Combination of Baseline LDH, Performance Status and Age as Integrated Algorithm to Identify Solid Tumor Patients with Higher Probability of Response to Anti PD-1 and PD-L1 Monoclonal Antibodies
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Ldh Evaluation
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Drent, M.; Cobben, N.A.; Henderson, R.F.; Wouters, E.F.; van Dieijen-Visser, M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur. Respir J. 1996, 9, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.; Donovan, J.; Higham, H.; Hooper, J. Biochemical markers of myocardial injury. Br. J. Anaesth. 2004, 93, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; McGowan, V.; Machado, R.F.; Little, J.A.; Taylor, V.I.J.; Morris, C.R.; Nichols, J.S.; Wang, X.; Poljakovic, M.; Morris, S.M.; et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood 2006, 107, 2279–2285. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; Bukowski, R.; Rini, B.I.; Hutson, T.E.; Barrios, C.H.; Lin, X.; Fly, K.; Matczak, E.; Gore, M.E. Prognostic factors for survival in 1059 patients treated with sunitinib for metastatic renal cell carcinoma. Br. J. Cancer 2013, 108, 2470–2477. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.B.; Wei, L.; Li, H.; Dong, M.; Lin, Q.; Ma, X.K.; Huang, P.Y.; Wen, J.Y.; Li, X.; Chen, J.; et al. High pretreatment serum lactate dehydrogenase level correlates with disease relapse and predicts an inferior outcome in locally advanced nasopharyngeal carcinoma. Eur. J. Cancer 2013, 49, 2356–2364. [Google Scholar] [CrossRef]
