Chromogranin A in the Laboratory Diagnosis of Pheochromocytoma and Paraganglioma
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. The Group of Patients
4.2. Laboratory Examination
4.3. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Modlin, I.M.; Gustafsson, B.I.; Moss, S.F.; Pavel, M.; Tsolakis, A.V.; Kidd, M. Chromogranin A-biological function and clinical utility in neuro endocrine tumor disease. Ann. Surg. Oncol. 2010, 17, 2427–2443. [Google Scholar] [CrossRef]
- Modlin, I.M.; Oberg, K.; Chung, D.C.; Jensen, R.T.; de Herder, W.W.; Thakker, R.V.; Caplin, M.; Delle Fave, G.; Kaltsas, G.A.; Krenning, E.P.; et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008, 9, 61–72. [Google Scholar] [CrossRef]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing but NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Di Giacinto, P.; Rota, F.; Rizza, L.; Campana, D.; Isidori, A.; Lania, A.; Lenzi, A.; Zuppi, P.; Baldelli, R. Chromogranin A: From Laboratory to Clinical Aspects of Patients with Neuroendocrine Tumors. Int. J. Endocrinol. 2018, 2018, 8126087. [Google Scholar] [CrossRef]
- Marotta, V.; Zatelli, M.C.; Sciammarella, C.; Ambrosio, M.R.; Bondanelli, M.; Colao, A.; Faggiano, A. Chromogranin A as circulating marker for diagnosis and management of neuroendocrine neoplasms: More flaws than fame. Endocr. Relat. Cancer 2018, 25, R11–R29. [Google Scholar] [CrossRef] [PubMed]
- Tischler, A.S. Molecular and cellular biology of pheochromocytomas and extra-adrenal paragangliomas. Endocr. Pathol. 2006, 17, 321–328. [Google Scholar] [CrossRef]
- Lenders, J.W.; Duh, Q.Y.; Eisenhofer, G.; Gimenez-Roqueplo, A.P.; Grebe, S.K.; Murad, M.H.; Naruse, M.; Pacak, K.; Young, W.F., Jr. Endocrine Society. Pheochromocytoma and paraganglioma: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014, 99, 1915–1942. [Google Scholar] [CrossRef]
- Pacak, K.; Tella, S.H. Pheochromocytoma and Paraganglioma. Available online: https://www.ncbi.nlm.nih.gov/books/NBK481899/ (accessed on 5 November 2018).
- Eisenhofer, G.; Lenders, J.W.; Pacak, K. Biochemical diagnosis of pheochromocytoma. Front. Horm. Res. 2004, 31, 76–106. [Google Scholar]
- Asa, S.L.; Ezzat, S.; Mete, O. The Diagnosis and Clinical Significance of Paragangliomas in Unusual Locations. J. Clin. Med. 2018, 7, 280. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sippel, R.S.; O’Dorisio, M.S.; Vinik, A.I.; Lloyd, R.V.; Pacak, K. North American Neuroendocrine Tumor Society (NANETS): The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: Pheochromocytoma; paraganglioma; and medullary thyroid cancer. Pancreas 2010, 39, 775–783. [Google Scholar] [CrossRef]
