Exosome Analysis in Tumor-Draining Pulmonary Vein Identifies NSCLC Patients with Higher Risk of Relapse after Curative Surgery
Abstract
:1. Introduction
2. Results
2.1. Patients
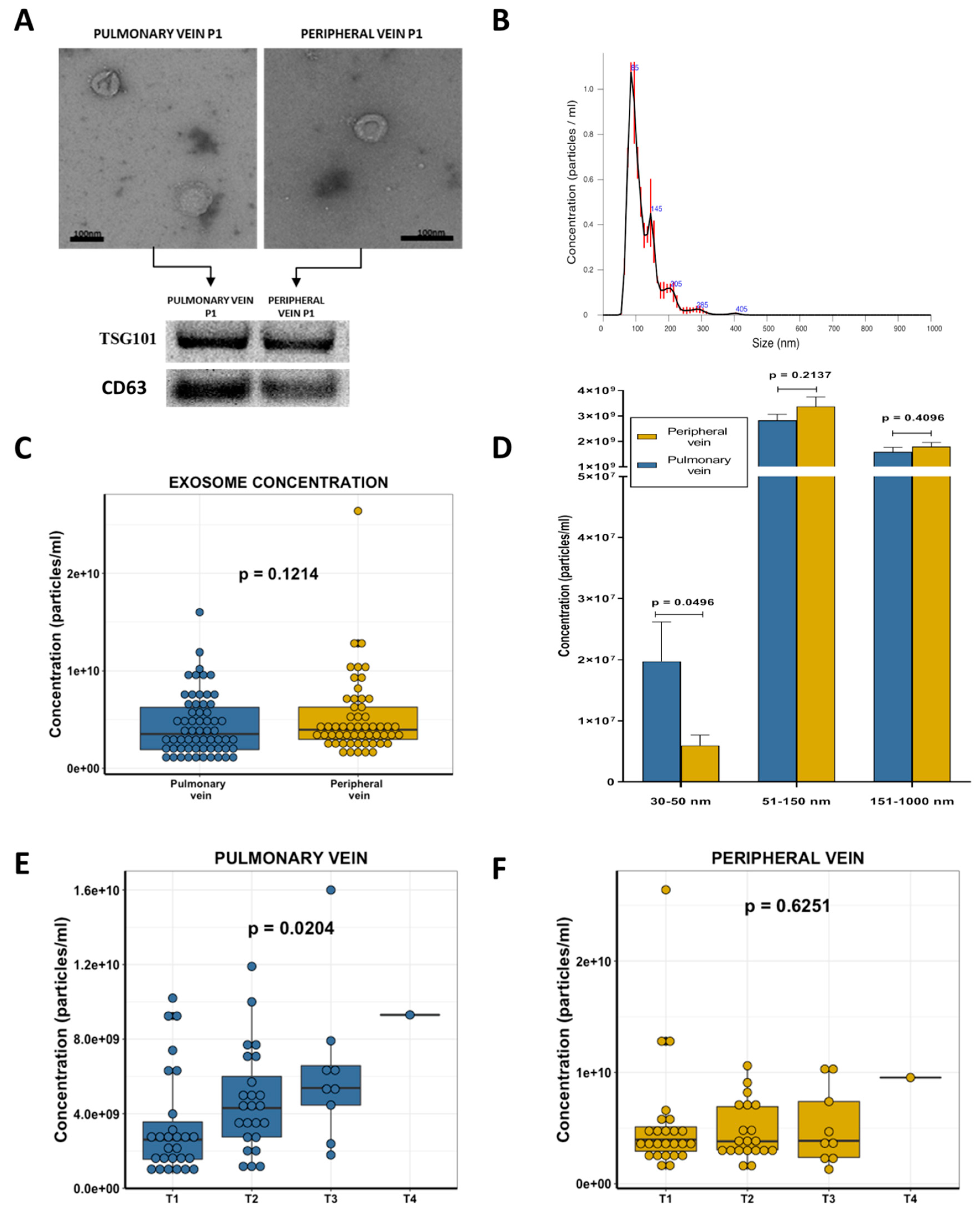
2.2. Exosome Characterization
2.3. Exosome Concentration and Clinical Characteristics
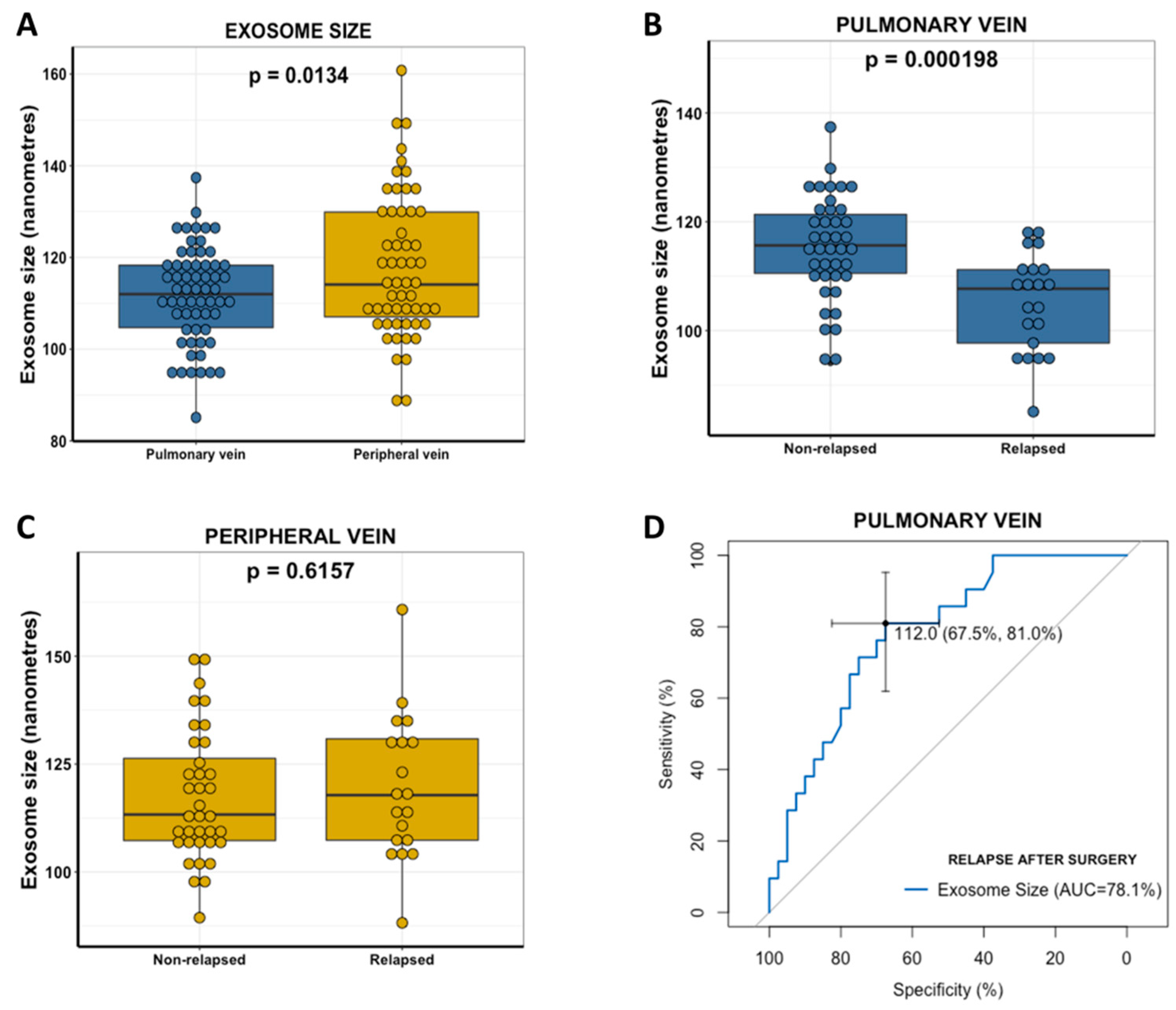
2.4. Pulmonary Vein Exosome Size Identifies Patients Who Relapse after Curative Surgery
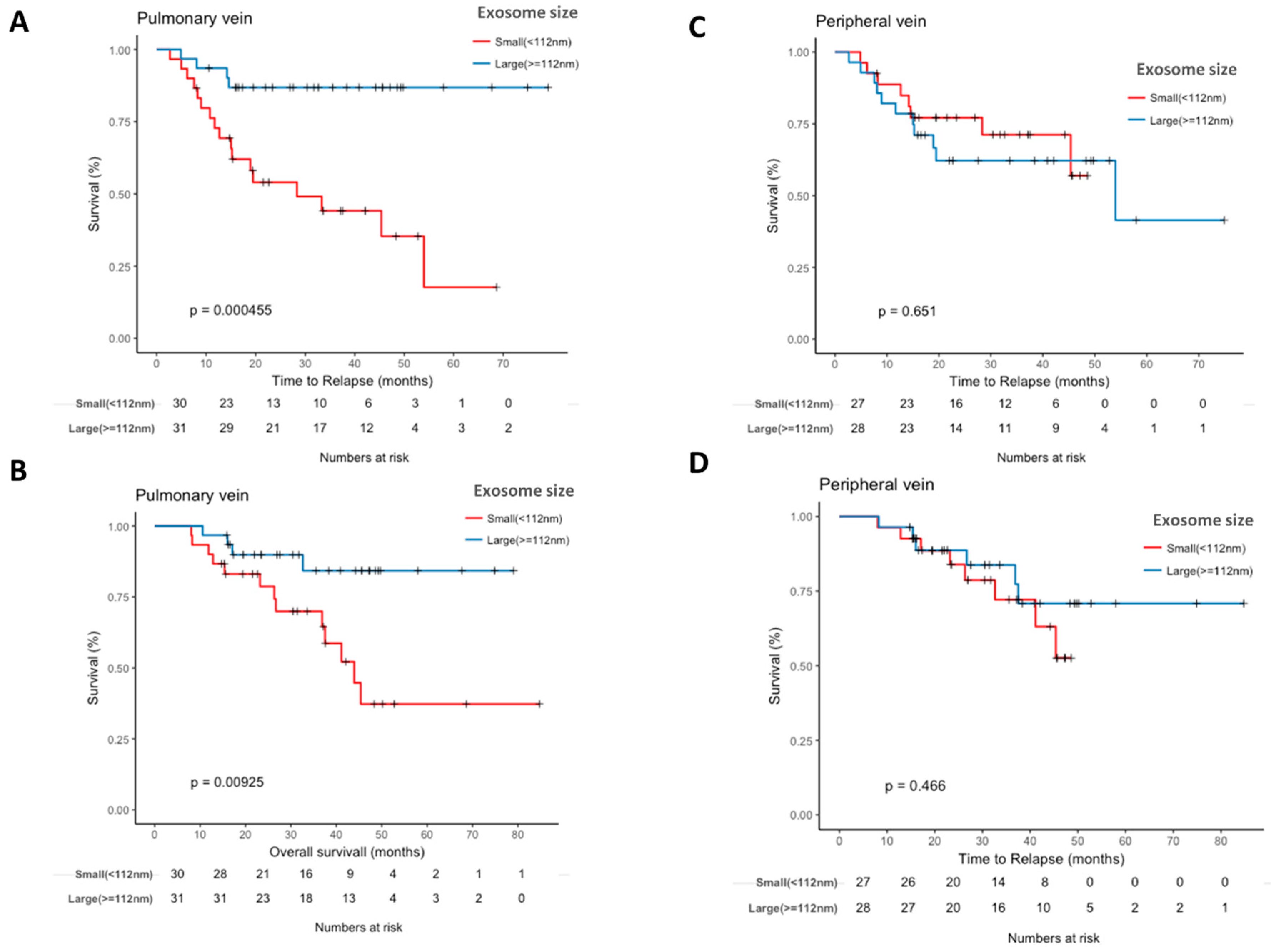
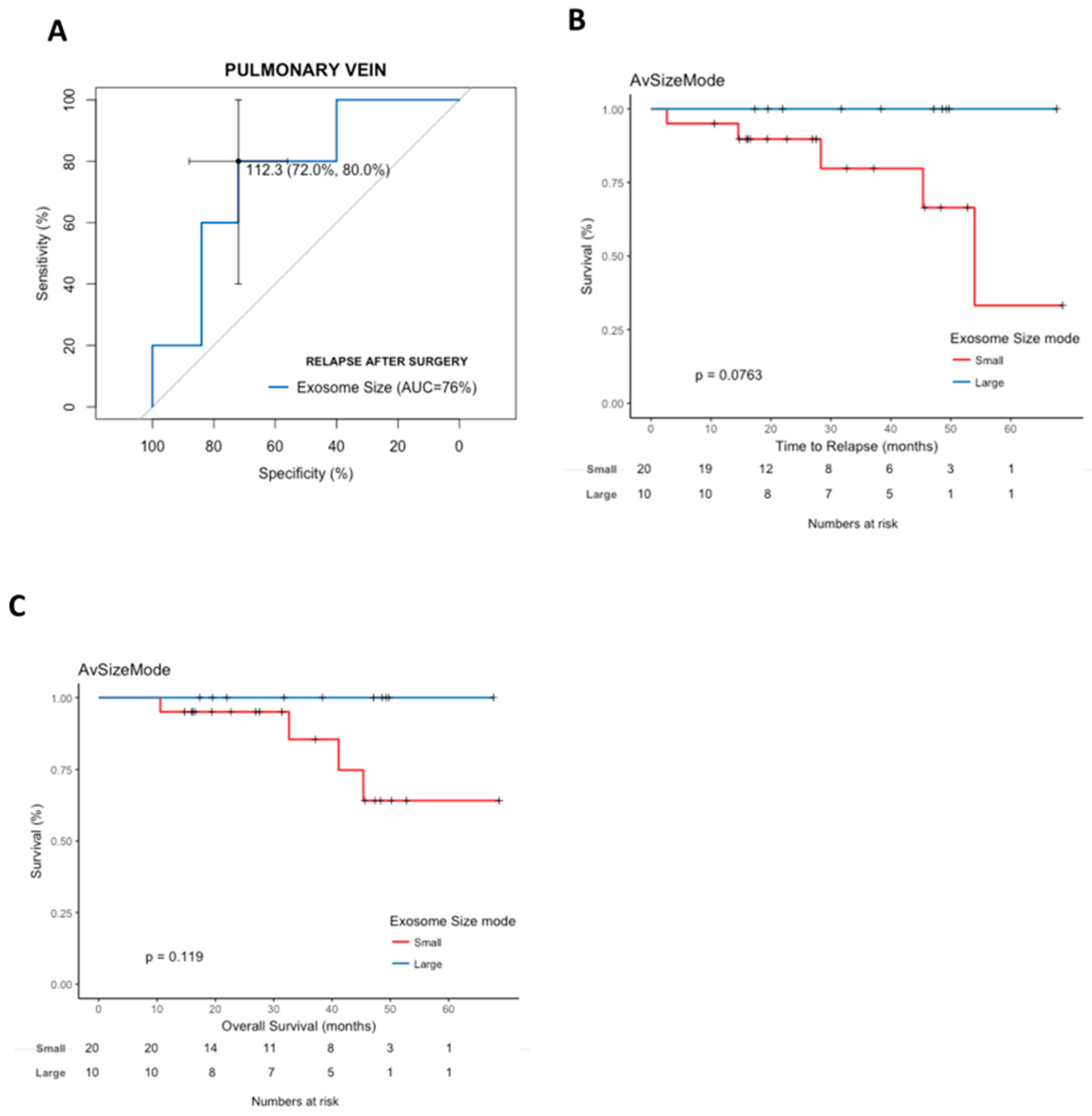
2.5. Pulmonary Vein Exosome Size Is Associated with Outcome after Curative Surgery
2.6. Cox Modeling of Relapse and Survival
3. Discussion
4. Material and Methods
4.1. Patient Samples
4.2. Exosome Purification and Characterization
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Vansteenkiste, J.; De Ruysscher, D.; Eberhardt, W.; Lim, E.; Senan, S.; Felip, E.; Peters, S.; Group, E.G.W. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi89–vi98. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.-Y.; Rosell, R.; De Lena, M.; Carpagnano, F.; Ramlau, R.; Gonzáles-Larriba, J.L.; Grodzki, T.; Pereira, J.R.; Le Groumellec, A.; Lorusso, V. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB–IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomised controlled trial. Lancet Oncol. 2006, 7, 719–727. [Google Scholar] [CrossRef]
