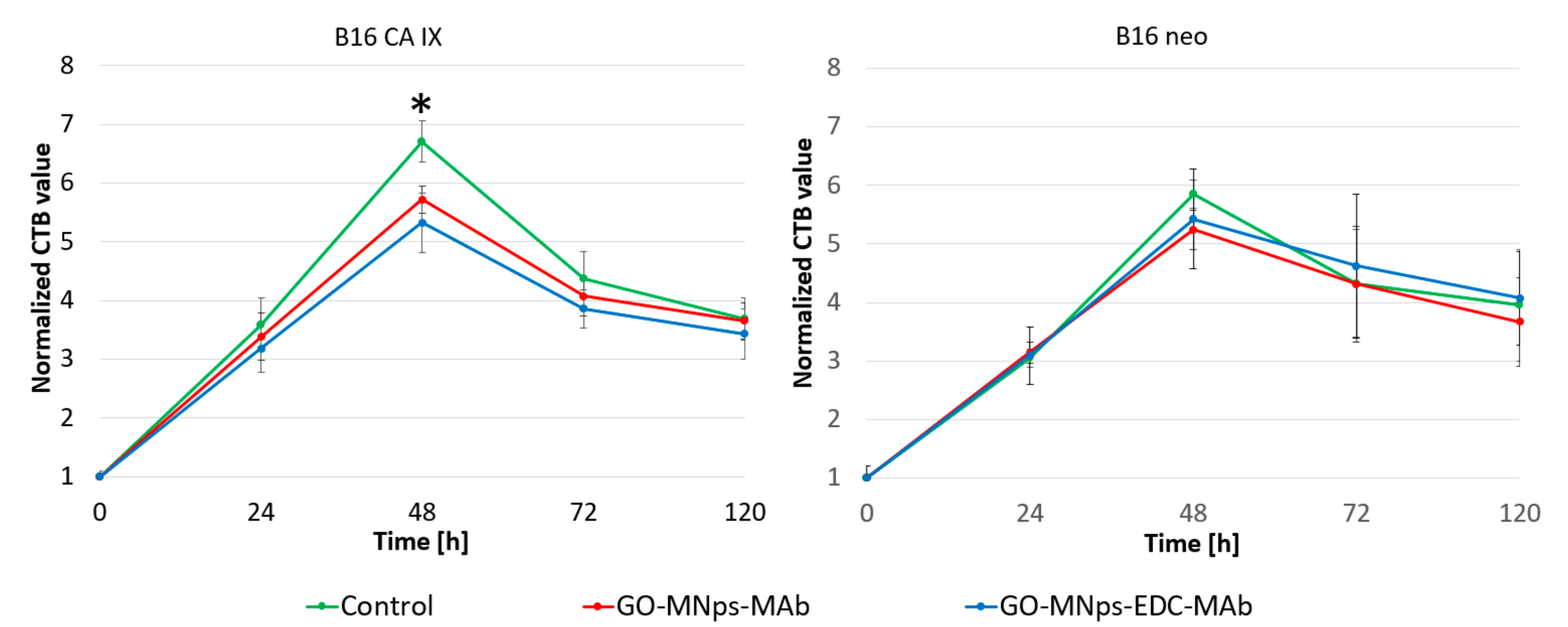
2.3. Cell Viability Assay
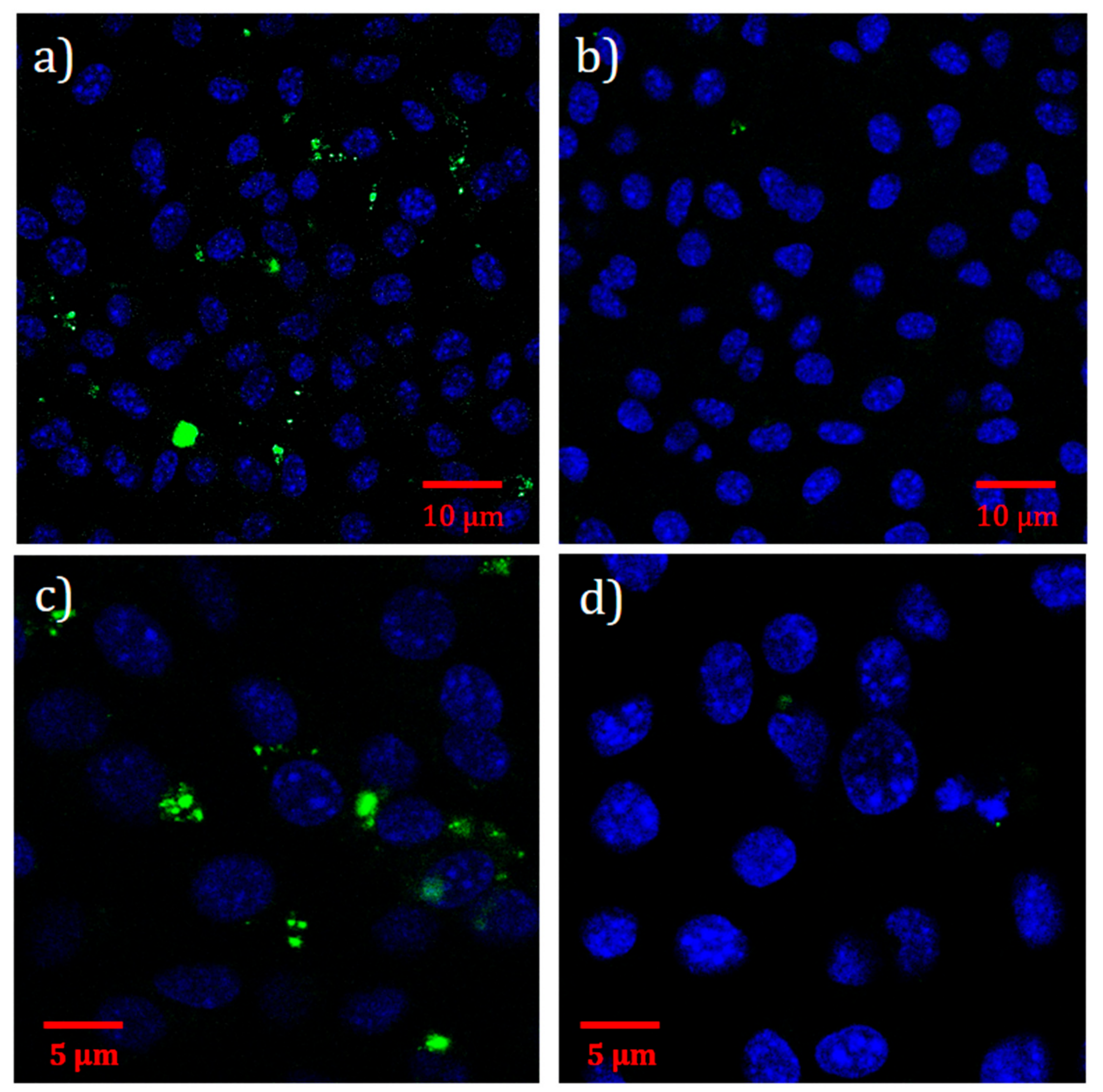
2.4. Immunofluorescence Assay
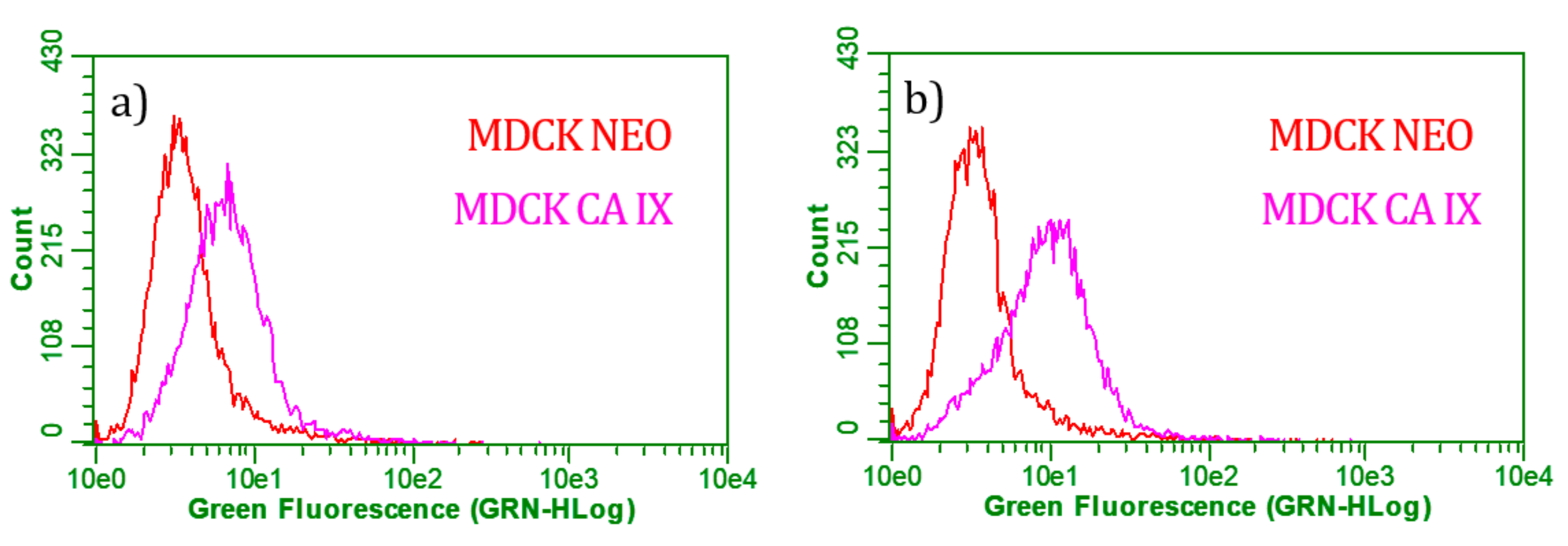
2.5. Flow Cytometry
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Preparation of Graphene Oxide
4.3. Modification of the Graphene Oxide Surface with Magnetic Nanoparticles
4.4. Synthesis of GO-MNps-MAb and GO-MNps-EDC-MAb
4.5. Characterization Techniques
4.6. Cell Viability Assay
4.7. Immunofluorescence Assay
4.8. Flow Cytometry Assay
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pardo, J.; Peng, Z.; Leblanc, R.; Pardo, J.; Peng, Z.; Leblanc, R.M. Cancer Targeting and Drug Delivery Using Carbon-Based Quantum Dots and Nanotubes. Molecules 2018, 23, 378. [Google Scholar] [CrossRef]
- Moreno, C.; Vilas-Varela, M.; Kretz, B.; Garcia-Lekue, A.; Costache, M.V.; Paradinas, M.; Panighel, M.; Ceballos, G.; Valenzuela, S.O.; Peña, D.; et al. Bottom-up synthesis of multifunctional nanoporous graphene. Science 2018, 360, 199–203. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Ghany, N.A.A.; Elsherif, S.A.; Handal, H.T. Revolution of Graphene for different applications: State-of-the-art. Surfaces Interfaces 2017, 9, 93–106. [Google Scholar] [CrossRef]
- Ubani, C.A.; Ibrahim, M.A.; Teridi, M.A.M.; Sopian, K.; Ali, J.; Chaudhary, K.T. Application of graphene in dye and quantum dots sensitized solar cell. Sol. Energy 2016, 137, 531–550. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, X. Chemically Functionalized Graphene and Their Applications in Electrochemical Energy Conversion and Storage. In Advances in Graphene Science; Aliofkhazraei, M., Ed.; InTechOpen: London, UK, 2013; pp. 161–189. ISBN 978-953-51-1182-5. [Google Scholar]
- Koyakutty, M.; Sasidharan, A.; Nair, S. Biomedical Applications of Graphene: Opportunities and Challenges. In Graphene: Synthesis, Properties, and Phenomena; Rao, C.N.R., Sood, A.K., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA.: Weinheim, Germany, 2012; pp. 373–408. ISBN 9783527332588. [Google Scholar]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer (Guildf) 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Gao, W. The chemistry of graphene oxide. Graphene Oxide Reduct. Recipes Spectrosc. Appl. 2015, 39, 61–95. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Kawakami, R.K.; Gmitra, M.; Fabian, J. Graphene spintronics. Nat. Nanotechnol. 2014, 9, 794–807. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Kostiuk, D.; Bodik, M.; Siffalovic, P.; Jergel, M.; Halahovets, Y.; Hodas, M.; Pelletta, M.; Pelach, M.; Hulman, M.; Spitalsky, Z.; et al. Reliable determination of the few-layer graphene oxide thickness using Raman spectroscopy. J. Raman Spectrosc. 2016, 47, 391–394. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A.; Ryon, S.; et al. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Gong, H.; Shi, X.; Wan, J.; Zhang, Y.; Liu, Z. In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials 2013, 34, 2787–2795. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Sood, A.K. Graphene: Synthesis, Properties, and Phenomena; Rao, C.N.R., Sood, A.K., Eds.; First Edit.; Wiley-VCH Verlag GmbH and Co. KGaA.: Weinheim, Germany, 2012; ISBN 9783527332588. [Google Scholar]
- Seabra, A.B.; Paula, A.J.; De Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Bonaccorso, F.; Falko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2014, 7, 4598–4810. [Google Scholar] [CrossRef]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Graphene: A versatile nanoplatform for biomedical applications. Nanoscale 2012, 4, 3833–3842. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, L.; Liu, M.; Zhang, Z. Biomedical applications of graphene. Theranostics 2012, 2, 283–294. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Deshmukh, Y.S.; Abdala, A.A. Recent advances in chemical modifications of graphene. Nano Res. 2015, 8, 1039–1074. [Google Scholar] [CrossRef]
- Chen, Y.; Su, Y.; Hu, S.; Chen, S. Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv. Drug Deliv. Rev. 2016, 105, 190–204. [Google Scholar] [CrossRef]
- Yang, Y.; Asiri, A.M.; Tang, Z.; Du, D.; Lin, Y. Graphene based materials for biomedical applications. Mater. Today 2013, 16, 365–373. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Water-soluble graphene covalently functionalized by biocompatible poly-L-lysine. Langmuir 2009, 25, 12030–12033. [Google Scholar] [CrossRef]