- Van Berkel, A.; Lenders, J.W.; Timmers, H.J. Diagnosis of endocrine disease: Biochemical diagnosis of phaeochromocytoma and paraganglioma. Eur. J. Endocrinol. 2014, 170, R109–R119. [Google Scholar] [CrossRef]
- Andersen, K.F.; Altaf, R.; Krarup-Hansen, A.; Kromann-Andersen, B.; Horn, T.; Christensen, N.J.; Hendel, H.W. Malignant pheochromocytomas and paragangliomas—The importance of a multidisciplinary approach. Cancer Treat. Rev. 2011, 37, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Lenders, J.W.; Eisenhofer, G.; Mannelli, M.; Pacak, K. Phaeochromocytoma. Lancet 2005, 366, 665–675. [Google Scholar] [CrossRef]
- Crona, J.; Taieb, D.; Pacak, K. New Perspectives on Pheochromocytoma and Paraganglioma: Toward a Molecular Classification. Endocr. Rev. 2017, 38, 489–515. [Google Scholar] [CrossRef] [PubMed]
- D’amico, M.A.; Ghinassi, B.; Izzicupo, P.; Manzoli, L.; Di Baldassarre, A. Biological function and clinical relevance of chromogranin A and derived peptides. Endocr. Connect. 2014, 3, R45–R54. [Google Scholar] [CrossRef] [PubMed]
- D’Herbomez, M.; Do Cao, C.; Vezzosi, D.; Borzon-Chasot, F.; Baudin, E.; Groupe des tumeurs endocrines (GTE France). Chromogranin A assay in clinical practice. Ann. Endocrinol. 2010, 71, 274–280. [Google Scholar] [CrossRef]
- Helman, L.J.; Ahn, T.G.; Levine, M.A.; Allison, A.; Cohen, P.S.; Cooper, M.J.; Cohn, D.V.; Israel, M.A. Molecular cloning and primary structure of human chromogranin A (secretory protein I) cDNA. J. Biol. Chem. 1988, 263, 11559–11563. [Google Scholar] [PubMed]
- Courel, M.; Rodemer, C.; Nguyen, S.T.; Pance, A.; Jackson, A.P.; O’Connor, D.T.; Taupenot, L. Secretory granule biogenesis in sympathoadrenal cells: Identification of a granulogenic determinant in the secretory prohormone chromogranin A. J. Biol. Chem. 2006, 281, 38038–38051. [Google Scholar] [CrossRef] [PubMed]
- Koshimizu, H.; Kim, T.; Cawley, N.X.; Loh, Y.P. Reprint of: Chromogranin A: A new proposal for trafficking; processing and induction of granule biogenesis. Regul. Pept. 2010, 165, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Corti, A.; Marcucci, F.; Bachetti, T. Circulating chromogranin A and its fragments as diagnostic and prognostic disease markers. Pflugers Arch. 2018, 470, 199–210. [Google Scholar] [CrossRef]
- Borges, R.; Díaz-Vera, J.; Domínguez, N.; Arnau, M.R.; Machado, J.D. Chromogranins as regulators of exocytosis. J. Neurochem. 2010, 114, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H. Secretory granules in inositol 1;4;5-trisphosphate-dependent Ca2+ signaling in the cytoplasm of neuroendocrine cells. FASEB J. 2010, 24, 653–664. [Google Scholar] [CrossRef]
