FGF21 Levels in Pheochromocytoma/Functional Paraganglioma
Abstract
1. Introduction
2. Results
2.1. Basic Characteristics of Groups
2.2. Effect of Tumor Removal in PPGL
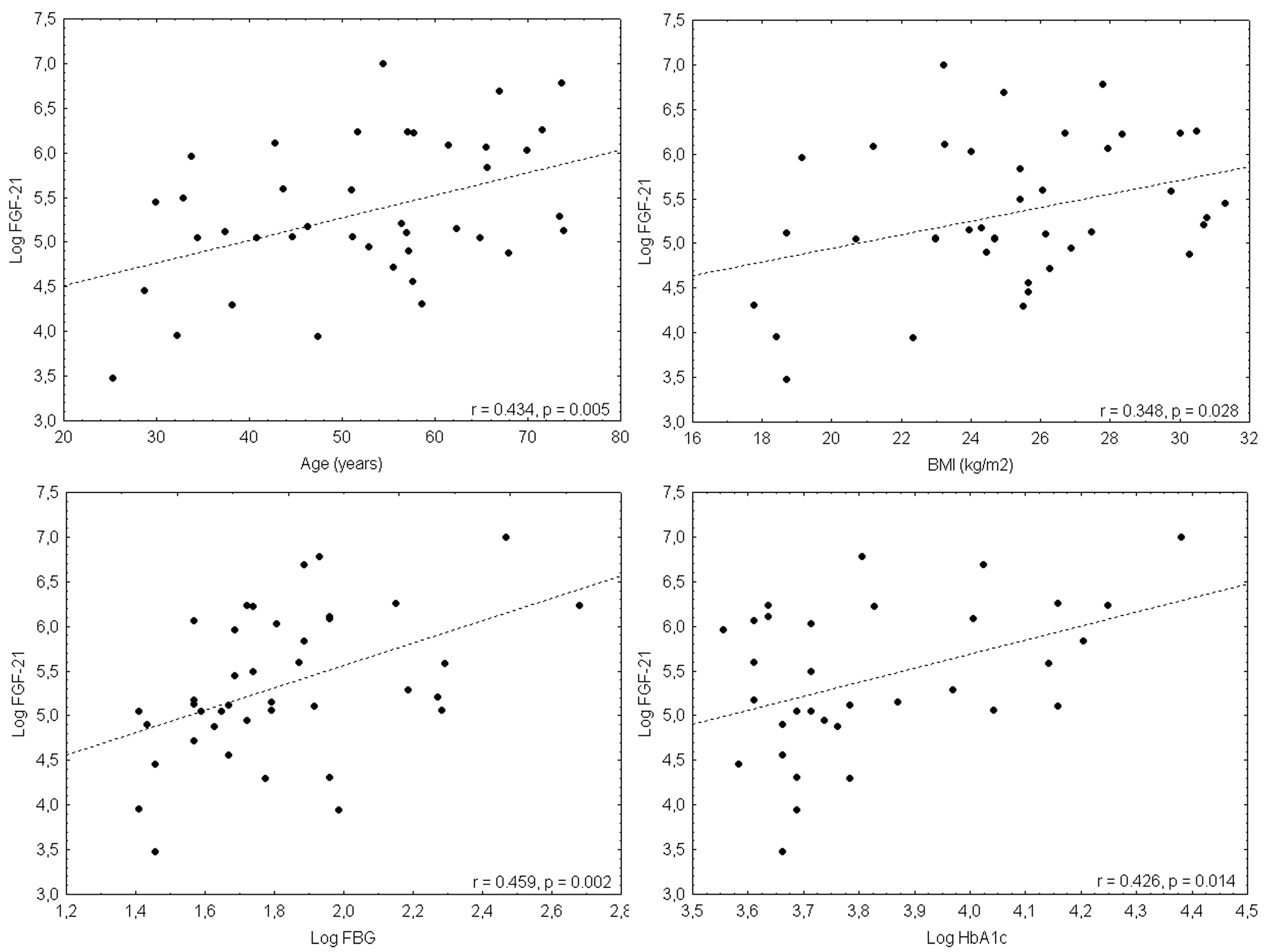
2.3. FGF21 Levels and Correlation
3. Discussion
4. Patients and Methods
4.1. Recruitment and Background
4.2. Anthropometric, Biochemical Measurements, and Indirect Calorimetry
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Staiger, H.; Keuper, M.; Berti, L.; Hrabe de Angelis, M.; Haring, H.U. Fibroblast Growth Factor 21-Metabolic Role in Mice and Men. Endocr. Rev. 2017, 38, 468–488. [Google Scholar] [CrossRef]
- BonDurant, L.D.; Potthoff, M.J. Fibroblast Growth Factor 21: A Versatile Regulator of Metabolic Homeostasis. Annu. Rev. Nutr. 2018, 38, 173–196. [Google Scholar] [CrossRef] [PubMed]
- Domouzoglou, E.M.; Maratos-Flier, E. Fibroblast growth factor 21 is a metabolic regulator that plays a role in the adaptation to ketosis. Am. J. Clin. Nutr. 2011, 93, 901S–905S. [Google Scholar] [CrossRef]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef]
- Hondares, E.; Iglesias, R.; Giralt, A.; Gonzalez, F.J.; Giralt, M.; Mampel, T.; Villarroya, F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J. Biol. Chem. 2011, 286, 12983–12990. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, M.; Ning, G.; Gu, W.; Su, T.; Xu, M.; Li, B.; Wang, W. Brown adipose tissue in humans is activated by elevated plasma catecholamines levels and is inversely related to central obesity. PLoS ONE 2011, 6, e21006. [Google Scholar] [CrossRef]
- Hadi, M.; Chen, C.C.; Whatley, M.; Pacak, K.; Carrasquillo, J.A. Brown fat imaging with (18)F-6-fluorodopamine PET/CT, (18)F-FDG PET/CT, and (123)I-MIBG SPECT: A study of patients being evaluated for pheochromocytoma. J. Nucl. Med. 2007, 48, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Hondares, E.; Gallego-Escuredo, J.M.; Flachs, P.; Frontini, A.; Cereijo, R.; Goday, A.; Perugini, J.; Kopecky, P.; Giralt, M.; Cinti, S.; et al. Fibroblast growth factor-21 is expressed in neonatal and pheochromocytoma-induced adult human brown adipose tissue. Metabolism 2014, 63, 312–317. [Google Scholar] [CrossRef] [PubMed]