- Shen, J.; Shi, M.; Ma, H.; Yan, B.; Li, N.; Hu, Y.; Ye, M. Synthesis of hydrophilic and organophilic chemically modified graphene oxide sheets. J. Colloid Interface Sci. 2010, 352, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jiang, H.-L.; Wang, X.-L.; Wang, X.; Xu, G.-J.; Zhang, B.-B.; Wang, L.-J.; Zhao, R.-S.; Lin, J.-M. Recent advances in graphene-based magnetic composites for magnetic solid-phase extraction. TrAC Trends Anal. Chem. 2018, 102, 60–74. [Google Scholar] [CrossRef]
- Chen, W.; Yi, P.; Zhang, Y.; Zhang, L.; Deng, Z.; Zhang, Z. Composites of Aminodextran-Coated Fe3O4 Nanoparticles and Graphene Oxide for Cellular Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2011, 3, 4085–4091. [Google Scholar] [CrossRef]
- Yu, D.; Yang, Y.; Durstock, M.; Baek, J.-B.; Dai, L. Soluble P3HT-Grafted Graphene for Efficient Bilayer–Heterojunction Photovoltaic Devices. ACS Nano 2010, 4, 5633–5640. [Google Scholar] [CrossRef]
- Závišová, V.; Koneracká, M.; Múčková, M.; Kopčanský, P.; Tomašovičová, N.; Lancz, G.; Timko, M.; Pätoprstá, B.; Bartoš, P.; Fabián, M. Synthesis and characterization of polymeric nanospheres loaded with the anticancer drug paclitaxel and magnetic particles. J. Magn. Magn. Mater. 2009, 321, 1613–1616. [Google Scholar] [CrossRef]
- Antal, I.; Kubovcikova, M.; Zavisova, V.; Koneracka, M.; Pechanova, O.; Barta, A.; Cebova, M.; Antal, V.; Diko, P.; Zduriencikova, M.; et al. Magnetic poly(d,l-lactide) nanoparticles loaded with aliskiren: A promising tool for hypertension treatment. J. Magn. Magn. Mater. 2015, 380, 280–284. [Google Scholar] [CrossRef]
- Koneracká, M.; Antošová, A.; Závišová, V.; Gažová, Z.; Lancz, G.; Juríková, A.; Tomašovčová, N.; Kováč, J.; Fabián, M.; Kopčanský, P. Preparation and characterization of albumin containing magnetic fluid as potential drug for amyloid diseases treatment. Phys. Procedia 2010, 9, 254–257. [Google Scholar] [CrossRef][Green Version]
- He, F.; Fan, J.; Ma, D.; Zhang, L.; Leung, C.; Chan, H.L. The attachment of Fe3O4 nanoparticles to graphene oxide by covalent bonding. Carbon N. Y. 2010, 48, 3139–3144. [Google Scholar] [CrossRef]