- Krebs, M.G.; Sloane, R.; Priest, L.; Lancashire, L.; Hou, J.-M.; Greystoke, A.; Ward, T.H.; Ferraldeschi, R.; Hughes, A.; Clack, G. Evaluation and prognostic significance of circulating tumor cells in patients with non–small-cell lung cancer. J. Clin. Oncol. 2011, 29, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Sienel, W.; Seen-Hibler, R.; Mutschler, W.; Pantel, K.; Passlick, B. Tumour cells in the tumour draining vein of patients with non-small cell lung cancer: Detection rate and clinical significance. Eur. J. Cardio-Thoracic Surg. 2003, 23, 451–456. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Patel, T.; Freedman, J.E. Circulating Extracellular Vesicles in Human Disease. N. Engl. J. Med. 2018, 379, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhang, Q.; Lou, Y.; Yang, J.; Nie, G.; Chen, Q.; Chen, Y.; Zhang, J.; Wang, J.; Wei, T. Primary tumor-derived exosomes facilitate metastasis by regulating adhesion of circulating tumor cells via SMAD3 in liver cancer. Oncogene 2018, 37, 6105–6118. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Gupta, R.C. Exosomal miRNAs as biomarkers of recurrent lung cancer. Tumor Biol. 2016, 37, 10703–10714. [Google Scholar] [CrossRef] [PubMed]
- Sandfeld-Paulsen, B.; Aggerholm-Pedersen, N.; Baek, R.; Jakobsen, K.; Meldgaard, P.; Folkersen, B.; Rasmussen, T.R.; Varming, K.; Jørgensen, M.; Sorensen, B. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol. Oncol. 2016, 10, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- König, L.; Kasimir-Bauer, S.; Bittner, A.