- Hagberg, H.; Siegbahn, A. Prognostic level of serum lactic dehydrogenase in non-Hodgkin’s lymphoma. Scand J. Haematol. 1983, 31, 49–56. [Google Scholar] [CrossRef]
- Simonsson, B.; Grenning, G.; Källander, C.; Ahre, A. Prognostic level of serum lactic dehydrogenase (S-LDH) in multiple myeloma. Eur. J. Clin. Investig. 1987, 17, 336–349. [Google Scholar] [CrossRef]
- Brereton, H.D.; Simon, R.; Pomeroy, T.C. Pretreatment lactate dehydrogenase predicting metastatic spread in Ewing’s sarcoma. Ann. Intern. Med. 1975, 83, 352–364. [Google Scholar] [CrossRef]
- Hermes, A.; Gatzemeier, U.; Waschki, B.; Reck, M. Lactate dehydrogenase as prognostic factor in limited and extensive disease stage small cell lung cancer e A retrospective single institution analysis. Respir. Med. 2010, 104, 1937–1942. [Google Scholar] [CrossRef]
- Giroux Leprieur, E.; Lavole, A.; Ruppert, A.M.; Gounant, V.; Wislez, M.; Cadranel, J.; Milleron, B. Factors associated with long-term survival of patients with advanced non-small cell lung cancer. Respirology 2012, 17, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Diem, S.; Kasenda, B.; Spain, L.; Martin-Liberal, J.; Marconcini, R.; Gore, M.; Larkin, J. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br. J. Cancer 2016, 114, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Kelderman, S.; Heemskerk, B.; van Tinteren, H.; van den Brom, R.R.; Hospers, G.A.; van den Eertwegh, A.J.; Kapiteijn, E.W.; de Groot, J.W.; Soetekouw, P.; Jansen, R.L.; et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol. Immunother. 