- Antal, I.; Koneracka, M.; Kubovcikova, M.; Zavisova, V.; Khmara, I.; Jelenska, L.; Vidlickova, I.; Pastorekova, S.; Zatovicova, M.; Bugarova, N.; et al. D,L-Lysine functionalized Fe3O4 nanoparticles for detection of cancer cells. Colloids Surfaces B Biointerfaces 2018, 163, 236–245. [Google Scholar] [CrossRef]
- Khmara, I.; Koneracka, M.; Kubovcikova, M.; Zavisova, V.; Antal, I.; Csach, K.; Kopcansky, P.; Vidlickova, I.; Csaderova, L.; Pastorekova, S.; et al. Preparation of poly-L-lysine functionalized magnetic nanoparticles and their influence on viability of cancer cells. J. Magn. Magn. Mater. 2017, 427, 114–121. [Google Scholar] [CrossRef]
- Tabish, T.; Pranjol, M.; Horsell, D.; Rahat, A.; Whatmore, J.; Winyard, P.; Zhang, S.; Tabish, T.A.; Pranjol, M.Z.I.; Horsell, D.W.; et al. Graphene Oxide-Based Targeting of Extracellular Cathepsin D and Cathepsin L As A Novel Anti-Metastatic Enzyme Cancer Therapy. Cancers (Basel) 2019, 11, 319. [Google Scholar] [CrossRef]
- Zatovicova, M.; Jelenska, L.; Hulikova, A.; Ditte, P.; Ditte, Z.; Csaderova, L.; Svastova, E.; Schmalix, W.; Boettger, V.; Bevan, P.; et al. Monoclonal antibody G250 targeting CA Ⅸ: Binding specificity, internalization and therapeutic effects in a non-renal cancer model. Int. J. Oncol. 2014, 45, 2455–2467. [Google Scholar] [CrossRef]
- Zatovicova, M.; Jelenska, L.; Hulikova, A.; Csaderova, L.; Ditte, Z.; Ditte, P.; Goliasova, T.; Pastorek, J.; Pastorekova, S. Carbonic Anhydrase IX as an Anticancer Therapy Target: Preclinical Evaluation of Internalizing Monoclonal Antibody Directed to Catalytic Domain. Curr. Pharm. Des. 2010, 16, 3255–3263. [Google Scholar] [CrossRef]
- Pastoreková, S.; Zaťovičová, M.; Pastorek, J. Cancer-associated carbonic anhydrases and their inhibition. Curr. Pharm. Des. 2008, 14, 685–698. [Google Scholar] [CrossRef]
- Chrastina, A.; Závada, J.; Parkkila, S.; Kaluz, Š.; Kaluzová, M.; Rajčáni, J.; Pastorek, J.; Pastoreková, S. Biodistribution and pharmacokinetics of 125I-labeled monoclonal antibody M75 specific for carbonic anhydrase IX, an intrinsic marker of hypoxia, in nude mice xenografted with human colorectal carcinoma. Int. J. Cancer 2003, 105, 873–881. [Google Scholar] [CrossRef]
- Yu, L.; Li, P.; Ding, X.; Zhang, Q. Graphene oxide and carboxylated graphene oxide: Viable two-dimensional nanolabels for lateral flow immunoassays. Talanta 2017, 165, 167–175. [Google Scholar] [CrossRef]