-K.; Hoffmann, O.; Wagner, B.; Santos Manvailer, L.F.; Kimmig, R.; Horn, P.A.; Rebmann, V. Elevated levels of extracellular vesicles are associated with therapy failure and disease progression in breast cancer patients undergoing neoadjuvant chemotherapy. Oncoimmunology 2018, 7, e1376153. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, P.A.; Shah, R.; Krysiak, P.; Zhou, C.; Morris, K.; Tugwood, J.; Booton, R.; Blackhall, F.; Dive, C. Circulating tumor cells detected in the tumor-draining pulmonary vein are associated with disease recurrence after surgical resection of NSCLC. J. Thoracic Oncol. 2016, 11, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Murlidhar, V.; Reddy, R.M.; Fouladdel, S.; Zhao, L.; Ishikawa, M.K.; Grabauskiene, S.; Zhang, Z.; Lin, J.; Chang, A.C.; Carrott, P. Poor prognosis indicated by venous circulating tumor cell clusters in early-stage lung cancers. Cancer Res. 2017, 77, 5194–5206. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.M.; Murlidhar, V.; Zhao, L.; Grabauskiene, S.; Zhang, Z.; Ramnath, N.; Lin, J.; Chang, A.C.; Carrott, P.; Lynch, W. Pulmonary venous blood sampling significantly increases the yield of circulating tumor cells in early-stage lung cancer. J. Thoracic Cardiovasc. Surg. 2016, 151, 852–858. [Google Scholar] [CrossRef]
- Kim, D.U.; San Lucas, F.A.; Cagley, M.; Stephens, B.; Mulu, F.C.; Castillo, J.; Bernard, V.; Varadhachary, G.R.; Katz, M.H.; Alvarez, H.A. Larger size extracellular vesicle as early biomarker in patients with metastatic pancreatic cancer. J. Clin. Oncol. 2017, 35, 234. [Google Scholar] [CrossRef]