- BonDurant, L.D.; Ameka, M.; Naber, M.C.; Markan, K.R.; Idiga, S.O.; Acevedo, M.R.; Walsh, S.A.; Ornitz, D.M.; Potthoff, M.J. FGF21 Regulates Metabolism Through Adipose-Dependent and -Independent Mechanisms. Cell Metab. 2017, 25, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Douris, N.; Stevanovic, D.M.; Fisher, F.M.; Cisu, T.I.; Chee, M.J.; Nguyen, N.L.; Zarebidaki, E.; Adams, A.C.; Kharitonenkov, A.; Flier, J.S.; et al. Central Fibroblast Growth Factor 21 Browns White Fat via Sympathetic Action in Male Mice. Endocrinology 2015, 156, 2470–2481. [Google Scholar] [CrossRef] [PubMed]
- Hsuchou, H.; Pan, W.; Kastin, A.J. The fasting polypeptide FGF21 can enter brain from blood. Peptides 2007, 28, 2382–2386. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Hallschmid, M.; Adya, R.; Kern, W.; Lehnert, H.; Randeva, H.S. Fibroblast growth factor 21 (FGF21) in human cerebrospinal fluid: Relationship with plasma FGF21 and body adiposity. Diabetes 2011, 60, 2758–2762. [Google Scholar] [CrossRef] [PubMed]
- Sarruf, D.A.; Thaler, J.P.; Morton, G.J.; German, J.; Fischer, J.D.; Ogimoto, K.; Schwartz, M.W. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 2010, 59, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Bookout, A.L.; de Groot, M.H.; Owen, B.M.; Lee, S.; Gautron, L.; Lawrence, H.L.; Ding, X.; Elmquist, J.K.; Takahashi, J.S.; Mangelsdorf, D.J.; et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 2013, 19, 1147–1152. [Google Scholar] [CrossRef]
- Liang, Q.; Zhong, L.; Zhang, J.; Wang, Y.; Bornstein, S.R.; Triggle, C.R.; Ding, H.; Lam, K.S.; Xu, A. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes 2014, 63, 4064–4075. [Google Scholar] [CrossRef] [PubMed]
- Martucci, V.L.; Pacak, K. Pheochromocytoma and paraganglioma: Diagnosis, genetics, management, and treatment. Curr. Probl. Cancer 2014, 38, 7–41. [Google Scholar] [CrossRef]
- Xu, J.; Lloyd, D.J.; Hale, C.; Stanislaus, S.; Chen, M.; Sivits, G.; Vonderfecht, S.; Hecht, R.; Li, Y.S.; Lindberg, R.A.; et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009, 58, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Straczkowski, M.; Karczewska-Kupczewska, M.; Adamska, A.; Otziomek, E.; Kowalska, I.; Nikolajuk, A. Serum fibroblast growth factor 21 in human obesity: Regulation by insulin infusion and relationship with glucose and lipid oxidation. Int. J. Obes. (Lond) 2013, 37, 1386–1390. [Google Scholar] [CrossRef][Green Version]
- Vienberg, S.G.; Brons, C.; Nilsson, E.; Astrup, A.; Vaag, A.; Andersen, B. Impact of short-term high-fat feeding and insulin-stimulated FGF21 levels in subjects with low birth weight and controls. Eur. J. Endocrinol. 2012, 167, 49–57. [Google Scholar] [CrossRef]