- Rauf, S.; Mishra, G.K.; Azhar, J.; Mishra, R.K.; Goud, K.Y.; Nawaz, M.A.H.; Marty, J.L.; Hayat, A. Carboxylic group riched graphene oxide based disposable electrochemical immunosensor for cancer biomarker detection. Anal. Biochem. 2018, 545, 13–19. [Google Scholar] [CrossRef]
- Tatiparti, K.; Sau, S.; Gawde, K.A.; Iyer, A.K. Copper-free ‘click’ chemistry-based synthesis and characterization of carbonic anhydrase-IX anchored albumin-paclitaxel nanoparticles for targeting tumor hypoxia. Int. J. Mol. Sci. 2018, 19, 838. [Google Scholar] [CrossRef]
- Shabana, A.M.; Mondal, U.K.; Alam, M.R.; Spoon, T.; Ross, C.A.; Madesh, M.; Supuran, C.T.; Ilies, M.A. PH-Sensitive Multiligand Gold Nanoplatform Targeting Carbonic Anhydrase IX Enhances the Delivery of Doxorubicin to Hypoxic Tumor Spheroids and Overcomes the Hypoxia-Induced Chemoresistance. ACS Appl. Mater. Interfaces 2018, 10, 17792–17808. [Google Scholar] [CrossRef]
- Liu, S.; Luo, X.; Liu, S.; Xu, P.; Wang, J.; Hu, Y. Acetazolamide-Loaded pH-Responsive Nanoparticles Alleviating Tumor Acidosis to Enhance Chemotherapy Effects. Macromol. Biosci. 2019, 19, 1–11. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Zeng, Z.; Xie, C.; Lyu, Y.; Pu, K. Organic Photodynamic Nanoinhibitor for Synergistic Cancer Therapy. Angew. Chemie Int. Ed. 2019. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Fujii, T.; de Groot, F.M.F.; Sawatzky, G.A.; Voogt, F.C.; Hibma, T.; Okada, K. In situ XPS analysis of various iron oxide films grown by NO2-assisted molecular-beam epitaxy. Phys. Rev. B 1999, 59, 3195–3202. [Google Scholar] [CrossRef]
- Bodik, M.; Zahoranova, A.; Micusik, M.; Bugarova, N.; Spitalsky, Z.; Omastova, M.; Majkova, E.; Jergel, M.; Siffalovic, P. Fast low-temperature plasma reduction of monolayer graphene oxide at atmospheric pressure. Nanotechnology 2017, 28, 145601. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Kadu, P.J.; Kushare, S.S.; Thacker, D.D.; Gattani, S.G. Enhancement of oral bioavailability of atorvastatin calcium by self-emulsifying drug delivery systems (SEDDS). Pharm. Dev. Technol. 2011, 16, 65–74. [Google Scholar] [CrossRef]