- Rabinowits, G.; Gerçel-Taylor, C.; Day, J.M.; Taylor, D.D.; Kloecker, G.H. Exosomal microRNA: A diagnostic marker for lung cancer. Clin. Lung Cancer 2009, 10, 42–46. [Google Scholar] [CrossRef]
- Rahman, M.A.; Barger, J.F.; Lovat, F.; Gao, M.; Otterson, G.A.; Nana-Sinkam, P. Lung cancer exosomes as drivers of epithelial mesenchymal transition. Oncotarget 2016, 7, 54852. [Google Scholar] [CrossRef]
- Rodríguez, M.; Silva, J.; López-Alfonso, A.; López-Muñiz, M.B.; Peña, C.; Domínguez, G.; García, J.M.; López-Gónzalez, A.; Méndez, M.; Provencio, M. Different exosome cargo from plasma/bronchoalveolar lavage in non-small-cell lung cancer. Genes Chromosomes Cancer 2014, 53, 713–724. [Google Scholar] [CrossRef]
- Monzo, M.; Santasusagna, S.; Moreno, I.; Martinez, F.; Hernáez, R.; Muñoz, C.; Castellano, J.J.; Moreno, J.; Navarro, A. Exosomal microRNAs isolated from plasma of mesenteric veins linked to liver metastases in resected patients with colon cancer. Oncotarget 2017, 8, 30859. [Google Scholar] [CrossRef]
- Ruiz-Martinez, M.; Navarro, A.; Marrades, R.M.; Viñolas, N.; Santasusagna, S.; Muñoz, C.; Ramírez, J.; Molins, L.; Monzo, M. YKT6 expression, exosome release, and survival in non-small cell lung cancer. Oncotarget 2016, 7, 51515. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Value | N = 61 | TTR | OS |
|---|---|---|---|---|
| N (%) | p-Value | p-Value | ||
| Sex | Male | 41 (67.2) | ||