- Ďurovcová, V.; Marek, J.; Hána, V.; Matoulek, M.; Zikán, V.; Haluzíková, D.; Kaválková, P.; Lacinová, Z.; Kršek, M.; Haluzík, M. Plasma concentrations of fibroblast growth factors 21 and 19 in patients with Cushing’s syndrome. Physiol. Res. 2010, 59, 415–422. [Google Scholar] [PubMed]
- Petrák, O.; Haluzíkova, D.; Kaválková, P.; Štrauch, B.; Rosa, J.; Holaj, R.; Brabcová Vranková, A.; Michalský, D.; Haluzík, M.; Zelinka, T.; et al. Changes in energy metabolism in pheochromocytoma. J. Clin. Endocrinol. Metab. 2013, 98, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Zelinka, T.; Petrák, O.; Štrauch, B.; Holaj, R.; Kvasnička, J.; Mazoch, J.; Pacák, K.; Widimský, J., Jr. Elevated inflammation markers in pheochromocytoma compared to other forms of hypertension. Neuroimmunomodulation 2007, 14, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Bosanská, L.; Petrák, O.; Zelinka, T.; Mráz, M.; Widimský, J., Jr.; Haluzík, M. The effect of pheochromocytoma treatment on subclinical inflammation and endocrine function of adipose tissue. Physiol. Res. 2009, 58, 319–325. [Google Scholar] [CrossRef]
- Tsujimoto, G.; Manger, W.M.; Hoffman, B.B. Desensitization of beta-adrenergic receptors by pheochromocytoma. Endocrinology 1984, 114, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Snavely, M.D.; Motulsky, H.J.; O’Connor, D.T.; Ziegler, M.G.; Insel, P.A. Adrenergic receptors in human and experimental pheochromocytoma. Clin. Exp. Hypertens. A 1982, 4, 829–848. [Google Scholar] [CrossRef]
- Mráz, M.; Bartlová, M.; Lacinová, Z.; Michalský, D.; Kasalický, M.; Haluziková, D.; Matoulek, M.; Dostalová, I.; Humenanská, V.; Haluzík, M. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin. Endocrinol. 2009, 71, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yeung, D.C.; Karpisek, M.; Stejskal, D.; Zhou, Z.G.; Liu, F.; Wong, R.L.; Chow, W.S.; Tso, A.W.; Lam, K.S.; et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008, 57, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Sun, K.; Egan, J.M.; Crasto, C.; Carlson, O.D.; Ferrucci, L. Relationship of serum fibroblast growth factor 21 with abnormal glucose metabolism and insulin resistance: The Baltimore Longitudinal Study of Aging. J. Clin. Endocrinol. Metab. 2012, 97, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Gong, Q.; Wu, C.; Yu, J.; Lu, T.; Pan, X.; Lin, S.; Li, X. Dynamic change of serum FGF21 levels in response to glucose challenge in human. J. Clin. Endocrinol. Metab. 2012, 97, E1224–E1228. [Google Scholar] [CrossRef] [PubMed]