2014, 63, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Manola, J.; Atkins, M.; Ibrahim, J.; Kirkwood, J. Prognostic factors in metastatic melanoma: A pooled analysis of Eastern Cooperative Oncology Group trials. J. Clin. Oncol. 2000, 18, 3782–3793. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tanikawa, T.; Kryczek, I.; Xia, H.; Li, G.; Wu, K.; Wei, S.; Zhao, L.; Vatan, L.; Wen, B. Aerobic Glycolysis Controls Myeloid-Derived Suppressor Cells and Tumor Immunity via a Specific CEBPB Isoform in Triple-Negative Breast Cancer. Cell Metab. 2018, 28, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Huang, Z.; Teng, F.; Xing, L.; Yu, J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev. 2015, 41, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Salvianti, F.; Pinzani, P.; Verderio, P.; Ciniselli, C.M.; Massi, D.; De Giorgi, V.; Grazzini, M.; Pazzagli, M.; Orlando, C. Multiparametric analysis of cell-free DNA in melanoma patients. PLoS ONE 2012, 7, e49843. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef] [PubMed]
- Daud, A.I.; Wolchok, J.D.; Robert, C.; Hwu, W.J.; Weber, J.S.; Ribas, A.; Hodi, F.S.; Joshua, A.M.; Kefford, R.; Hersey, P.; et al. Programmed Death-Ligand 1 Expression and Response to the Anti-Programmed Death 1 Antibody Pembrolizumab in Melanoma. J. Clin. Oncol. 2016, 34, 4102–4109. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, M.; Giampieri, R.; Loupakis, F.; Prochilo, T.; Salvatore, L.; Faloppi, L.; Bianconi, M.; Bittoni, A.; Aprile, G.; Zaniboni, A.; et al. Prognostic clinical factors in pretreated colorectal cancer patients receiving regorafenib: Implications for clinical management. Oncotarget 2015, 6, 33982–33992. [Google Scholar] [CrossRef]