| Female | 20 (32.8) | 0.5427 | 0.6291 | |
| Age, y | Mean (Range) | 63 (32–80) | ||
| ≤65 | 34 (55.7) | |||
| >65 | 27 (44.3) | 0.8 | 0.2253 | |
| ECOG PS | 0 | 26 (42.6) | ||
| 1 | 35 (57.4) | 0.4723 | 0.3479 | |
| Pathological Stage | I | 30 (49.2) | ||
| II | 24 (39.3) | |||
| III | 7 (11.5) | 0.0069 | 0.0082 | |
| Histology | Adenocarcinoma | 35 (57.4) | ||
| Squamous cell carcinoma | 16 (26.2) | |||
| Others | 10 (16.4) | 0.7562 | 0.9579 | |
| Type of surgery | Segmentectomy | 5 (8.2) | ||
| Lobectomy/bilobectomy | 48 (78.7) | |||
| Pneumonectomy | 7 (11.5) | |||
| Atypical Resection | 1 (1.6) | 0.3963 | 0.4178 | |
| Smoking history | Current Smoker | 32 (52.5) | ||
| Former Smoker | 24 (39.3) | |||
| Never smoker | 5 (8.2) | 0.1118 | 0.1881 | |
| FEV1 | Liters (±SD) | 2.4 (±0.6) | ||
| %pred (±SD) | 76.8 (±16.1) | - | - | |
| FVC | Liters (±SD) | 3.56 (±0.74) | ||
| %pred (±SD) | 88 (±16.42) | - | - | |
| FEV1/FVC | Ratio (±SD) | 67.2 (±9.74) | - | - |
| Tumor size | mm (±SD) | 38.3 (±23.2) | - | - |
| Received adjuvant chemotherapy | Yes | 23 (37.7) | ||
| No | 38 (62.3) | 0.0113 | 0.1958 | |
| Experienced recurrence | No | 40 (65.6) | ||
| Yes | 21 (34.4) | - | - |
| Time to Relapse | Hazard Ratio (HR, 5% CI) | p-Value |
|---|---|---|
| Stage | 2.42 (1.16–5.03) | 0.0180 |
| Adjuvant chemotherapy | 1.59 (0.59–4.26) | 0.3563 |
| Exosome size in pulmonary vein <112 nm | 6.66 (2.06–21.51) | 0.0015 |
| Overall Survival | HR (95% CI) | p-value |
| Stage | 2.93 (1.46–5.84) | 0.0024 |