- Vienberg, S.G.; Jacobsen, S.H.; Worm, D.; Hvolris, L.E.; Naver, L.; Almdal, T.; Hansen, D.L.; Wulff, B.S.; Clausen, T.R.; Madsbad, S.; et al. Increased glucose-stimulated FGF21 response to oral glucose in obese nondiabetic subjects after Roux-en-Y gastric bypass. Clin. Endocrinol. 2017, 86, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Dushay, J.R.; Toschi, E.; Mitten, E.K.; Fisher, F.M.; Herman, M.A.; Maratos-Flier, E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol. Metab. 2015, 4, 51–57. [Google Scholar] [CrossRef] [PubMed]
- von Holstein-Rathlou, S.; BonDurant, L.D.; Peltekian, L.; Naber, M.C.; Yin, T.C.; Claflin, K.E.; Urizar, A.I.; Madsen, A.N.; Ratner, C.; Holst, B.; et al. FGF21 Mediates Endocrine Control of Simple Sugar Intake and Sweet Taste Preference by the Liver. Cell Metab. 2016, 23, 335–343. [Google Scholar] [CrossRef]
- Samms, R.J.; Lewis, J.E.; Norton, L.; Stephens, F.B.; Gaffney, C.J.; Butterfield, T.; Smith, D.P.; Cheng, C.C.; Perfield, J.W., 2nd; Adams, A.C.; et al. FGF21 Is an Insulin-Dependent Postprandial Hormone in Adult Humans. J. Clin. Endocrinol. Metab. 2017, 102, 3806–3813. [Google Scholar] [CrossRef]
- Colwell, J.A. Inhibition of insulin secretion by catecholamines in pheochromocytoma. Ann. Intern. Med. 1969, 71, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Isles, C.G.; Johnson, J.K. Phaeochromocytoma and diabetes mellitus: Further evidence that alpha 2 receptors inhibit insulin release in man. Clin. Endocrinol. 1983, 18, 37–41. [Google Scholar] [CrossRef]
- Cryer, P.E. Adrenaline: A physiological metabolic regulatory hormone in humans? Int. J. Obes Relat Metab Disord 1993, 17 (Suppl. 3), S43–S46; discussion S68. [Google Scholar] [PubMed]
- Komada, H.; Hirota, Y.; So, A.; Nakamura, T.; Okuno, Y.; Fukuoka, H.; Iguchi, G.; Takahashi, Y.; Sakaguchi, K.; Ogawa, W. Insulin Secretion and Insulin Sensitivity Before and After Surgical Treatment of Pheochromocytoma or Paraganglioma. J. Clin. Endocrinol. Metab. 2017, 102, 3400–3405. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Chui, P.C.; Antonellis, P.J.; Bina, H.A.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 2010, 59, 2781–2789. [Google Scholar] [CrossRef]