- Malvern Instruments Ltd. Stabilita Suspenzí a Disperzí—Proč Jsou Parametry jako Velikost Částic, Zeta Potenciál a Reologické Vlastnosti tak Důležité? Chemagazín 2011, 21, 14–16. [Google Scholar]
- Hermanson, G.T. Bioconjugate Technicques, 3rd Edition; Audet, J., Preap, M., Eds.; Third edit.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9780123822390. [Google Scholar]
- Sohová, M.E.; Bodík, M.; Siffalovic, P.; Bugarova, N.; Zaťovičová, M.; Hianik, T.; Omastová, M.; Majkova, E. Label-Free Tracking of Nanosized Graphene Oxide Cellular Uptake by Confocal Raman Microscopy. Analyst 2018, 143, 3686–3692. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef]
- Sasidharan, A.; Panchakarla, L.S.; Chandran, P.; Menon, D.; Nair, S.; Rao, C.N.R.; Koyakutty, M. Differential nano-bio interactions and toxicity effects of pristine versus functionalized graphene. Nanoscale 2011, 3, 2461–2464. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, M.K.; Kulkarni, P.P.; Sonkar, V.K.; Grácio, J.J.A.; Dash, D. Amine-Modified Graphene: Thrombo-Protective Safer Alternative to Graphene Oxide for Biomedical. ACS Nano 2012, 6, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Van Kuijk, S.J.A.; Yaromina, A.; Houben, R.; Niemans, R.; Lambin, P.; Dubois, L.J. Prognostic Significance of Carbonic Anhydrase IX Expression in Cancer Patients: A Meta-Analysis. Front. Oncol. 2016, 6, 69. [Google Scholar] [CrossRef]
- Pastorekova, S.; Gillies, R.J. The role of carbonic anhydrase IX in cancer development: Links to hypoxia, acidosis and beyond. Cancer Metastasis Rev. 2019, (in press). [CrossRef]
- Tokárová, V.; Pittermannová, A.; Král, V.; Řezáčová, P.; Štěpánek, F. Feasibility and constraints of particle targeting using the antigen-antibody interaction. Nanoscale 2013, 5, 11490–11498. [Google Scholar] [CrossRef] [PubMed]
- Kostiv, U.; Patsula, V.; Šlouf, M.; Pongrac, I.M.; Škokić, S.; Radmilović, M.D.; Pavičić, I.; Vrček, I.V.; Gajović, S.; Horák, D. Physico-chemical characteristics, biocompatibility, and MRI applicability of novel monodisperse PEG-modified magnetic Fe3O4 &SiO 2 core–shell nanoparticles. RSC Adv. 2017, 7, 8786–8797. [Google Scholar] [CrossRef]




| Sample | Surface Chemical Composition [at. %] | |||||