- Nelson, N.; Harvey, W.R. Vacuolar and plasma membrane proton-adenosinetriphosphatases. Physiol. Rev. 1999, 79, 361–385. [Google Scholar] [CrossRef]
- Kimura, N.; Takekoshi, K.; Naruse, M. Risk Stratification on Pheochromocytoma and Paraganglioma from Laboratory and Clinical Medicine. J. Clin. Med. 2018, 7, 242. [Google Scholar] [CrossRef]
- Zuber, S.; Wesley, R.; Prodanov, T.; Eisenhofer, G.; Pacak, K.; Kantorovich, V. Clinical utility of chromogranin A in SDHx-related paragangliomas. Eur. J. Clin. Investig. 2014, 44, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.D. Phaeochromocytoma of the adrenal gland scoring scale (PASS) to separate benign from malignant neoplasms. A clinicopathologic and immunophenotypic study of 100 cases. Am. J. Surg. Pathol. 2002, 26, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Szalat, A.; Fraenkel, M.; Doviner, V.; Salmon, A.; Gross, D.J. Malignant pheochromocytoma: Predictive factors of malignancy and clinical course in 16 patients at a single tertiary medical center. Endocrine 2011, 39, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, Y.; Li, Z.; Cheng, C.; Yang, T.; Wang, C.; Liu, L.; Liu, S. Diagnostic value of circulating chromogranin a for neuroendocrine tumors: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0124884. [Google Scholar] [CrossRef]
- Bilek, R.; Safarik, L.; Ciprova, V.; Vlcek, P.; Lisa, L. Chromogranin A; a member of neuroendocrine secretory proteins as a selective marker for laboratory diagnosis of pheochromocytoma. Physiol. Res. 2008, 57, S171–S179. [Google Scholar]
- D’Herbomez, M.; Gouze, V.; Huglo, D.; Nocaudie, M.; Pattou, F.; Proye, C.; Wemeau, J.L.; Marchandise, X. Chromogranin A assay and (131)I-MIBG scintigraphy for diagnosis and follow-up of pheochromocytoma. J. Nucl. Med. 2001, 42, 993–997. [Google Scholar] [PubMed]
- Giovanella, L.; Squin, N.; Ghelfo, A.; Ceriani, L. Chromogranin A immunoradiometric assay in diagnosis of pheochromocytoma: Comparison with plasma metanephrines and 123I-MIBG scan. Q. J. Nucl. Med. Mol. Imaging 2006, 50, 344–347. [Google Scholar] [PubMed]
- Vinik, A.I.; Woltering, E.A.; Warner, R.R.; Caplin, M.; O’Dorisio, T.M.; Wiseman, G.A.; Coppola, D.; Go, V.L.; North American Neuroendocrine Tumor Society (NANETS). North American Neuroendocrine Tumor Society (NANETS). NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas 2010, 39, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Vacante, M.; Fichera, R.; Cappellani, A.; Cristaldi, E.; Motta, M. Chromogranin A (CgA) serum level as a marker of progression in hepatocellular carcinoma (HCC) of elderly patients. Arch. Gerontol. Geriatr. 2010, 51, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.; Gustafsson, B.I.; Kidd, M.; Pavel, M.; Svejda, B.; Modlin, I.M. The clinical relevance of chromogranin A as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrinol. Metab. Clin. N. Am. 2011, 40, 111–134. [Google Scholar] [CrossRef]