- Hale, C.; Chen, M.M.; Stanislaus, S.; Chinookoswong, N.; Hager, T.; Wang, M.; Veniant, M.M.; Xu, J. Lack of overt FGF21 resistance in two mouse models of obesity and insulin resistance. Endocrinology 2012, 153, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Markan, K.R. Defining “FGF21 Resistance” during obesity: Controversy, criteria and unresolved questions. F1000Res 2018, 7, 289. [Google Scholar] [CrossRef] [PubMed]
- Markan, K.R.; Naber, M.C.; Ameka, M.K.; Anderegg, M.D.; Mangelsdorf, D.J.; Kliewer, S.A.; Mohammadi, M.; Potthoff, M.J. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 2014, 63, 4057–4063. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Lenders, J.W.; Goldstein, D.S.; Mannelli, M.; Csako, G.; Walther, M.M.; Brouwers, F.M.; Pacak, K. Pheochromocytoma catecholamine phenotypes and prediction of tumor size and location by use of plasma free metanephrines. Clin. Chem. 2005, 51, 735–744. [Google Scholar] [CrossRef] [PubMed]

| Factors | Controls n = 26 | Obese n = 21 | PPGL n = 40 | p |
|---|---|---|---|---|
| Female (n, %) | 21 (81) | 18 (86) | 21 (53) | 0.008 |
| Age (years) | 48.5 ± 10 | 54.6 ± 14 | 52.4 ± 14 | 0.262 |
| Body mass index (kg/m²) | 23.9 ± 3 | 44.9 ± 9 *,† | 25.1 ± 4 | <0.001 |
| Body fat percentage (%) | 27.5 ± 11 | 51.4 ± 11 *,† | 29.5 ± 8 | <0.001 |
| Resting energy expenditure (Kcal/day) | 1467 ± 165 | 1943 ± 398 +,* | 1691 ± 327 | <0.001 |
| REE/BEE (%) | 98.7 ± 8 | 101.9 ± 18 ‡ | 110.3 ± 12 • | 0.007 |
| Systolic blood pressure (mmHg) | 114.7 ± 15 | 132.9 ± 17 ‡ | 131.7 ± 18 • | 0.002 |
| Diastolic blood pressure (mmHg) | 71.3 ± 15 | 78.8 ± 10 | 75.9 ± 11 | 0.106 |
| Mean arterial pressure (mmHg) | 85.7 ± 12 | 96.8 ± 11 ‡ | 94.8 ± 13 • | 0.013 |
| Pulse pressure (mmHg) | 50.1 ± 9 | 54.0 ± 10 | 55.7 ± 12 | 0.439 |
| Fasting blood glucose (mmol/L) | 4.2 (0.9) | 6.0 (3) * | 5.8 (2.1) ° | <0.001 |
| HbA1c (mmol/mol) | 34.0 (6) | 44.0 (25) * | 42.0 (16) • | <0.001 |
| Insulin (mIU/L) | 6.1 (4) | 16.3 (9) ‡ | 3.7 (3) •,† | <0.001 |
| HOMA-IR | 1.2 (1) | 4.9 (7) †,* | 0.95 (1) | <0.001 |
| Total cholesterol (mmol/L) | 5.1 ± 0.8 | 4.9 ± 1 | 4.6 ± 1 | 0.180 |
| HDL cholesterol (mmol/L) | 1.6 ± 0.4 | 1.1 ± 0.3 +,* | 1.5 ± 0,5 | <0.001 |
| LDL cholesterol (mmol/L) | 3 ± 0.7 | 2.9 ± 0.9 | 2.6 ± 1 | 0.144 |
| Triglycerides (mmol/L) | 1.0 (0.4) | 1.7 (0.4) *,† | 0.9 (0.7) | <0.001 |
| Fibroblast growth factor 21 (pg/mL) | 107.9 (116) | 160.4 (180) * | 174.2 (283) ° | <0.001 |
| Hypertension (n, %) | - | 18 (86) | 26 (65) | 0.185 |
