Casticin-Induced Inhibition of Cell Growth and Survival Are Mediated through the Dual Modulation of Akt/mTOR Signaling Cascade
Abstract
1. Introduction
2. Results
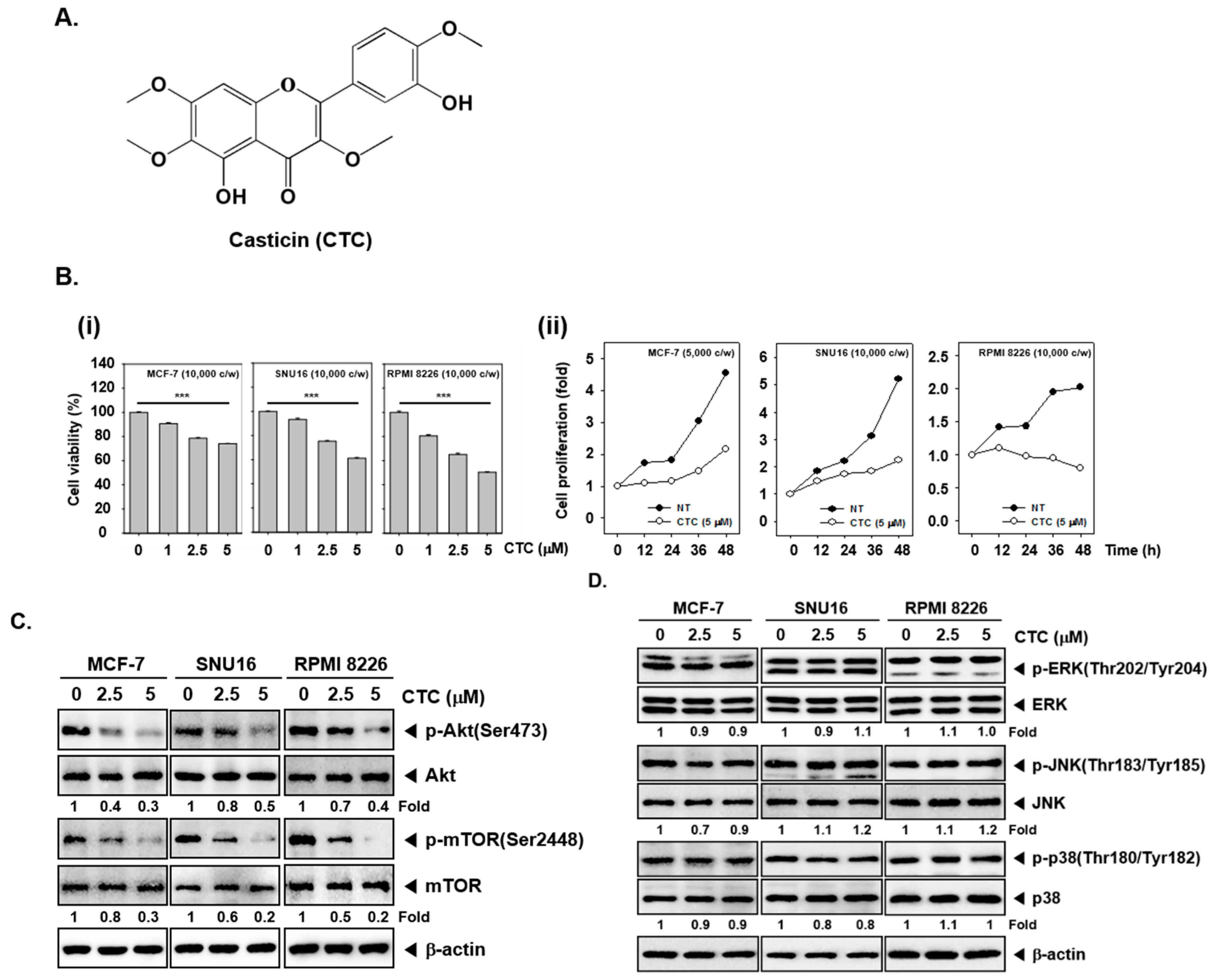
2.1. CTC Inhibits Cellular Growth in Several Human Cancer Cells
2.2. CTC Suppresses Activation of Akt/mTOR Signaling Pathway
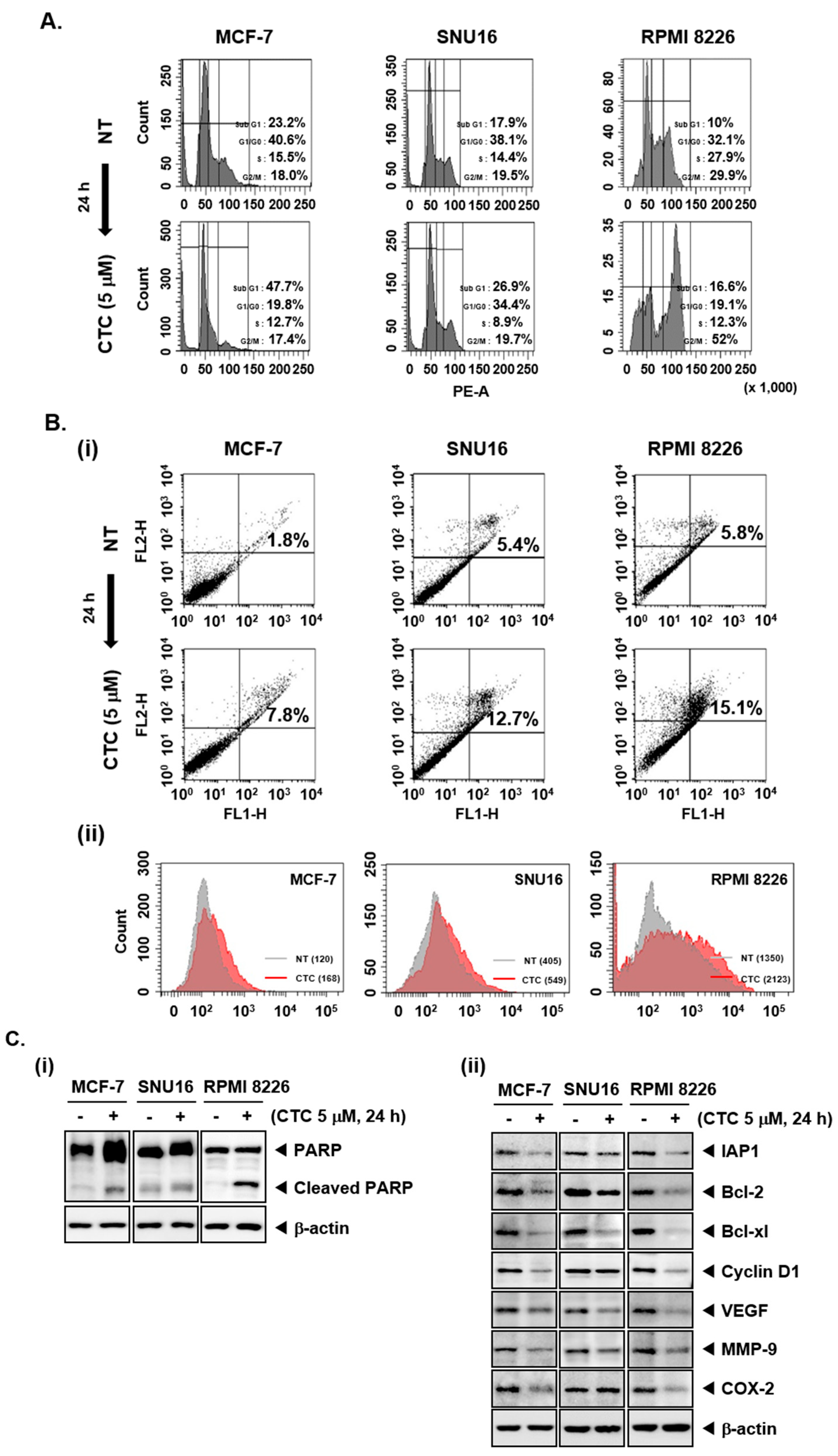
2.3. CTC Increases Accumulation of Cells in Sub-G1 Phase and Induces Apoptosis
2.4. CTC Induces Apoptosis via the Suppression of Various Oncogenic Proteins
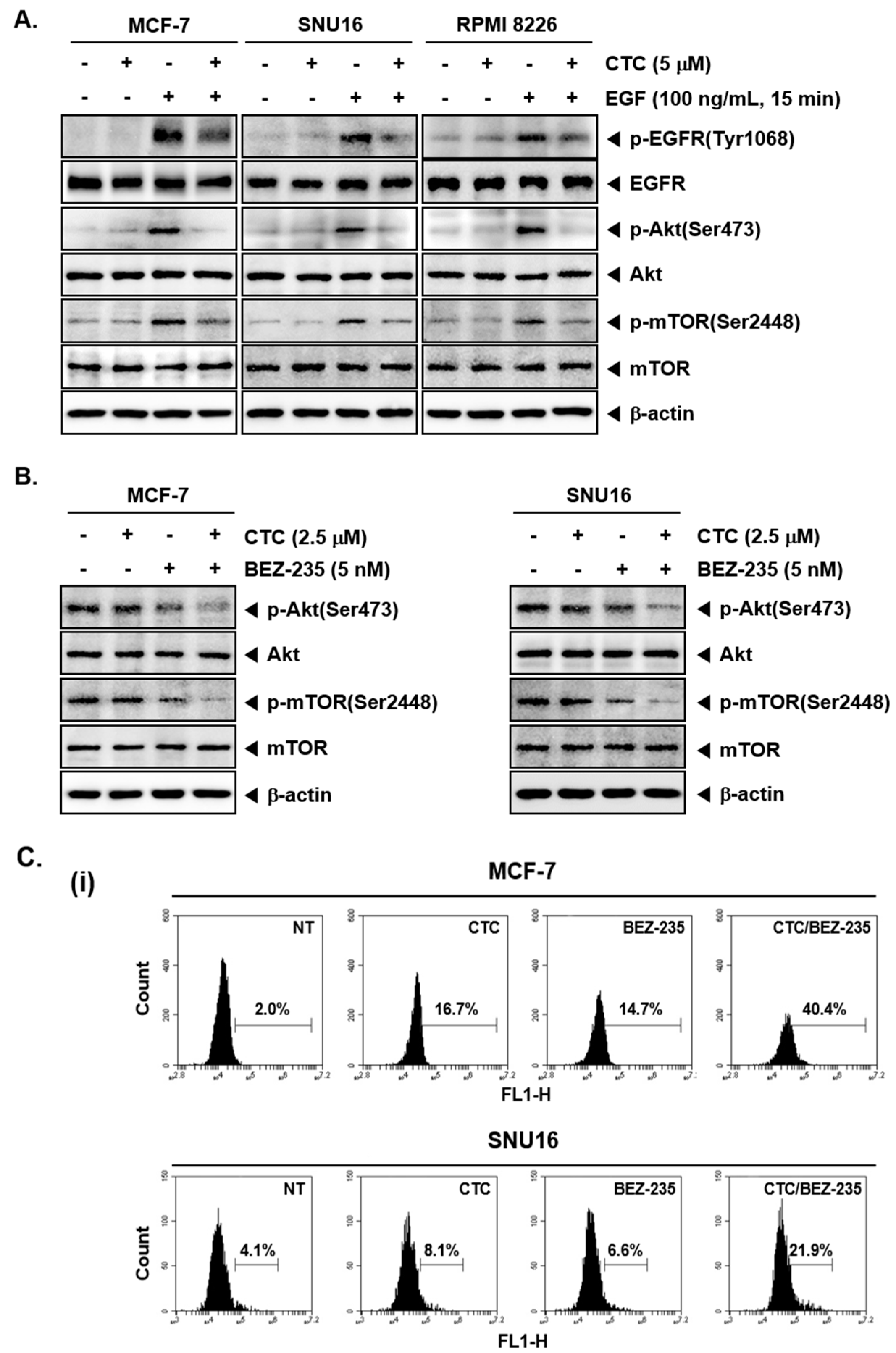
2.5. Pre-Treatment with CTC Abrogates EGF-Induced Oncogenic Signaling Cascade
2.6. CTC and BEZ-235 Efficiently Inhibits Akt/mTOR Signaling Pathway
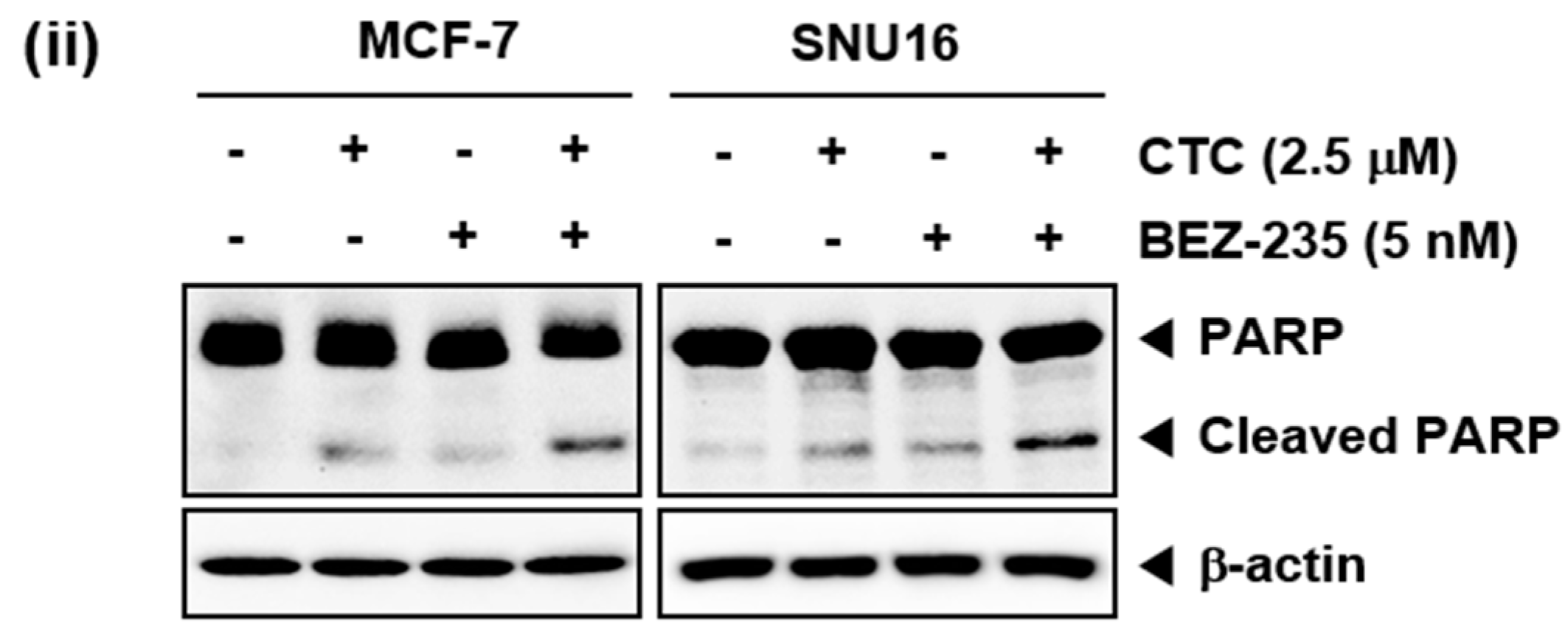
2.7. CTC and BEZ-235 Can Exhibit Enhanced Apoptosis in MCF-7 and SNU16 Cells
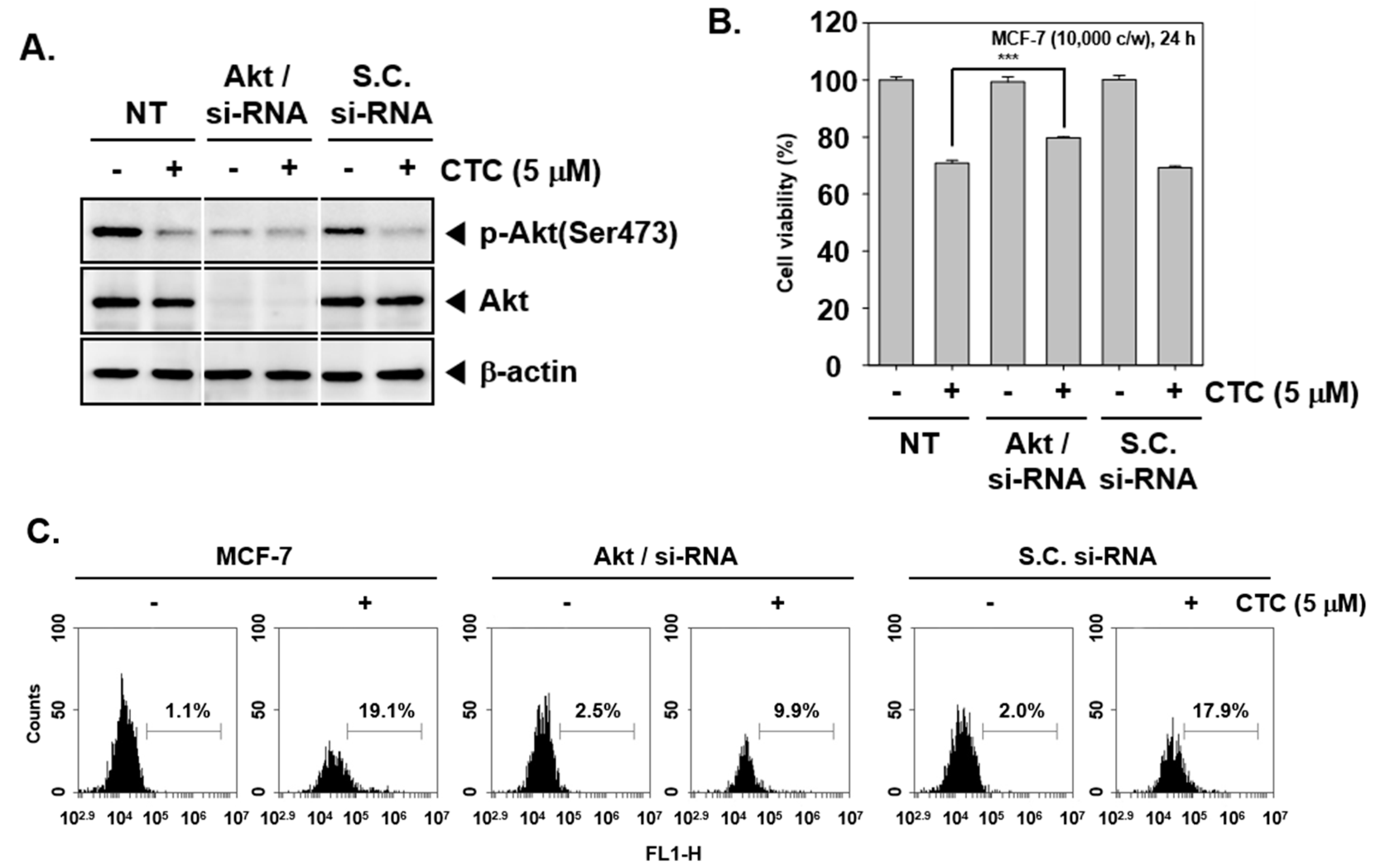
2.8. Knockdown of Akt by si-RNA Reverses the Growth Inhibitory and Apoptotic Effects of CTC
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Lines and Culture Conditions
4.3. Cell Viability Assays
4.4. Western Blot Analysis
4.5. Cell Cycle Analysis
4.6. Annexin V and TUNEL Assays
4.7. Drug Combination Analyses
4.8. siRNA Transfection
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Tolentino Ede, S.; Centurion, B.S.; Ferreira, L.H.; Souza, A.P.; Damante, J.H.; Rubira-Bullen, I.R. Oral adverse effects of head and neck radiotherapy: Literature review and suggestion of a clinical oral care guideline for irradiated patients. J. Appl. Oral Sci. 2011, 19, 448–454. [Google Scholar] [PubMed]
- Pearce, A.; Haas, M.; Viney, R.; Pearson, S.A.; Haywood, P.; Brown, C.; Ward, R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS ONE 2017, 12, e0184360. [Google Scholar] [CrossRef] [PubMed]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Christen, P.; Cuendet, M. Plants as a source of therapeutic and health products. Chimia (Aarau) 2012, 66, 320–323. [Google Scholar] [CrossRef]