| Exosome size in pulmonary vein <112 nm | 4.55 (1.43–14.49) | 0.0104 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, A.; Molins, L.; Marrades, R.M.; Moises, J.; Viñolas, N.; Morales, S.; Canals, J.; Castellano, J.J.; Ramírez, J.; Monzo, M. Exosome Analysis in Tumor-Draining Pulmonary Vein Identifies NSCLC Patients with Higher Risk of Relapse after Curative Surgery. Cancers 2019, 11, 249. https://doi.org/10.3390/cancers11020249
Navarro A, Molins L, Marrades RM, Moises J, Viñolas N, Morales S, Canals J, Castellano JJ, Ramírez J, Monzo M. Exosome Analysis in Tumor-Draining Pulmonary Vein Identifies NSCLC Patients with Higher Risk of Relapse after Curative Surgery. Cancers. 2019; 11(2):249. https://doi.org/10.3390/cancers11020249
Chicago/Turabian StyleNavarro, Alfons, Laureano Molins, Ramon M. Marrades, Jorge Moises, Nuria Viñolas, Sara Morales, Jordi Canals, Joan J. Castellano, José Ramírez, and Mariano Monzo. 2019. "Exosome Analysis in Tumor-Draining Pulmonary Vein Identifies NSCLC Patients with Higher Risk of Relapse after Curative Surgery" Cancers 11, no. 2: 249. https://doi.org/10.3390/cancers11020249
APA StyleNavarro, A., Molins, L., Marrades, R. M., Moises, J., Viñolas, N., Morales, S., Canals, J., Castellano, J. J., Ramírez, J., & Monzo, M. (2019). Exosome Analysis in Tumor-Draining Pulmonary Vein Identifies NSCLC Patients with Higher Risk of Relapse after Curative Surgery. Cancers, 11(2), 249. https://doi.org/10.3390/cancers11020249