- Armstrong, A.J.; George, D.J.; Halabi, S. Serum lactate dehydrogenase predicts for overall survival benefit in patients with metastatic renal cell carcinoma treated with inhibition of mammalian target of rapamycin. J. Clin. Oncol. 2012, 30, 3402–3417. [Google Scholar] [CrossRef]
- Faloppi, L.; Bianconi, M.; Giampieri, R.; Sobrero, A.; Labianca, R.; Ferrari, D.; Barni, S.; Aitini, E.; Zaniboni, A.; Boni, C.; et al. The value of lactate dehydrogenase serum levels as a prognostic and predictive factor for advanced pancreatic cancer patients receiving sorafenib. Oncotarget 2015, 6, 35087–35094. [Google Scholar] [CrossRef]
- Faloppi, L.; Scartozzi, M.; Bianconi, M.; Svegliati Baroni, G.; Toniutto, P.; Giampieri, R.; Del Prete, M.; De Minicis, S.; Bitetto, D.; Loretelli, M.; et al. The role of LDH serum levels in predicting global outcome in HCC patients treated with sorafenib: implications for clinical management. PLoS ONE 2012, 7, e32653. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Peiris-Pagés, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer metabolism: a therapeutic perspective. Nat. Rev. Clin. Oncol. 2017, 14, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Vernieri, C.; Casola, S.; Foiani, M.; Pietrantonio, F.; de Braud, F.; Longo, V. Targeting Cancer Metabolism: Dietary and Pharmacologic Interventions. Cancer Discov. 2016, 6, 1315–1333. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Shi, L.; Wang, B.; Yang, J.; Xiao, Z.; Du, P.; Wang, Q.; Yang, W. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: A systematic review and meta-analysis of 66 cohort studies. Cancer Treat. Rev. 2017, 58, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Freeman, G.J. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov. 2015, 5, 16–28. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Abbott, T.; Fishman, D.; McMurray, W.; Mor, G.; Stone, K.; Ward, D.; Williams, K.; Zhao, H. Comparison of statistical methods for classification of ovarian cancer using mass spectrometry data. Bioinformatics 2003, 19, 1636–1643. [Google Scholar] [CrossRef]
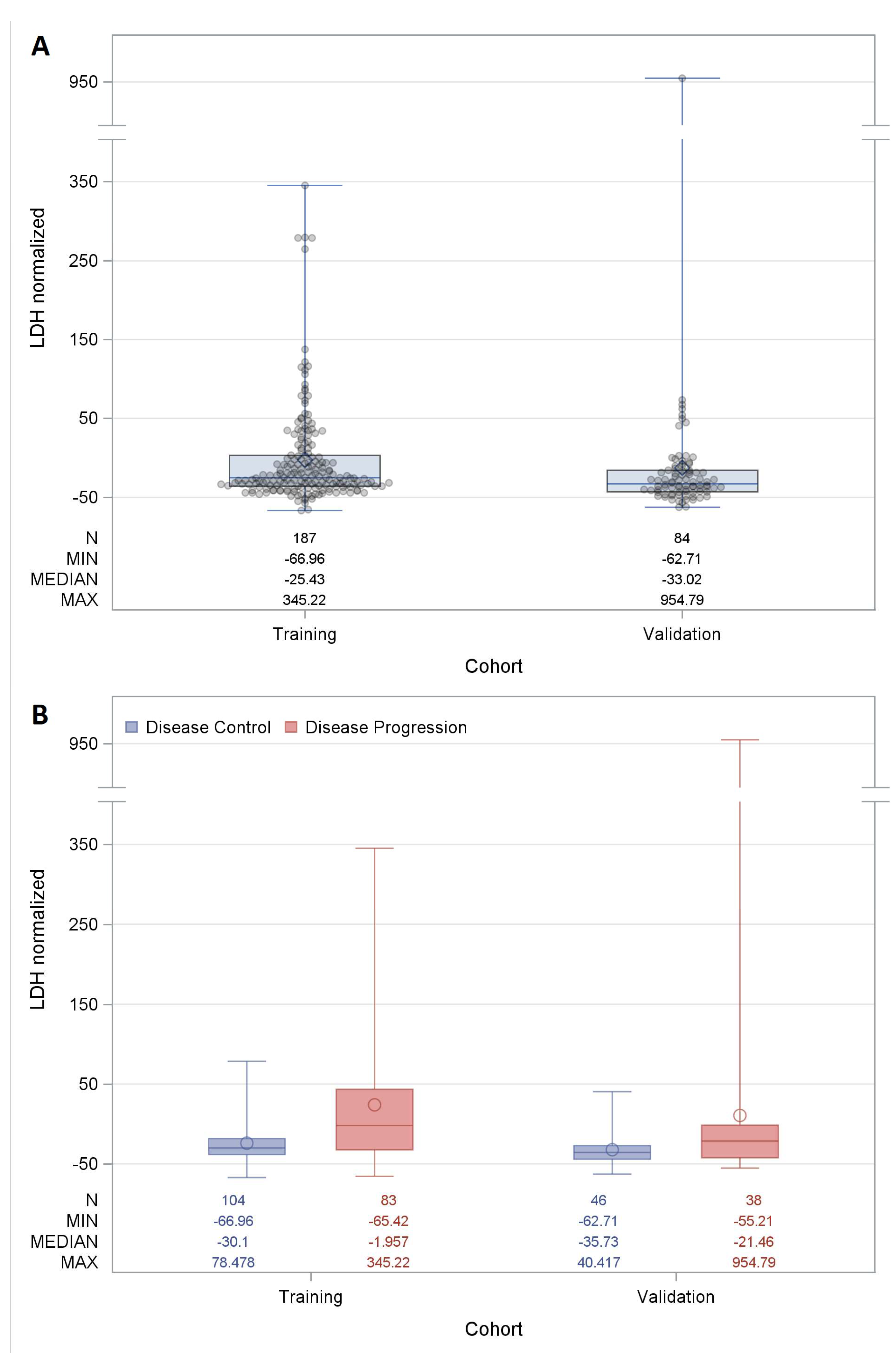
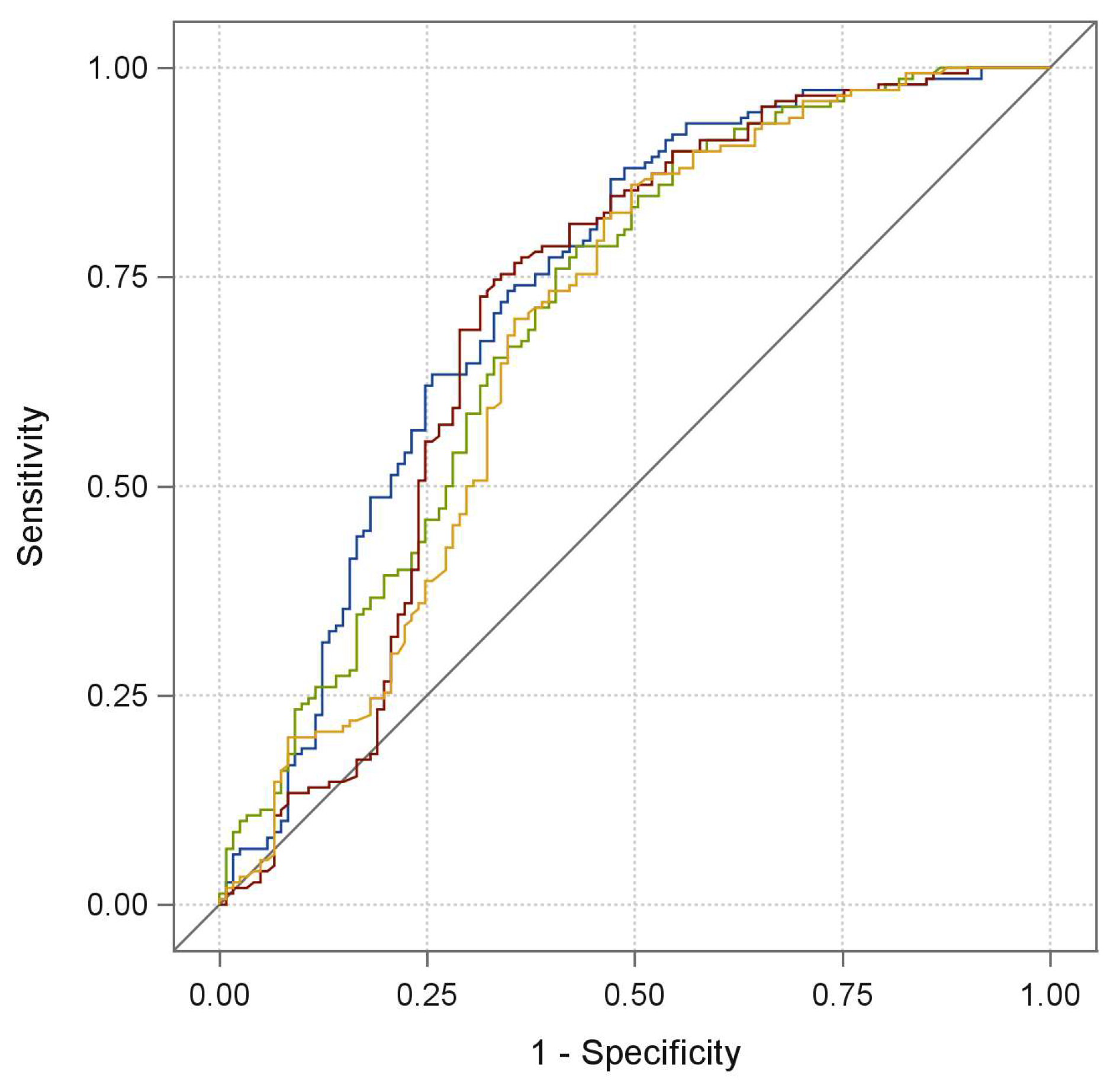
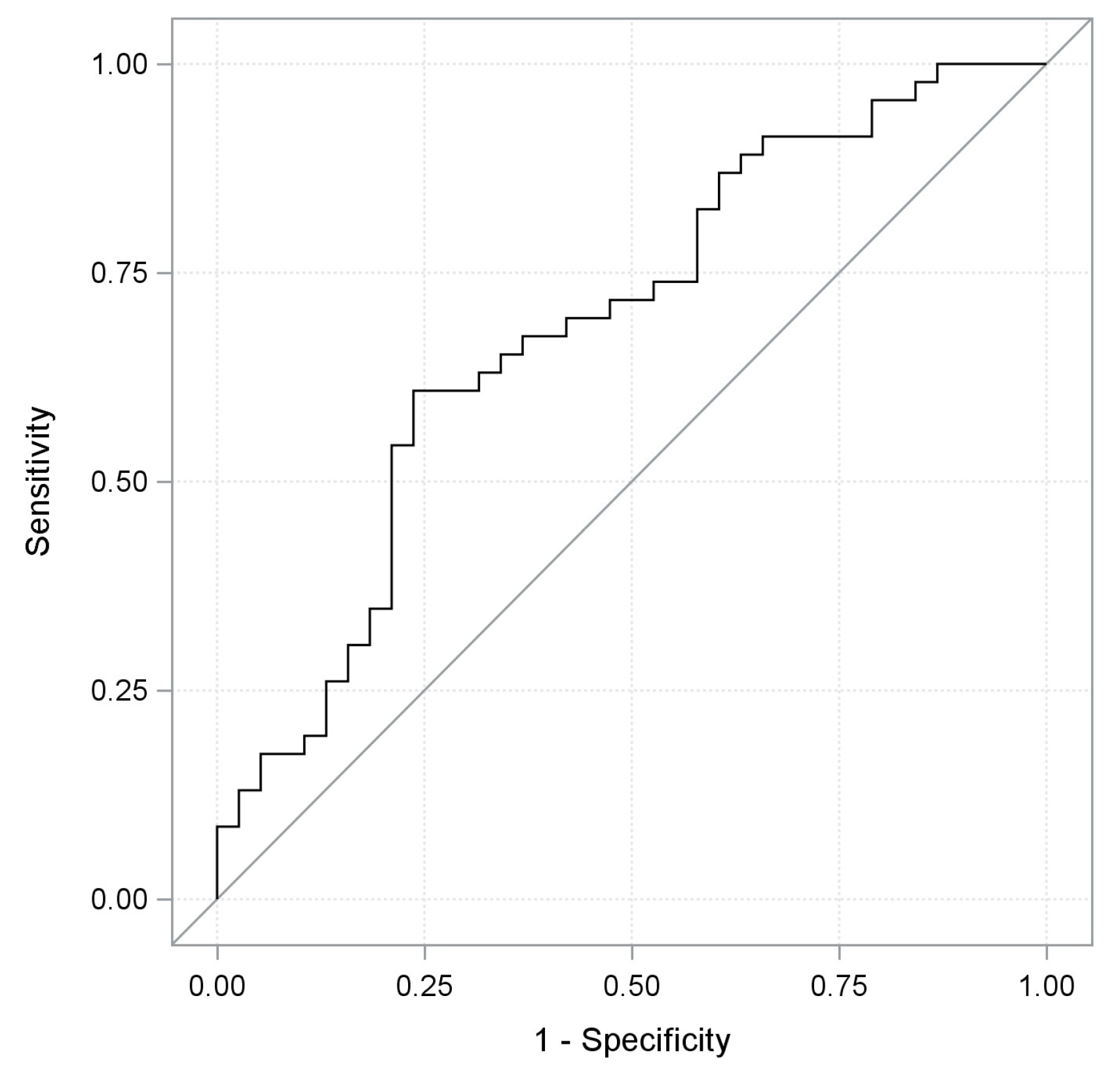
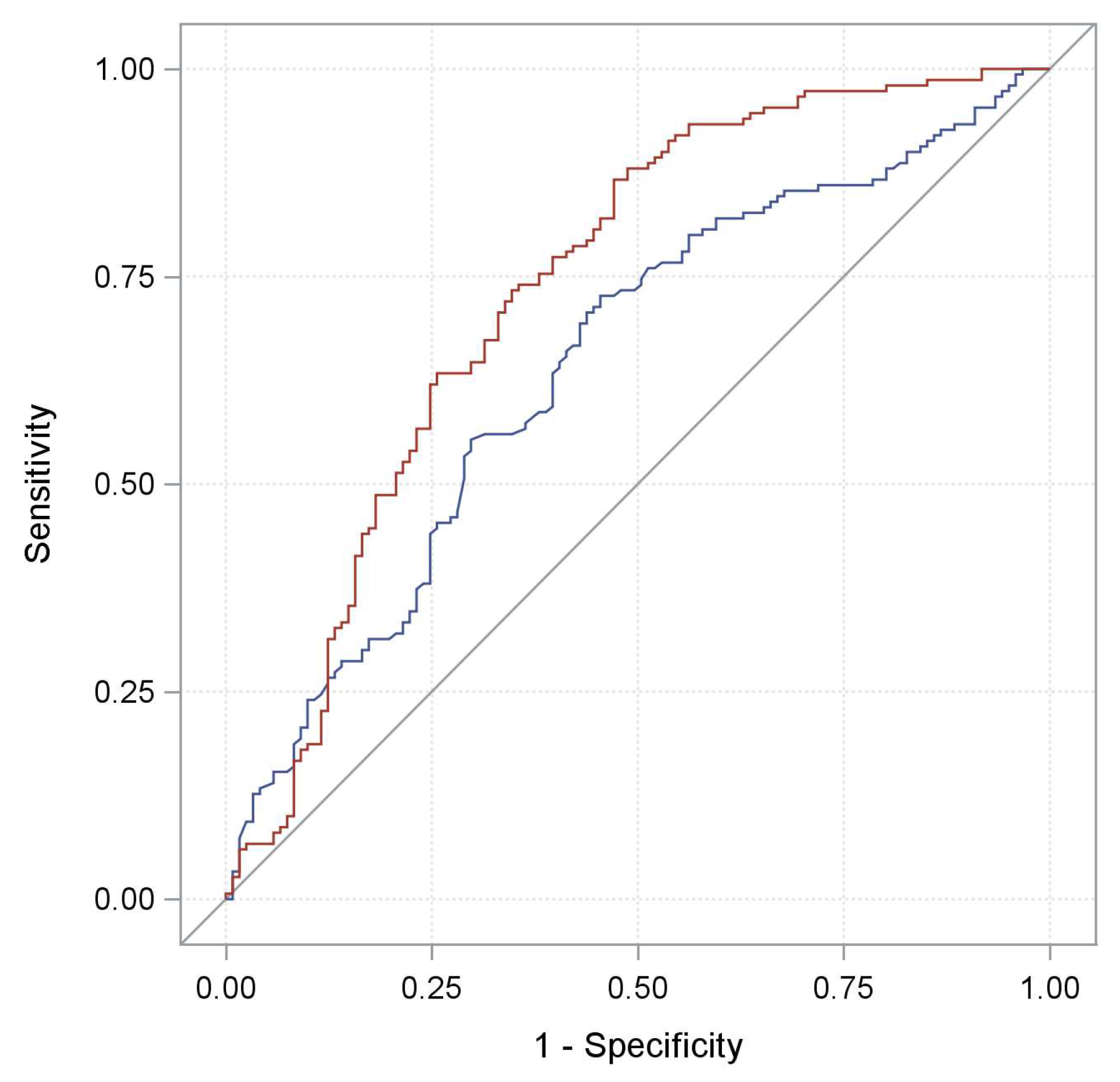
| Characteristics | Training Cohort | Validation Cohort | Total Cohort | |||
|---|---|---|---|---|---|---|
| Categorical Variables | Freq | % | Freq | % | Freq | % |
| Sex | ||||||
| Female | 78 | 41.71 | 32 | 38.1 | 110 | 40.59 |
| Male | 109 | 58.29 | 52 | 61.9 | 161 | 59.41 |
| Tumor Site | ||||||
| Melanoma | 36 | 19.25 | 24 | 28.57 | 60 | 22.14 |
| Lung | 93 | 49.73 | 24 | 28.57 | 117 | 43.17 |
| Others * | 58 | 31.02 | 36 | 42.86 | 94 | 34.69 |
| Treatment | ||||||
| PD-1 | 117 | 62.57 | 51 | 60.71 | 168 | 61.99 |
| PD-L1 | 70 | 37.43 | 33 | 39.29 | 103 | 38.01 |
| Line of therapy | ||||||
| 1 | 8 | 4.3 | 37 | 44.0 | 45 | 16.61 |
| 2 | 56 | 29.9 | 33 | 39.3 | 89 | 32.84 |
| ≥3 | 123 | 65.8 | 14 | 16.7 | 137 | 50.55 |
| Number of Metastatic sites | ||||||