- Giovinazzo, F.; Schimmack, S.; Svejda, B.; Alaimo, D.; Pfragner, R.; Modlin, I.; Kidd, M. Chromogranin A and its fragments as regulators of small intestinal neuroendocrine neoplasm proliferation. PLoS ONE 2013, 8, e81111. [Google Scholar] [CrossRef]
- Korse, C.M.; Muller, M.; Taal, B.G. Discontinuation of proton pump inhibitors during assessment of chromogranin A levels in patients with neuroendocrine tumours. Br. J. Cancer 2011, 105, 1173–1175. [Google Scholar] [CrossRef][Green Version]
- Mosli, H.H.; Dennis, A.; Kocha, W.; Asher, L.J.; Van Uum, S.H. Effect of short-term proton pump inhibitor treatment and its discontinuation on chromogranin A in healthy subjects. J. Clin. Endocrinol. Metab. 2012, 97, E1731–E1735. [Google Scholar] [CrossRef]
- O’Connor, D.T.; Pandlan, M.R.; Carlton, E.; Cervenka, J.H.; Hslao, R.J. Rapid radioimmunoassay of circulating chromogranin A: In vitro stability; exploration of the neuroendocrine character of neoplasia; and assessment of the effects of organ failure. Clin. Chem. 1989, 35, 1631–1637. [Google Scholar]
- Mikkelsen, G.; Asberg, A.; Hultström, M.E.; Aasarod, K.; Hov, G.G. Reference limits for chromogranin A; CYFRA 21–1; CA 125; CA 19–9 and carcinoembryonic antigen in patients with chronic kidney disease. Int. J. Biol. Markers 2017, 32, e461–e466. [Google Scholar] [CrossRef]
- Peracchi, M.; Gebbia, C.; Basilisco, G.; Quatrini, M.; Tarantino, C.; Vescarelli, C.; Massironi, S.; Conte, D. Plasma chromogranin A in patients with autoimmune chronic atrophic gastritis; enterochromaffin-like cell lesions and gastric carcinoids. Eur. J. Endocrinol. 2005, 152, 443–448. [Google Scholar] [CrossRef][Green Version]
- Massironi, S.; Fraquelli, M.; Paggi, S.; Sangiovanni, A.; Conte, D.; Sciola, V.; Ciafardini, C.; Colombo, M.; Peracchi, M. Chromogranin A levels in chronic liver disease and hepatocellular carcinoma. Dig. Liver. Dis. 2009, 41, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, R.; McAlindon, M.E.; Leeds, J.S.; Skilling, J.; Sanders, D.S. The role of serum chromogranin A in diarrhoea predominant irritable bowel syndrome. J. Gastrointestin. Liver. Dis. 2009, 18, 23–26. [Google Scholar]
- Estensen, M.E.; Hognestad, A.; Syversen, U.; Squire, I.; Ng, L.; Kjekshus, J.; Dickstein, K.; Omland, T. Prognostic value of plasma chromogranin A levels in patients with complicated myocardial infarction. Am. Heart J. 2006, 152, e1–e6. [Google Scholar] [CrossRef]