|---|---|---|---|---|---|---|
| C1s | O1s | S2p | N1s | Fe2p | K 2s/Cl2p/Al2p/P2p/Si2s/Na1s/Br3d | |
| Graphite G5 | 90.2 | 9.8 | — | — | — | —/—/—/—/—/—/— |
| GO | 61.7 | 33.7 | 1.9 | 0.9 | — | 0.5/0.4/1.0/—/—/—/— |
| MNPs | 29.6 | 41.2 | 0.8 | 6.8 | 19.7 | —/—/——/—/1.0/1.0 |
| MNps-MAb | 32.7 | 39.7 | — | 4.3 | 12.0 | 0.1/3.4/—/2.2/0.3/5.3/— |
| GO-MNps-MAb | 69.2 | 26.6 | 0.6 | 1.0 | 0.2 | —/0.6/1.4/—/0.1/0.3/— |
| GO-MNps-EDC-MAb | 62.5 | 32.8 | 0.8 | 0.7 | 0.2 | —/0.6/2.4/—/—/0.1/— |
| Purified GO | 70.8 | 28.2 | 0.2 | 0.1 | — | —/—/0.9/—/—/—/— |
| pH | 2 | 3 | 4 | 5 | 6,5 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|
| GO | −12 | −15.8 | −39.3 | −35.3 | −41.3 | −46.3 | −44.4 | −42.8 | −44.9 |
| No. | Sample | MDCK Cell Line | |
|---|---|---|---|
| CA IX [%] | Neo [%] | ||
| 1 | GO, neg. control | 1.12 | 1.43 |
| 2 | A488, neg. control | 0.10 | 0.03 |
| 3 | GO-MNps-MAb | 22.16 | 6.07 |
| 4 | GO-MNps-EDC-MAb | 48.63 | 6.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugárová, N.; Špitálsky, Z.; Mičušík, M.; Bodík, M.; Šiffalovič, P.; Koneracká, M.; Závišová, V.; Kubovčíková, M.; Kajanová, I.; Zaťovičová, M.; et al. A Multifunctional Graphene Oxide Platform for Targeting Cancer. Cancers 2019, 11, 753. https://doi.org/10.3390/cancers11060753
Bugárová N, Špitálsky Z, Mičušík M, Bodík M, Šiffalovič P, Koneracká M, Závišová V, Kubovčíková M, Kajanová I, Zaťovičová M, et al. A Multifunctional Graphene Oxide Platform for Targeting Cancer. Cancers. 2019; 11(6):753. https://doi.org/10.3390/cancers11060753
Chicago/Turabian StyleBugárová, Nikola, Zdenko Špitálsky, Matej Mičušík, Michal Bodík, Peter Šiffalovič, Martina Koneracká, Vlasta Závišová, Martina Kubovčíková, Ivana Kajanová, Miriam Zaťovičová, and et al. 2019. "A Multifunctional Graphene Oxide Platform for Targeting Cancer" Cancers 11, no. 6: 753. https://doi.org/10.3390/cancers11060753
APA StyleBugárová, N., Špitálsky, Z., Mičušík, M., Bodík, M., Šiffalovič, P., Koneracká, M., Závišová, V., Kubovčíková, M., Kajanová, I., Zaťovičová, M., Pastoreková, S., Šlouf, M., Majková, E., & Omastová, M. (2019). A Multifunctional Graphene Oxide Platform for Targeting Cancer. Cancers, 11(6), 753. https://doi.org/10.3390/cancers11060753