| 1 | 31 | 16.6 | 11 | 13.1 | 42 | 15.5 |
| 2 | 83 | 44.4 | 37 | 44.0 | 120 | 44.28 |
| ≥3 | 73 | 39.0 | 36 | 42.9 | 109 | 40.22 |
| PS (ECOG) | ||||||
| 0 | 123 | 65.78 | 54 | 64.29 | 177 | 65.31 |
| ≥1 | 64 | 34.22 | 30 | 35.71 | 94 | 34.69 |
| Best Response | ||||||
| DC | 104 | 55.61 | 46 | 54.76 | 150 | 55.35 |
| DP | 83 | 44.39 | 38 | 45.24 | 121 | 44.65 |
| Continuos variables | Median | Range | Median | Range | Median | Range |
| Age, years | 61 | 16; 84 | 66 | 34; 83 | 62 | 16; 84 |
| N/L ratio | 3.44 | 0.65; 39.50 | 3.39 | 0.78; 28.33 | 3.44 | 0.65; 39.50 |
| LDH serum level | 353.00 | 152.00; 2048.00 | 321.50 | 179.00; 5063.00 | 343.00 | 152.00; 5063.00 |
| Effect | OR | 95% CI | |
|---|---|---|---|
| LDH normalized for a 10% increment | 0.810 | 0.744 | 0.883 |
| Age for a ten-years increment | 1.305 | 1.038 | 1.641 |
| PS (ECOG) 1 vs. 0 score | 0.481 | 0.274 | 0.846 |
| Variable | Value |
|---|---|
| Kit Characteristic | |
| Upper limit of normal reference range | 460 |
| Patients Characteristics | |
| LDH serum value | 77 |
| ECOG PS score [17] | 1 |
| Age | 60 |
| Estimated Probability % | 76.39 |
| PS (ECOG) | Baseline LDH | Age (years) | Estimated Probability of Response (%) | Best Response | Microsatellite Instability |
|---|---|---|---|---|---|
| 0 | 1063 | 60 | 8.21 | DP | Yes |
| 0 | 354 | 67 | 69.45 | SD | Yes |