- Jansson, A.M.; Rosjo, H.; Omland, T.; Karlsson, T.; Hartford, M.; Flyvbjerg, A.; Caidahl, K. Prognostic value of circulating chromogranin A levels in acute coronary syndromes. Eur. Heart J. 2009, 30, 25–32. [Google Scholar] [CrossRef]
- Zelinka, T.; Petrak, O.; Turkova, H.; Holaj, R.; Strauch, B.; Krsek, M.; Vrankova, A.B.; Musil, Z.; Dusková, J.; Kubinyi, J.; et al. High incidence of cardiovascular complications in pheochromocytoma. Horm. Metab. Res. 2012, 44, 379–384. [Google Scholar] [CrossRef]
- Bilek, R.; Zelinka, T.; Vlcek, P.; Duskova, J.; Michalsky, D.; Novak, K.; Vaclavikova, E.; Widimsky, J., Jr. Radioimmunoassay of chromogranin A and free metanephrines in diagnosis of pheochromocytoma. Physiol. Res. 2017, 66, S397–S408. [Google Scholar]
- Eisenhofer, G.; Lenders, J.W.; Goldstein, D.S.; Mannelli, M.; Csako, G.; Walther, M.M.; Brouwers, F.M.; Pacak, K. Pheochromocytoma catecholamine phenotypes and prediction of tumor size and location by use of plasma free metanephrines. Clin. Chem. 2005, 51, 735–744. [Google Scholar] [CrossRef]
- Van Duinen, N.; Kema, I.P.; Romijn, J.A.; Corssmit, E.P. Plasma chromogranin A levels are increased in a small portion of patients with hereditary head and neck paragangliomas. Clin. Endocrinol. 2011, 74, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Stenman, A.; Zedenius, J.; Juhlin, C.C. The Value of Histological Algorithms to Predict the Malignancy Potential of Pheochromocytomas and Abdominal Paragangliomas. A Meta-Analysis and Systematic Review of the Literature. Cancers 2019, 11, 225. [Google Scholar] [CrossRef]
- Plouin, P.F.; Amar, L.; Dekkers, O.M.; Fassnacht, M.; Gimenez-Roqueplo, A.P.; Lenders, J.W.; Lussey-Lepoutre, C.; Steichen, O.; Guideline Working Group. European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma. Eur. J. Endocrinol. 2016, 174, G1–G10. [Google Scholar] [CrossRef]
- Yoo, S.H.; Albanesi, J.P. Ca2(+)-induced conformational change and aggregation of chromogranin A. J. Biol. Chem. 1990, 265, 14414–14421. [Google Scholar] [PubMed]
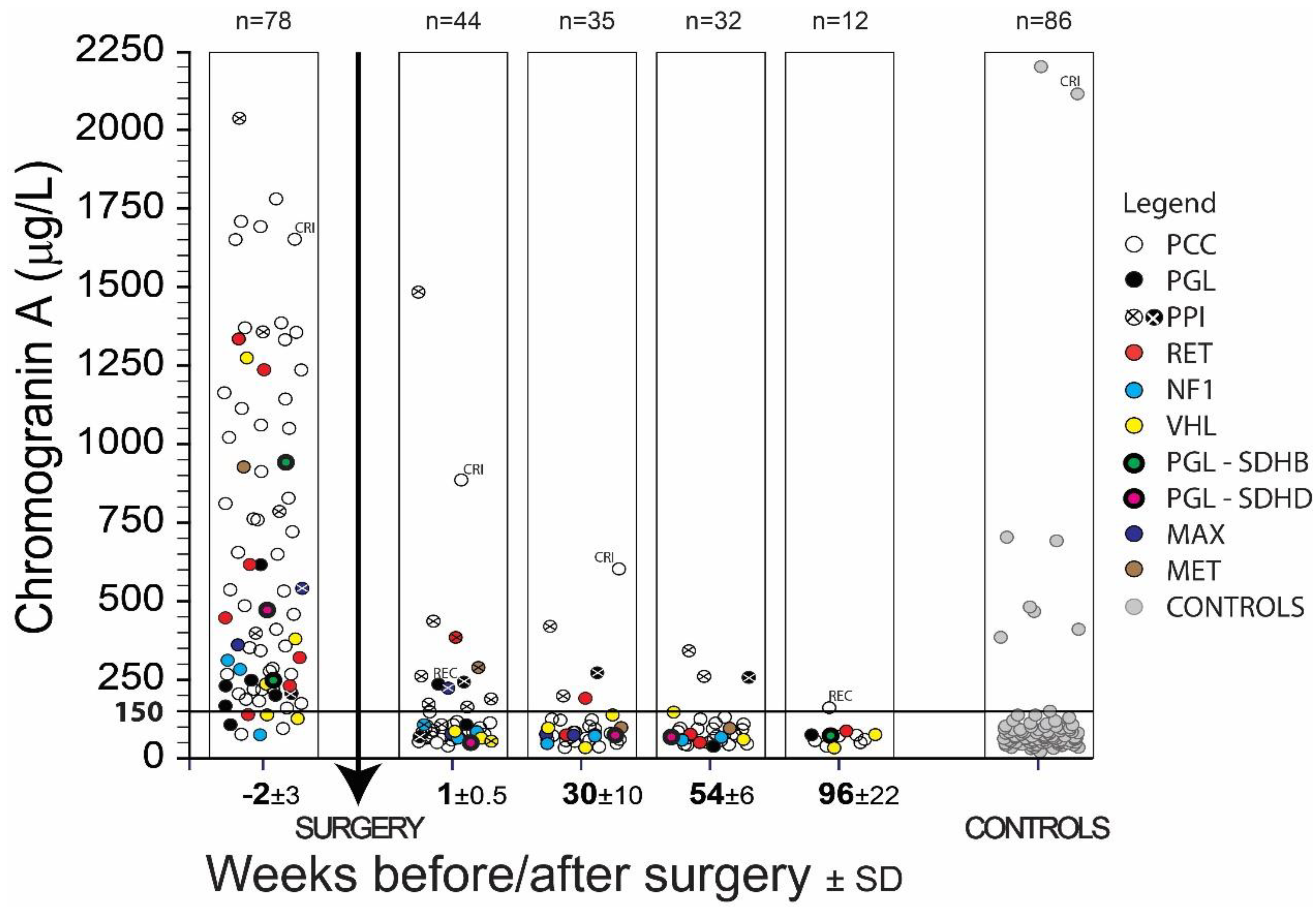
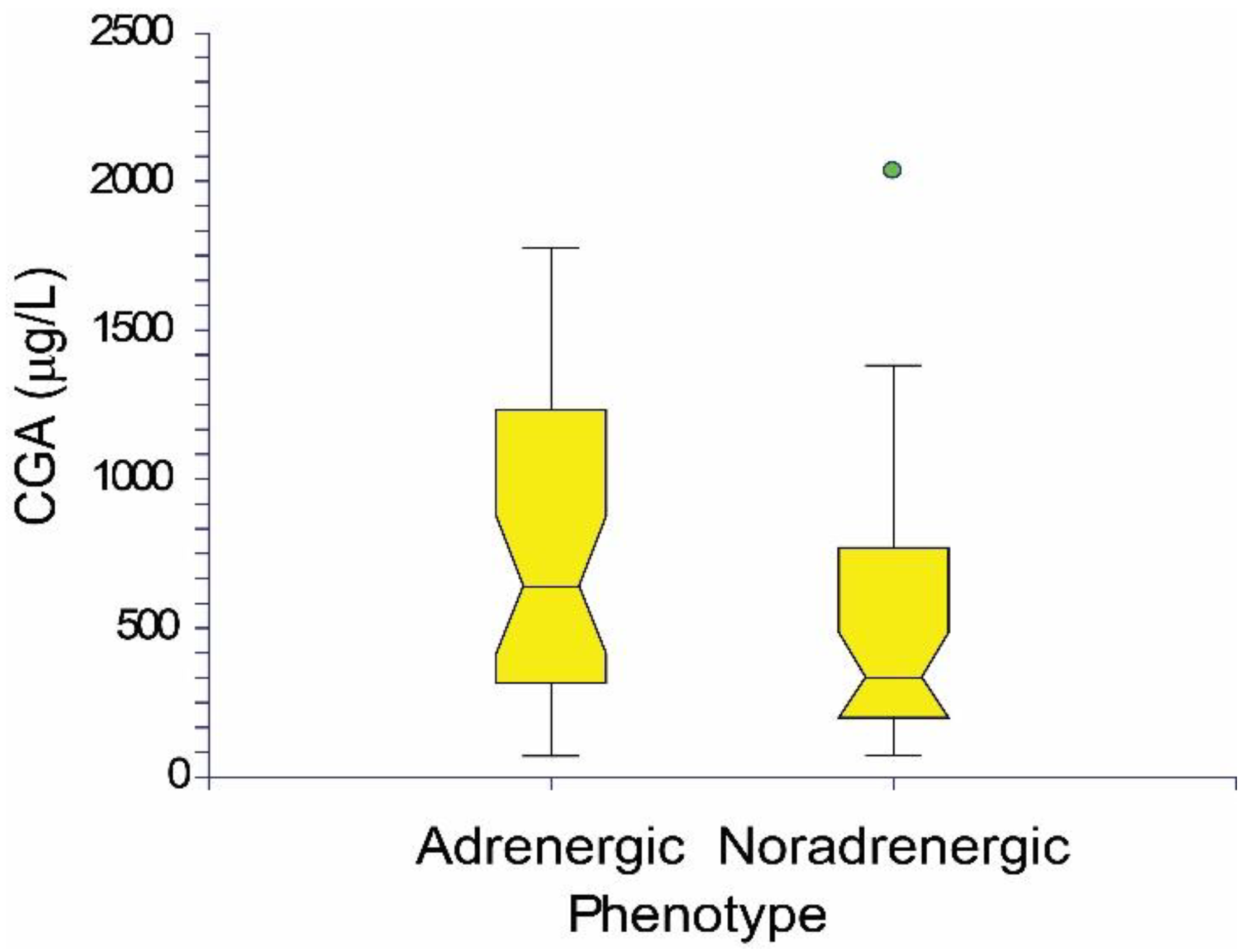
| PPGL—Patients with Pheochromocytoma (PCC) or Paraganglioma (PGL) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease | Genetics | Gender | n | 2 ± 3 Weeks before Surgery | 1 ± 0.5 Week after Surgery | 6 Months (30 ± 10 Weeks) after Surgery | 1 Year (54 ± 6 Weeks) after Surgery | 2 Years (96 ± 22 Weeks) after Surgery | ||||||||||||||||