| Diabetes Mellitus (n, %) | - | 11 (52) | 13 (33) | 0.757 |
| Obesity (n, %) | - | 21 (100) | 6 (15) | - |
| Dyslipidemia (n, %) | - | 18 (86) | 23 (58) | 0.718 |
| Metabolic syndrome (n, %) | - | 19 (90) | 20 (50) | 0.118 |
| Use of PAD (n, %) | - | 10 (48) | 10 (26) | 0.025 |
| Use of insulin (n, %) | - | 4 (19) | 3 (8) | - |
| Use of statins (n, %) | - | 10 (48) | 16 (40) | 0.863 |
| Number of antihypertensives (n) | - | 2.38 ± 1.5 | 1.85 ± 1.1 | 0.038 |
| Use of alpha blockers (n, %) | - | 4 (19) | 28 (70) | - |
| Use of beta blockers (n, %) | - | 12 (57) | 14 (35) | 0.017 |
| Factors | Before n = 27 | After n = 27 | p |
|---|---|---|---|
| Female (n, %) | 15 (56) | 15 (56) | - |
| Age (years) | 51.9 ± 13 | 53.0 ± 13 | <0.001 |
| Body mass index (kg/m²) | 24.7 ± 3 | 26.2 ± 4 | <0.001 |
| Body fat percentage (%) | 29.3 ± 9 | 32.3 ± 9 | 0.034 |
| BEE (Kcal/day) | 1509 ± 252 | 1543 ± 266 | 0.001 |
| REE (Kcal/day) | 1655 ± 311 | 1477 ± 216 | <0.001 |
| REE/BEE (%) | 110.8 ± 12 | 96.5 ± 7 | <0.001 |
| Systolic BP (mmHg) | 131.8 ± 19 | 124.9 ± 17 | 0.084 |
| Diastolic BP (mmHg) | 75.7 ± 11 | 74.5 ± 11 | 0.598 |
| MAP (mmHg) | 94.4 ± 13 | 91.3 ± 12 | 0.253 |
| Pulse pressure (mmHg) | 56.11 ± 13 | 55.6 ± 19 | 0.888 |
| FBG (mmol/L) | 5.7 (1.7) | 4.8 (0.8) | <0.001 |
| HbA1c (mmol/mol) | 42.0 (17) | 40.0 (6) | 0.018 |
| Insulin (mIU/L) | 3.3 ± 3 | 2.8 ± 3 | <0.001 |
| HOMA-IR | 0.9 ± 0.9 | 0.6 ± 0.7 | <0.001 |
| Total cholesterol (mmol/L) | 4.8 ± 1 | 4.6 ± 1.1 | 0.436 |
| HDLc (mmol/L) | 1.5 ± 0.5 | 1.4 ± 0.8 | 0.237 |
| LDLc (mmol/L) | 2.7 ± 1 | 2.7 ± 0.8 | 0.771 |
| Triglycerides (mmol/L) | 0.8 (0.6) | 1.2 (0.7) | 0.017 |
| FGF21 (pg/mL) | 176.4 (284) | 131.3 (225) | <0.001 |
| P-Metanephrine (nmol/L) | 3.1 (9) | 0.17 (0.2) | <0.001 |
| P-Normetanephrine (nmol/L) | 11.6 (14) | 0.33 (0.4) | <0.001 |
| Chromogranine (ng/mL) | 334.8 (489) | 39.6 (38) | <0.001 |
| Hypertension (%) | 19 (70) | 6 (22) | 0.071 |
| Diabetes mellitus (%) | 9 (33) | 2 (7) | 0.037 |
| Obesity (n, %) | 2 (7) | 2 (7) | 1.000 |
| Dyslipidemia (n, %) | 17 (63) | 18 (67) | 0.775 |
| MS (n, %) | 14 (52) | 6 (22) | 0.241 |
| Use of PAD (n, %) | 5 (19) | 2 (7) | 0.203 |
| Use of insulin (n, %) | 3 (11) | 0 (0) | 0.074 |
| Use of statins (n, %) | 11 (41) | 13 (48) | 0.583 |
| Use of AHT (n) | 1.93 ± 1 | 0.37 ± 0.7 | <0.001 |
| Factors | PPGL (n = 40) | |
|---|---|---|
| r | p | |