- Zaffiri, L.; Gardner, J.; Toledo-Pereyra, L.H. History of antibiotics. From salvarsan to cephalosporins. J. Investig. Surg. 2012, 25, 67–77. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Kannaiyan, R.; Sethi, G. Targeting cell signaling and apoptotic pathways by dietary agents: Role in the prevention and treatment of cancer. Nutr. Cancer 2011, 63, 161–173. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sethi, G.; Baladandayuthapani, V.; Krishnan, S.; Shishodia, S. Targeting cell signaling pathways for drug discovery: An old lock needs a new key. J. Cell. Biochem. 2007, 102, 580–592. [Google Scholar] [CrossRef]
- Deorukhkar, A.; Krishnan, S.; Sethi, G.; Aggarwal, B.B. Back to basics: How natural products can provide the basis for new therapeutics. Expert Opin. Investig. Drugs 2007, 16, 1753–1773. [Google Scholar] [CrossRef]
- Yang, S.F.; Weng, C.J.; Sethi, G.; Hu, D.N. Natural bioactives and phytochemicals serve in cancer treatment and prevention. Evid. Based Complement. Altern. Med. 2013, 2013, 698190. [Google Scholar] [CrossRef]
- Tang, C.H.; Sethi, G.; Kuo, P.L. Novel medicines and strategies in cancer treatment and prevention. Biomed. Res. Int. 2014, 2014, 474078. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Yang, S.F.; Sethi, G.; Hu, D.N. Natural bioactives in cancer treatment and prevention. Biomed. Res. Int. 2015, 2015, 182835. [Google Scholar] [CrossRef] [PubMed]
- Yarla, N.S.; Bishayee, A.; Sethi, G.; Reddanna, P.; Kalle, A.M.; Dhananjaya, B.L.; Dowluru, K.S.; Chintala, R.; Duddukuri, G.R. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin. Cancer Biol. 2016, 40–41, 48–81. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Lee, J.H.; Chai, E.Z.; Kanchi, M.M.; Kar, S.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Ramar, P.S.; Looi, C.Y.; et al. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin. Cancer Biol. 2016, 40–41, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Hasanpourghadi, M.; Looi, C.Y.; Pandurangan, A.K.; Sethi, G.; Wong, W.F.; Mustafa, M.R. Phytometabolites Targeting the Warburg Effect in Cancer Cells: A Mechanistic Review. Curr. Drug Targets 2017, 18, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Warrier, S.; Kumar, A.P.; Sethi, G.; Arfuso, F. Potential Role of Natural Compounds as Anti-Angiogenic Agents in Cancer. Curr. Vasc. Pharmacol. 2017, 15, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Zhao, B.J.; Liu, J.; Liu, B.; Sun, J.X.; Li, J.; Li, X.M. Molecular mechanisms of casticin action: An update on its antitumor functions. Asian Pac. J. Cancer Prev. 2014, 15, 9049–9058. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.; Ko, J.H.; Jung, Y.Y.; Jung, S.H.; Kim, E.; Kong, M.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; et al. Casticin inhibits growth and enhances ionizing radiation-induced apoptosis through the suppression of STAT3 signaling cascade. J. Cell. Biochem. 2018. [Google Scholar] [CrossRef]
- Mesaik, M.A.; Azizuddin; Murad, S.; Khan, K.M.; Tareen, R.B.; Ahmed, A.; Atta-ur, R.; Choudhary, M.I. Isolation and immunomodulatory properties of a flavonoid, casticin from Vitex agnus-castus. Phytother. Res. 2009, 23, 1516–1520. [Google Scholar] [CrossRef]
- You, K.M.; Son, K.H.; Chang, H.W.; Kang, S.S.; Kim, H.P. Vitexicarpin, a flavonoid from the fruits of Vitex rotundifolia, inhibits mouse lymphocyte proliferation and growth of cell lines in vitro. Planta Med. 1998, 64, 546–550. [Google Scholar] [CrossRef]
- Ko, W.G.; Kang, T.H.; Lee, S.J.; Kim, N.Y.; Kim, Y.C.; Sohn, D.H.; Lee, B.H. Polymethoxyflavonoids from Vitex rotundifolia inhibit proliferation by inducing apoptosis in human myeloid leukemia cells. Food Chem. Toxicol. 2000, 38, 861–865. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yuan, B.; Nishimura, Y.; Imai, M.; Furutani, R.; Kamoi, S.; Seno, M.; Fukushima, S.; Hazama, S.; Hirobe, C.; et al. Cytotoxicity of Vitex agnus-castus fruit extract and its major component, casticin, correlates with differentiation status in leukemia cell lines. Int. J. Oncol. 2013, 43, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Song, X.L.; Zhang, Y.J.; Wang, X.F.; Zhang, W.J.; Wang, Z.; Zhang, F.; Zhang, Y.J.; Lu, J.H.; Mei, J.W.; Hu, Y.P.; et al. Casticin induces apoptosis and G0/G1 cell cycle arrest in gallbladder cancer cells. Cancer Cell Int. 2017, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Cao, X.C.; Cao, J.G.; Liu, F.; Quan, M.F.; Sheng, X.F.; Ren, K.Q. Casticin induces ovarian cancer cell apoptosis by repressing FoxM1 through the activation of FOXO3a. Oncol. Lett. 2013, 5, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Tian, L.; Liu, F.; Cao, J.; Quan, M.; Sheng, X. Induction of apoptosis by casticin in cervical cancer cells: Reactive oxygen species-dependent sustained activation of Jun N-terminal kinase. Acta Biochim. Biophys. Sin. (Shanghai) 2012, 44, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Zhong, M.Z.; Yuan, G.J.; Hou, S.P.; Yin, L.L.; Jiang, H.; Yu, Z. Casticin, a flavonoid, potentiates TRAIL-induced apoptosis through modulation of anti-apoptotic proteins and death receptor 5 in colon cancer cells. Oncol. Rep. 2013, 29, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jung, K.H.; Lee, H.; Park, S.; Choi, W.; Bae, H. Casticin, an active compound isolated from Vitex Fructus, ameliorates the cigarette smoke-induced acute lung inflammatory response in a murine model. Int. Immunopharmacol. 2015, 28, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, H.; Han, T.; Wu, J.Z.; Rahman, K.; Qin, L.P. In vivo effect of casticin on acute inflammation. Zhong Xi Yi Jie He Xue Bao 2007, 5, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.L.; Chou, H.M.; Chou, H.C.; Lu, H.F.; Chu, Y.L.; Shang, H.S.; Chung, J.G. Casticin impairs cell migration and invasion of mouse melanoma B16F10 cells via PI3K/AKT and NF-kappaB signaling pathways. Environ. Toxicol. 2017, 32, 2097–2112. [Google Scholar] [CrossRef] [PubMed]
- Shiue, Y.W.; Lu, C.C.; Hsiao, Y.P.; Liao, C.L.; Lin, J.P.; Lai, K.C.; Yu, C.C.; Huang, Y.P.; Ho, H.C.; Chung, J.G. Casticin Induced Apoptosis in A375.S2 Human Melanoma Cells through the Inhibition of NF-[Formula: See text]B and Mitochondria-Dependent Pathways In Vitro and Inhibited Human Melanoma Xenografts in a Mouse Model In Vivo. Am. J. Chin. Med. 2016, 44, 637–661. [Google Scholar] [CrossRef] [PubMed]
- Carnero, A.; Blanco-Aparicio, C.; Renner, O.; Link, W.; Leal, J.F. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets 2008, 8, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Polivka, J., Jr.; Janku, F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol. Ther. 2014, 142, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Yap, W.N.; Arfuso, F.; Kar, S.; Wang, C.; Cai, W.; Dharmarajan, A.M.; Sethi, G.; Kumar, A.P. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: A reality for personalized medicine? World J. Gastroenterol. 2015, 21, 12261–12273. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.S.; Wang, L.Z.; Dai, X.; Tseng, S.H.; Loo, S.J.; Sethi, G. Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front. Pharmacol. 2016, 7, 395. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Ko, J.H.; Lee, J.H.; Kim, C.; Lee, H.; Nam, D.; Lee, J.; Lee, S.G.; Yang, W.M.; Um, J.Y.; et al. Ginkgolic Acid Inhibits Invasion and Migration and TGF-beta-Induced EMT of Lung Cancer Cells Through PI3K/Akt/mTOR Inactivation. J. Cell. Physiol. 2017, 232, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.D.; Srinivasa, V.; Rangappa, S.; Mervin, L.; Mohan, S.; Paricharak, S.; Baday, S.; Li, F.; Shanmugam, M.K.; Chinnathambi, A.; et al. Trisubstituted-Imidazoles Induce Apoptosis in Human Breast Cancer Cells by Targeting the Oncogenic PI3K/Akt/mTOR Signaling Pathway. PLoS ONE 2016, 11, e0153155. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Ahn, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Yap, W.N.; Kumar, A.P.; Fong, C.W.; Tergaonkar, V.; Hui, K.M.; et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget 2014, 5, 1897–1911. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, S.M.; Bae, H.; Nam, D.; Lee, J.H.; Lee, S.G.; Shim, B.S.; Kim, S.H.; Ahn, K.S.; Choi, S.H.; et al. Embelin inhibits growth and induces apoptosis through the suppression of Akt/mTOR/S6K1 signaling cascades. Prostate 2013, 73, 296–305. [Google Scholar] [CrossRef]
- Park, K.R.; Nam, D.; Yun, H.M.; Lee, S.G.; Jang, H.J.; Sethi, G.; Cho, S.K.; Ahn, K.S. Beta-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.M.; Garcia-Haro, L.; Sparks, C.A.; Guertin, D.A. mTOR-dependent cell survival mechanisms. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, M.G.; Sun, X.M.; Brown, D.; Snowden, R.T.; Cohen, G.M. Quantification of apoptosis and necrosis by flow cytometry. Acta Oncol. 1993, 32, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Oliver, F.J.; de la Rubia, G.; Rolli, V.; Ruiz-Ruiz, M.C.; de Murcia, G.; Murcia, J.M. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J. Biol. Chem. 1998, 273, 33533–33539. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Ahn, K.S.; Chaturvedi, M.M.; Aggarwal, B.B. Epidermal growth factor (EGF) activates nuclear factor-kappaB through IkappaBalpha kinase-independent but EGF receptor-kinase dependent tyrosine 42 phosphorylation of IkappaBalpha. Oncogene 2007, 26, 7324–7332. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Syn, N.L.; Subhash, V.V.; Any, Y.; Thuya, W.L.; Cheow, E.S.H.; Kong, L.; Yu, F.; Peethala, P.C.; Wong, A.L.; et al. Pan-HDAC inhibition by panobinostat mediates chemosensitization to carboplatin in non-small cell lung cancer via attenuation of EGFR signaling. Cancer Lett. 2018, 417, 152–160. [Google Scholar] [CrossRef]