| AGE (Years) | CGA (µg/L) | Clinical Sensitivity | n | CGA (µg/L) | Clinical Specificity | n | CGA (µg/L) | Clinical Specificity | n | CGA (µg/L) | Clinical Specificity | n | CGA (µg/L) | Clinical Specificity | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | mean | SD | Mean | SD | |||||||||||||
| PPGL | total | total | 71 | 49 | 17 | 618.9 | 479.7 | 90 | 27 | 76 | 24.4 | 100 | 31 | 75.9 | 33.8 | 97 | 29 | 73.1 | 28.6 | 100 | 11 | 59.2 | 16.7 | 100 |
| males | 38 | 47 | 16 | 638.7 | 512 | 92 | 15 | 69.1 | 21.5 | 100 | 17 | 71.6 | 28.6 | 100 | 16 | 73.1 | 31 | 100 | 6 | 64.1 | 16.6 | 100 | ||
| females | 33 | 50 | 18 | 596.1 | 446.5 | 88 | 12 | 84.6 | 26 | 100 | 14 | 81.2 | 39.7 | 93 | 13 | 73.2 | 26.6 | 100 | 5 | 53.4 | 16.7 | 100 | ||
| PCC | total | total | 62 | 48 | 17 | 657.1 | 492.7 | 90 | 25 | 76 | 24 | 100 | 30 | 76.2 | 34.3 | 97 | 27 | 74.9 | 28.6 | 100 | 9 | 57.0 | 17.9 | 100 |
| males | 35 | 48 | 16 | 667.4 | 522.8 | 91 | 14 | 70.7 | 21.3 | 100 | 16 | 71.8 | 29.5 | 100 | 15 | 73.7 | 32 | 100 | 4 | 61.5 | 20.8 | 100 | ||
| females | 27 | 48 | 18 | 643.7 | 460.4 | 89 | 11 | 82.8 | 26.5 | 100 | 14 | 81.2 | 39.7 | 93 | 12 | 76.4 | 25 | 100 | 5 | 53.4 | 16.7 | 100 | ||
| not specified | total | 45 | 52 | 16 | 718.9 | 506 | 96 | 20 | 77.1 | 26.4 | 100 | 20 | 71.2 | 27.3 | 100 | 20 | 74.8 | 27.5 | 100 | 6 | 55.0 | 13.6 | 100 | |
| males | 25 | 52 | 15 | 758.4 | 543.4 | 100 | 10 | 70.6 | 24.6 | 100 | 11 | 70.7 | 24.8 | 100 | 10 | 70 | 30 | 100 | 3 | 54.3 | 18.2 | 100 | ||
| females | 20 | 52 | 16 | 669.6 | 464.2 | 90 | 10 | 83.7 | 27.8 | 100 | 9 | 71.8 | 31.5 | 100 | 10 | 79.6 | 25.5 | 100 | 3 | 55.7 | 11.4 | 100 | ||
| RET | total | 7 | 34 | 9 | 614.8 | 482.6 | 86 | - | - | - | - | 2 | 129 | 82.8 | 50 | 2 | 60.5 | 20 | 100 | 1 | 83.3 | - | 100 | |
| males | 3 | 42 | 6 | 693.7 | 603 | 67 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 83.3 | - | 100 | ||
| females | 4 | 28 | 5 | 555.6 | 460.9 | 100 | - | - | - | - | 2 | 129 | 82.8 | 50 | 2 | 60.5 | 20 | 100 | - | - | - | - | ||
| VHL | total | 5 | 32 | 21 | 427.9 | 482.5 | 60 | 2 | 70.9 | 14.9 | 100 | 3 | 85 | 52.2 | 100 | 2 | 99.1 | 62.6 | 100 | 2 | 49.9 | 28.5 | 100 | |
| males | 3 | 22 | 2 | 244.2 | 127.4 | 67 | 2 | 70.9 | 14.9 | 100 | 2 | 82.3 | 73.5 | 100 | 2 | 99.1 | 62.6 | 100 | - | - | - | - | ||
| females | 2 | 46 | 34 | 703.6 | 803.5 | 50 | - | - | - | 100 | 1 | 90.3 | - | 100 | - | - | - | - | 2 | 49.9 | 28.5 | 100 | ||
| NF1 | total | 3 | 54 | 10 | 220.2 | 128.8 | 67 | 2 | 71.4 | 15.4 | 100 | 2 | 56.2 | 16.9 | 100 | 2 | 57.8 | 7.5 | 100 | - | - | - | - | |
| males | 3 | 54 | 10 | 220.2 | 128.8 | 67 | 2 | 71.4 | 15.4 | 100 | 2 | 56.2 | 16.9 | 100 | 2 | 57.8 | 7.5 | 100 | - | - | - | - | ||