| Age | 0.435 | 0.005 |
| Weight | 0.267 | 0.095 |
| Body mass index | 0.348 | 0.028 |
| P-Metanephrine | 0.212 | 0.194 |
| P-Normetanephrine | 0.086 | 0.602 |
| Respiratory Quotient | −0.121 | 0.474 |
| BEE (Kcal/day) | 0.169 | 0.316 |
| REE (Kcal/day) | 0.163 | 0.336 |
| REE/BEE (%) | 0.018 | 0.915 |
| Systolic blood pressure | 0.194 | 0.231 |
| Diastolic blood pressure | −0.047 | 0.755 |
| Mean arterial pressure | 0.058 | 0.721 |
| Pulse pressure | 0.338 | 0.032 |
| Fasting blood glucose | 0.459 | 0.002 |
| HbA1c | 0.426 | 0.014 |
| Insulin | 0.097 | 0.551 |
| HOMA-IR | 0.248 | 0.121 |
| Total cholesterol | −0.045 | 0.785 |
| HDL cholesterol | 0.009 | 0.593 |
| LDL cholesterol | −0.211 | 0.196 |
| Triglycerides | 0.255 | 0.113 |
| Category | Occurrence | n | FGF21 | p |
|---|---|---|---|---|
| Dyslipidemia | Yes | 23 | 264.9 (343) | <0.001 |
| No | 17 | 133.7 (102) | ||
| Diabetes mellitus | Yes | 13 | 438.2 (337) | 0.001 |
| No | 27 | 158.0 (170) | ||
| Central Obesity | Yes | 6 | 214.2 (326) | 0.486 |
| No | 34 | 167.1 (280) | ||
| Metabolic syndrome | Yes | 20 | 377.9 (333) | <0.001 |
| No | 20 | 147.8 (94) | ||
| Hypertension | Yes | 26 | 214.2 (348) | 0.085 |
| No | 14 | 160.4 (131) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klímová, J.; Zelinka, T.; Rosa, J.; Štrauch, B.; Haluzíková, D.; Haluzík, M.; Holaj, R.; Krátká, Z.; Kvasnička, J.; Ďurovcová, V.; et al. FGF21 Levels in Pheochromocytoma/Functional Paraganglioma. Cancers 2019, 11, 485. https://doi.org/10.3390/cancers11040485
Klímová J, Zelinka T, Rosa J, Štrauch B, Haluzíková D, Haluzík M, Holaj R, Krátká Z, Kvasnička J, Ďurovcová V, et al. FGF21 Levels in Pheochromocytoma/Functional Paraganglioma. Cancers. 2019; 11(4):485. https://doi.org/10.3390/cancers11040485
Chicago/Turabian StyleKlímová, Judita, Tomáš Zelinka, Ján Rosa, Branislav Štrauch, Denisa Haluzíková, Martin Haluzík, Robert Holaj, Zuzana Krátká, Jan Kvasnička, Viktorie Ďurovcová, and et al. 2019. "FGF21 Levels in Pheochromocytoma/Functional Paraganglioma" Cancers 11, no. 4: 485. https://doi.org/10.3390/cancers11040485
APA StyleKlímová, J., Zelinka, T., Rosa, J., Štrauch, B., Haluzíková, D., Haluzík, M., Holaj, R., Krátká, Z., Kvasnička, J., Ďurovcová, V., Matoulek, M., Novák, K., Michalský, D., Widimský Jr., J., & Petrák, O. (2019). FGF21 Levels in Pheochromocytoma/Functional Paraganglioma. Cancers, 11(4), 485. https://doi.org/10.3390/cancers11040485