- Guo, Y.H.; Zhang, C.; Shi, J.; Xu, M.H.; Liu, F.; Yuan, H.H.; Wang, J.Y.; Jiang, B.; Gao, F.H. Abnormal activation of the EGFR signaling pathway mediates the downregulation of miR145 through the ERK1/2 in non-small cell lung cancer. Oncol. Rep. 2014, 31, 1940–1946. [Google Scholar] [CrossRef]
- Ryu, N.H.; Park, K.R.; Kim, S.M.; Yun, H.M.; Nam, D.; Lee, S.G.; Jang, H.J.; Ahn, K.S.; Kim, S.H.; Shim, B.S.; et al. A hexane fraction of guava Leaves (Psidium guajava L.) induces anticancer activity by suppressing AKT/mammalian target of rapamycin/ribosomal p70 S6 kinase in human prostate cancer cells. J. Med. Food 2012, 15, 231–241. [Google Scholar] [CrossRef]
- Ben-Sasson, S.A.; Sherman, Y.; Gavrieli, Y. Identification of dying cells--in situ staining. Methods Cell Biol. 1995, 46, 29–39. [Google Scholar] [PubMed]
- Haidara, K.; Zamir, L.; Shi, Q.W.; Batist, G. The flavonoid Casticin has multiple mechanisms of tumor cytotoxicity action. Cancer Lett. 2006, 242, 180–190. [Google Scholar] [CrossRef]
- Liu, F.; Cao, X.; Liu, Z.; Guo, H.; Ren, K.; Quan, M.; Zhou, Y.; Xiang, H.; Cao, J. Casticin suppresses self-renewal and invasion of lung cancer stem-like cells from A549 cells through down-regulation of pAkt. Acta Biochim. Biophys. Sin. (Shanghai) 2014, 46, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Kempf, R.C.; Long, J.; Laidler, P.; Mijatovic, S.; Maksimovic-Ivanic, D.; Stivala, F.; Mazzarino, M.C.; et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 2011, 3, 192–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Liou, C.J.; Huang, W.C. Casticin inhibits interleukin-1beta-induced ICAM-1 and MUC5AC expression by blocking NF-kappaB, PI3K-Akt, and MAPK signaling in human lung epithelial cells. Oncotarget 2017, 8, 101175–101188. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001, 15, 2177–2196. [Google Scholar] [CrossRef]
- Chou, G.L.; Peng, S.F.; Liao, C.L.; Ho, H.C.; Lu, K.W.; Lien, J.C.; Fan, M.J.; La, K.C.; Chung, J.G. Casticin impairs cell growth and induces cell apoptosis via cell cycle arrest in human oral cancer SCC-4 cells. Environ. Toxicol. 2018, 33, 127–141. [Google Scholar] [CrossRef]