| females | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| MAX | total | 1 | 60 | - | 357.9 | - | 100 | 1 | 74.2 | - | 100 | 2 | 71.5 | 2.1 | 100 | - | - | - | - | - | - | - | - | |
| males | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| females | 1 | 60 | - | 357.9 | - | 100 | 1 | 74.2 | - | 100 | 2 | 71.5 | 2.1 | 100 | - | - | - | - | - | - | - | - | ||
| MET | total | 1 | 47 | - | 925 | - | 100 | - | - | - | - | 1 | 94.4 | - | 100 | 1 | 92 | - | 100 | - | - | - | - | |
| males | 1 | 47 | - | 925 | - | 100 | - | - | - | - | 1 | 94.4 | - | 100 | 1 | 92 | - | 100 | - | - | - | - | ||
| females | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| PGL | total | total | 9 | 52 | 20 | 355.9 | 270.8 | 89 | 2 | 74.9 | 40.4 | 100 | 1 | 67.3 | - | 100 | 2 | 49.3 | 20.7 | 100 | 2 | 69.1 | 2.8 | 100 |
| males | 3 | 36 | 12 | 303.7 | 145.1 | 100 | 1 | 46.4 | - | 100 | 1 | 67.3 | - | 100 | 1 | 64 | - | 100 | 2 | 69.1 | 2.8 | 100 | ||
| females | 6 | 61 | 18 | 382 | 326.3 | 83 | 1 | 103.5 | - | 100 | - | - | - | - | 1 | 34.6 | - | 100 | - | - | - | - | ||
| not specified | total | 6 | 56 | 22 | 258.2 | 181.1 | 83 | 1 | 103.5 | - | 100 | - | - | - | - | 1 | 34.6 | - | 100 | 1 | 71.1 | - | 100 | |
| males | 1 | 32 | - | 197.1 | - | 100 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 71.1 | - | 100 | ||
| females | 5 | 61 | 20 | 270.5 | 199.7 | 80 | 1 | 103.5 | - | 100 | - | - | - | - | 1 | 34.6 | - | 100 | - | - | - | - | ||
| SDHB | total | 2 | 55 | 7 | 592.2 | 190.9 | 100 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 67.1 | - | 100 | |
| males | 1 | 50 | - | 245.1 | - | 100 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 67.1 | - | 100 | ||
| females | 1 | 60 | - | 939.4 | - | 100 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| SDHD | total | 1 | 26 | - | 469 | - | 100 | 1 | 46.4 | - | 100 | 1 | 67.3 | - | 100 | 1 | 64 | - | 100 | - | - | - | - | |
| males | 1 | 26 | - | 469 | - | 100 | 1 | 46.4 | - | 100 | 1 | 67.3 | - | 100 | 1 | 64 | - | 100 | - | - | - | - | ||
| females | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Controls | |||||||
|---|---|---|---|---|---|---|---|
| Syndrome | Gender | n | AGE (years) | CGA µg/L) | Clinical Specificity | ||
| Mean | SD | Mean | SD | ||||
| total | total | 85 | 53 | 19 | 125.3 | 259.3 | 92 |
| males | 29 | 53 | 21 | 102.6 | 128.9 | 93 | |
| females | 56 | 54 | 18 | 134 | 306.3 | 91 | |
| adrenocortical adenoma | total | 44 | 63 | 11 | 178.5 | 352.7 | 84 |
| males | 15 | 65 | 6 | 135.2 | 173.3 | 87 | |
| females | 29 | 62 | 13 | 200.9 | 417.7 | 83 | |