| 1 | 925 | 47 | 5.29 | DP | No |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cona, M.S.; Lecchi, M.; Cresta, S.; Damian, S.; Del Vecchio, M.; Necchi, A.; Poggi, M.M.; Raggi, D.; Randon, G.; Ratta, R.; et al. Combination of Baseline LDH, Performance Status and Age as Integrated Algorithm to Identify Solid Tumor Patients with Higher Probability of Response to Anti PD-1 and PD-L1 Monoclonal Antibodies. Cancers 2019, 11, 223. https://doi.org/10.3390/cancers11020223
Cona MS, Lecchi M, Cresta S, Damian S, Del Vecchio M, Necchi A, Poggi MM, Raggi D, Randon G, Ratta R, et al. Combination of Baseline LDH, Performance Status and Age as Integrated Algorithm to Identify Solid Tumor Patients with Higher Probability of Response to Anti PD-1 and PD-L1 Monoclonal Antibodies. Cancers. 2019; 11(2):223. https://doi.org/10.3390/cancers11020223
Chicago/Turabian StyleCona, Maria Silvia, Mara Lecchi, Sara Cresta, Silvia Damian, Michele Del Vecchio, Andrea Necchi, Marta Maria Poggi, Daniele Raggi, Giovanni Randon, Raffaele Ratta, and et al. 2019. "Combination of Baseline LDH, Performance Status and Age as Integrated Algorithm to Identify Solid Tumor Patients with Higher Probability of Response to Anti PD-1 and PD-L1 Monoclonal Antibodies" Cancers 11, no. 2: 223. https://doi.org/10.3390/cancers11020223
APA StyleCona, M. S., Lecchi, M., Cresta, S., Damian, S., Del Vecchio, M., Necchi, A., Poggi, M. M., Raggi, D., Randon, G., Ratta, R., Signorelli, D., Vernieri, C., de Braud, F., Verderio, P., & Di Nicola, M. (2019). Combination of Baseline LDH, Performance Status and Age as Integrated Algorithm to Identify Solid Tumor Patients with Higher Probability of Response to Anti PD-1 and PD-L1 Monoclonal Antibodies. Cancers, 11(2), 223. https://doi.org/10.3390/cancers11020223