- Laroche, A.; Chaire, V.; Algeo, M.P.; Karanian, M.; Fourneaux, B.; Italiano, A. MDM2 antagonists synergize with PI3K/mTOR inhibition in well-differentiated/dedifferentiated liposarcomas. Oncotarget 2017, 8, 53968–53977. [Google Scholar] [CrossRef]
- Maira, S.M.; Stauffer, F.; Brueggen, J.; Furet, P.; Schnell, C.; Fritsch, C.; Brachmann, S.; Chene, P.; De Pover, A.; Schoemaker, K.; et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 2008, 7, 1851–1863. [Google Scholar] [CrossRef]
- Moon du, G.; Lee, S.E.; Oh, M.M.; Lee, S.C.; Jeong, S.J.; Hong, S.K.; Yoon, C.Y.; Byun, S.S.; Park, H.S.; Cheon, J. NVP-BEZ235, a dual PI3K/mTOR inhibitor synergistically potentiates the antitumor effects of cisplatin in bladder cancer cells. Int. J. Oncol. 2014, 45, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Narayanan, S.; Sethuraman, S.; Krishnan, U.M. Combinations of plant polyphenols & anti-cancer molecules: A novel treatment strategy for cancer chemotherapy. Anticancer Agents Med. Chem. 2013, 13, 281–295. [Google Scholar] [PubMed]
- Sak, K.; Everaus, H. Chemomodulating Effects of Flavonoids in Human Leukemia Cells. Anticancer Agents Med. Chem. 2015, 15, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Sak, K.; Everaus, H. Role of flavonoids in future anticancer therapy by eliminating the cancer stem cells. Curr. Stem Cell Res. Ther. 2015, 10, 271–282. [Google Scholar] [CrossRef]
- Harikumar, K.B.; Kunnumakkara, A.B.; Sethi, G.; Diagaradjane, P.; Anand, P.; Pandey, M.K.; Gelovani, J.; Krishnan, S.; Guha, S.; Aggarwal, B.B. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int. J. Cancer 2010, 127, 257–268. [Google Scholar] [PubMed]
- Yang, M.Y.; Wang, C.J.; Chen, N.F.; Ho, W.H.; Lu, F.J.; Tseng, T.H. Luteolin enhances paclitaxel-induced apoptosis in human breast cancer MDA-MB-231 cells by blocking STAT3. Chem. Biol. Interact. 2014, 213, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. NF-kappaB in cancer therapy. Arch. Toxicol. 2015, 89, 711–731. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Fong, C.W.; Kumar, A.P.; Tan, P.; Sethi, G. First evidence that gamma-tocotrienol inhibits the growth of human gastric cancer and chemosensitizes it to capecitabine in a xenograft mouse model through the modulation of NF-kappaB pathway. Clin. Cancer Res. 2012, 18, 2220–2229. [Google Scholar] [CrossRef]