| adrenocortical adenoma + MTC, FTC | total | 1 | 71 | - | 31.8 | - | 100 |
| males | - | - | - | - | - | - | |
| females | 1 | 71 | - | 31.8 | - | 100 | |
| adrenocortical adenoma + PTC | total | 1 | 75 | - | 108.4 | - | 100 |
| males | - | - | - | - | - | - | |
| females | 1 | 75 | - | 108.4 | - | 100 | |
| hypertension | total | 8 | 45 | 16 | 43.2 | 11 | 100 |
| males | 4 | 41 | 22 | 50.2 | 9.1 | 100 | |
| females | 4 | 48 | 8 | 36.3 | 8.3 | 100 | |
| MEN 2A | total | 2 | 10 | 4 | 70.6 | 15.2 | 100 |
| males | 1 | 13 | - | 81.3 | - | 100 | |
| females | 1 | 7 | - | 59.8 | - | 100 | |
| MEN 2A + MTC | total | 7 | 26 | 14 | 69.5 | 35.5 | 100 |
| males | 3 | 20 | 12 | 71.8 | 24.9 | 100 | |
| females | 4 | 30 | 16 | 67.7 | 45.7 | 100 | |
| MEN 2B + MTC | total | 3 | 30 | 16 | 90.7 | 53.9 | 100 |
| males | 1 | 17 | - | 146.2 | - | 100 | |
| females | 2 | 37 | 16 | 62.9 | 34.4 | 100 | |
| MTC | total | 9 | 56 | 13 | 76.9 | 36.2 | 100 |
| males | 2 | 51 | 17 | 51.3 | 10.9 | 100 | |
| females | 7 | 57 | 13 | 84.2 | 38.1 | 100 | |
| MTC + PTC | total | 1 | 70 | - | 42.3 | - | 100 |
| males | 1 | 70 | - | 42.3 | - | 100 | |
| females | - | - | - | - | - | - | |
| PTC | total | 4 | 58 | 22 | 81.1 | 40.4 | 100 |
| males | 1 | 80 | - | 102.6 | - | 100 | |
| females | 3 | 50 | 20 | 73.9 | 46.2 | 100 | |
| thyroid disorders | total | 5 | 36 | 18 | 70.2 | 12.2 | 100 |
| males | 1 | 51 | - | 54.6 | - | 100 | |
| females | 4 | 32 | 18 | 74.1 | 9.9 | 100 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bílek, R.; Vlček, P.; Šafařík, L.; Michalský, D.; Novák, K.; Dušková, J.; Václavíková, E.; Widimský, J., Jr.; Zelinka, T. Chromogranin A in the Laboratory Diagnosis of Pheochromocytoma and Paraganglioma. Cancers 2019, 11, 586. https://doi.org/10.3390/cancers11040586
Bílek R, Vlček P, Šafařík L, Michalský D, Novák K, Dušková J, Václavíková E, Widimský J Jr., Zelinka T. Chromogranin A in the Laboratory Diagnosis of Pheochromocytoma and Paraganglioma. Cancers. 2019; 11(4):586. https://doi.org/10.3390/cancers11040586
Chicago/Turabian StyleBílek, Radovan, Petr Vlček, Libor Šafařík, David Michalský, Květoslav Novák, Jaroslava Dušková, Eliška Václavíková, Jiří Widimský, Jr., and Tomáš Zelinka. 2019. "Chromogranin A in the Laboratory Diagnosis of Pheochromocytoma and Paraganglioma" Cancers 11, no. 4: 586. https://doi.org/10.3390/cancers11040586
APA StyleBílek, R., Vlček, P., Šafařík, L., Michalský, D., Novák, K., Dušková, J., Václavíková, E., Widimský, J., Jr., & Zelinka, T. (2019). Chromogranin A in the Laboratory Diagnosis of Pheochromocytoma and Paraganglioma. Cancers, 11(4), 586. https://doi.org/10.3390/cancers11040586