- Sethi, G.; Shanmugam, M.K.; Ramachandran, L.; Kumar, A.P.; Tergaonkar, V. Multifaceted link between cancer and inflammation. Biosci. Rep. 2012, 32, 1–15. [Google Scholar] [CrossRef]
- Li, F.; Sethi, G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim. Biophys. Acta 2010, 1805, 167–180. [Google Scholar]
- Sethi, G.; Tergaonkar, V. Potential pharmacological control of the NF-kappaB pathway. Trends Pharmacol. Sci. 2009, 30, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Siveen, K.S.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Arfuso, F.; Kumar, A.P.; et al. Isorhamnetin augments the anti-tumor effect of capeciatbine through the negative regulation of NF-kappaB signaling cascade in gastric cancer. Cancer Lett. 2015, 363, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.G.; Yang, W.M.; Arfuso, F.; Um, J.Y.; Kumar, A.P.; Bian, J.; Sethi, G.; Ahn, K.S. Formononetin-induced oxidative stress abrogates the activation of STAT3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Lett. 2018, 431, 123–141. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Kim, C.; Um, J.-Y.; Sethi, G.; Ahn, K.S. Casticin-Induced Inhibition of Cell Growth and Survival Are Mediated through the Dual Modulation of Akt/mTOR Signaling Cascade. Cancers 2019, 11, 254. https://doi.org/10.3390/cancers11020254
Lee JH, Kim C, Um J-Y, Sethi G, Ahn KS. Casticin-Induced Inhibition of Cell Growth and Survival Are Mediated through the Dual Modulation of Akt/mTOR Signaling Cascade. Cancers. 2019; 11(2):254. https://doi.org/10.3390/cancers11020254
Chicago/Turabian StyleLee, Jong Hyun, Chulwon Kim, Jae-Young Um, Gautam Sethi, and Kwang Seok Ahn. 2019. "Casticin-Induced Inhibition of Cell Growth and Survival Are Mediated through the Dual Modulation of Akt/mTOR Signaling Cascade" Cancers 11, no. 2: 254. https://doi.org/10.3390/cancers11020254
APA StyleLee, J. H., Kim, C., Um, J.-Y., Sethi, G., & Ahn, K. S. (2019). Casticin-Induced Inhibition of Cell Growth and Survival Are Mediated through the Dual Modulation of Akt/mTOR Signaling Cascade. Cancers, 11(2), 254. https://doi.org/10.3390/cancers11020254






