Zoledronic Acid Modulation of TRPV1 Channel Currents in Osteoblast Cell Line and Native Rat and Mouse Bone Marrow-Derived Osteoblasts: Cell Proliferation and Mineralization Effect
Abstract
1. Introduction
2. Results
2.1. In Vitro Cell Viability Experiments on RAW264.7 and MC3T3-E1 Cell Lines
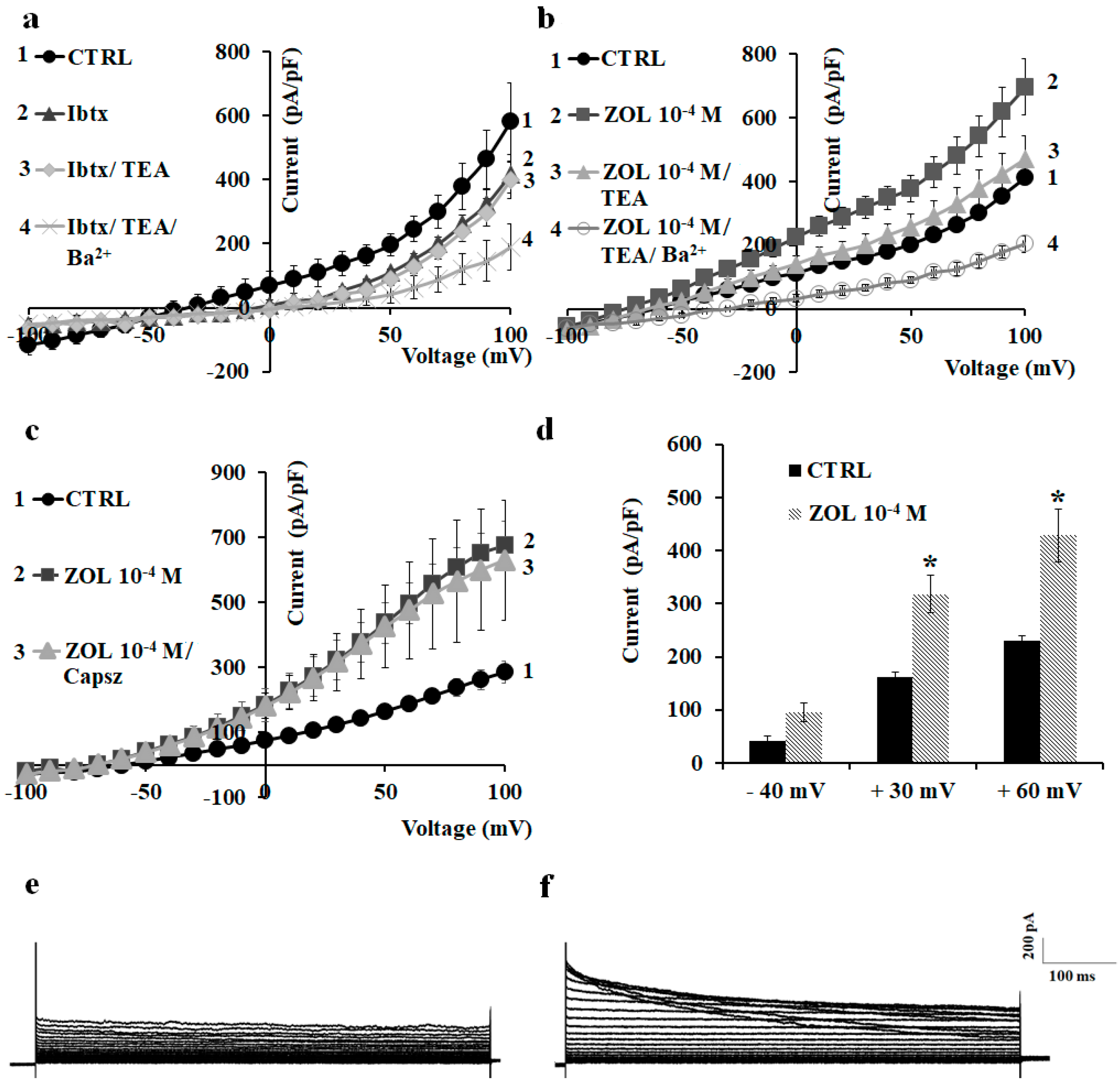
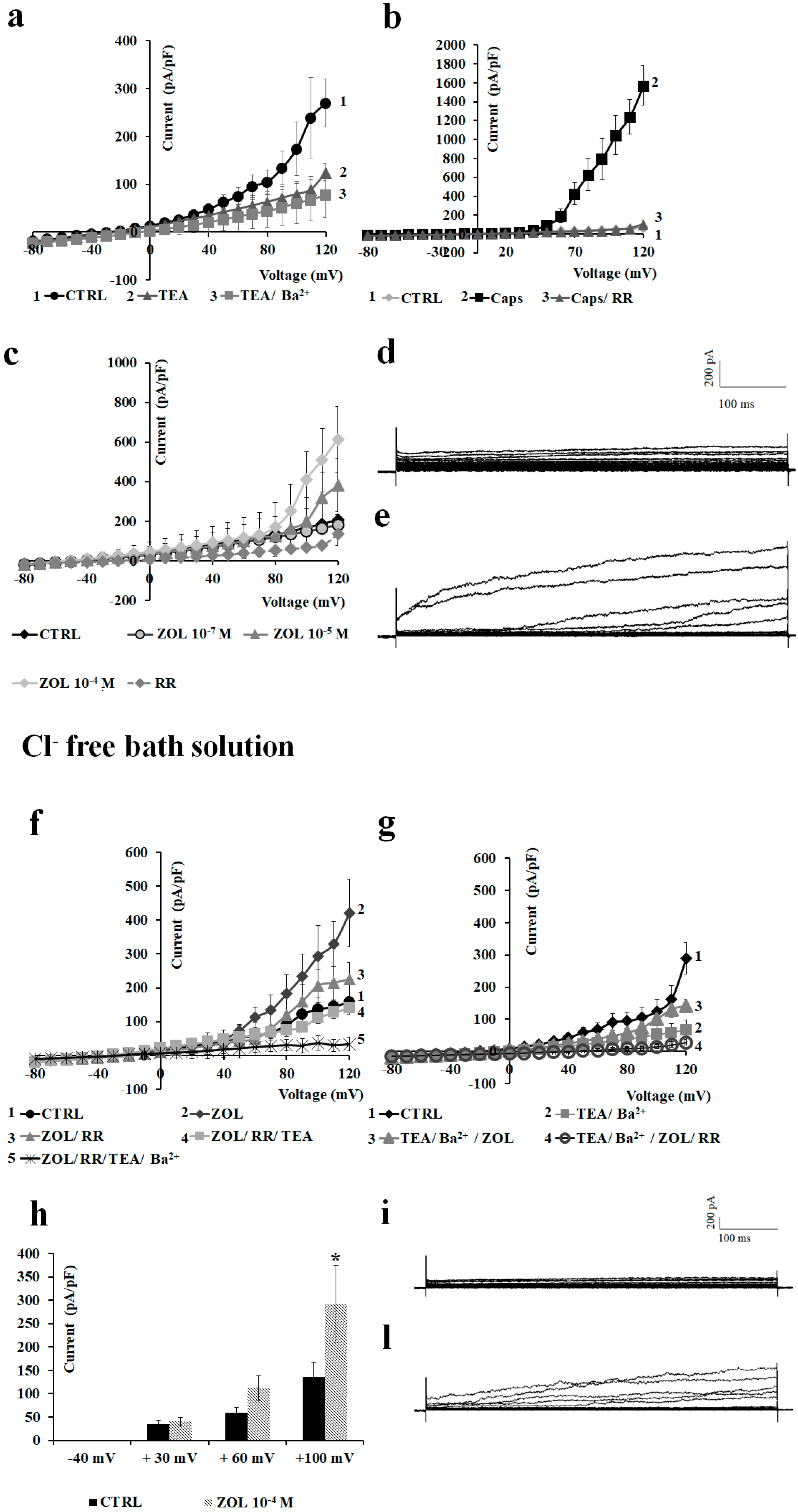
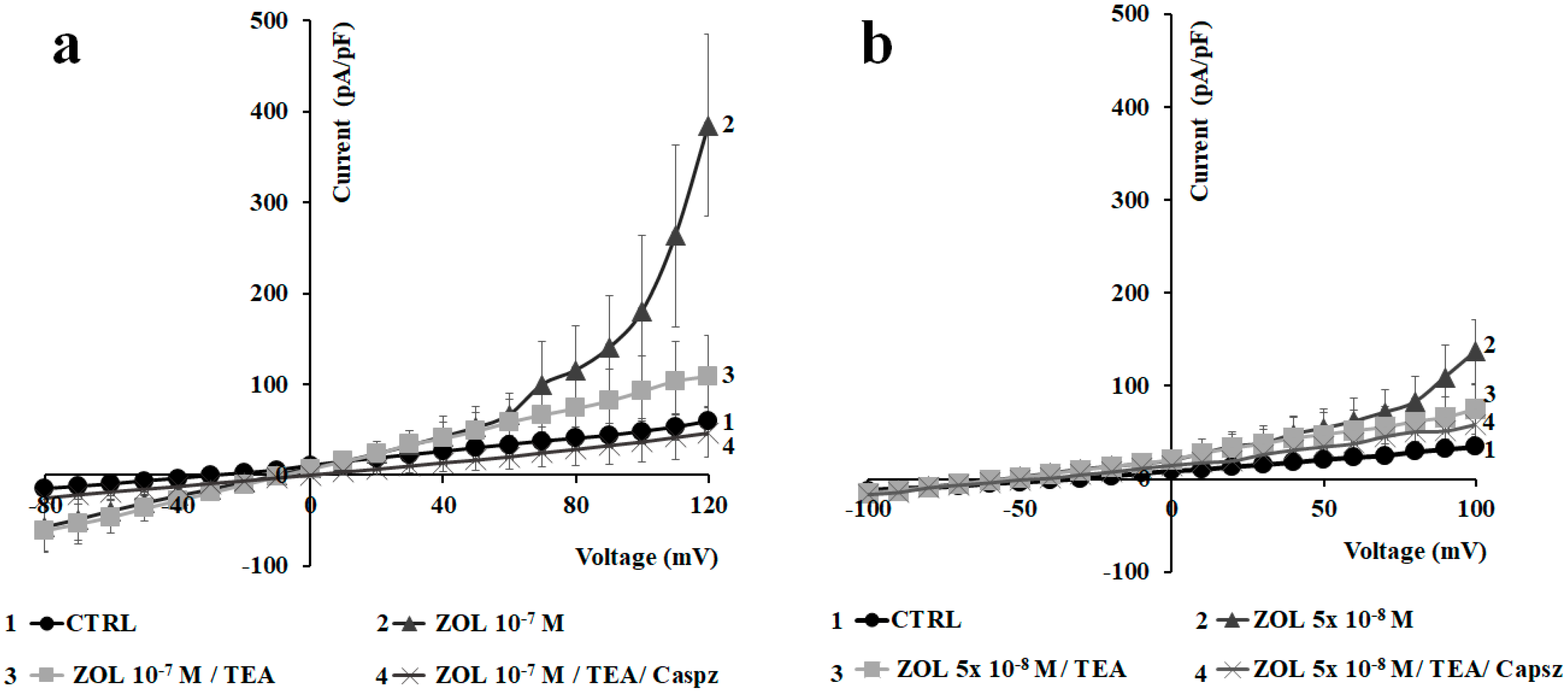
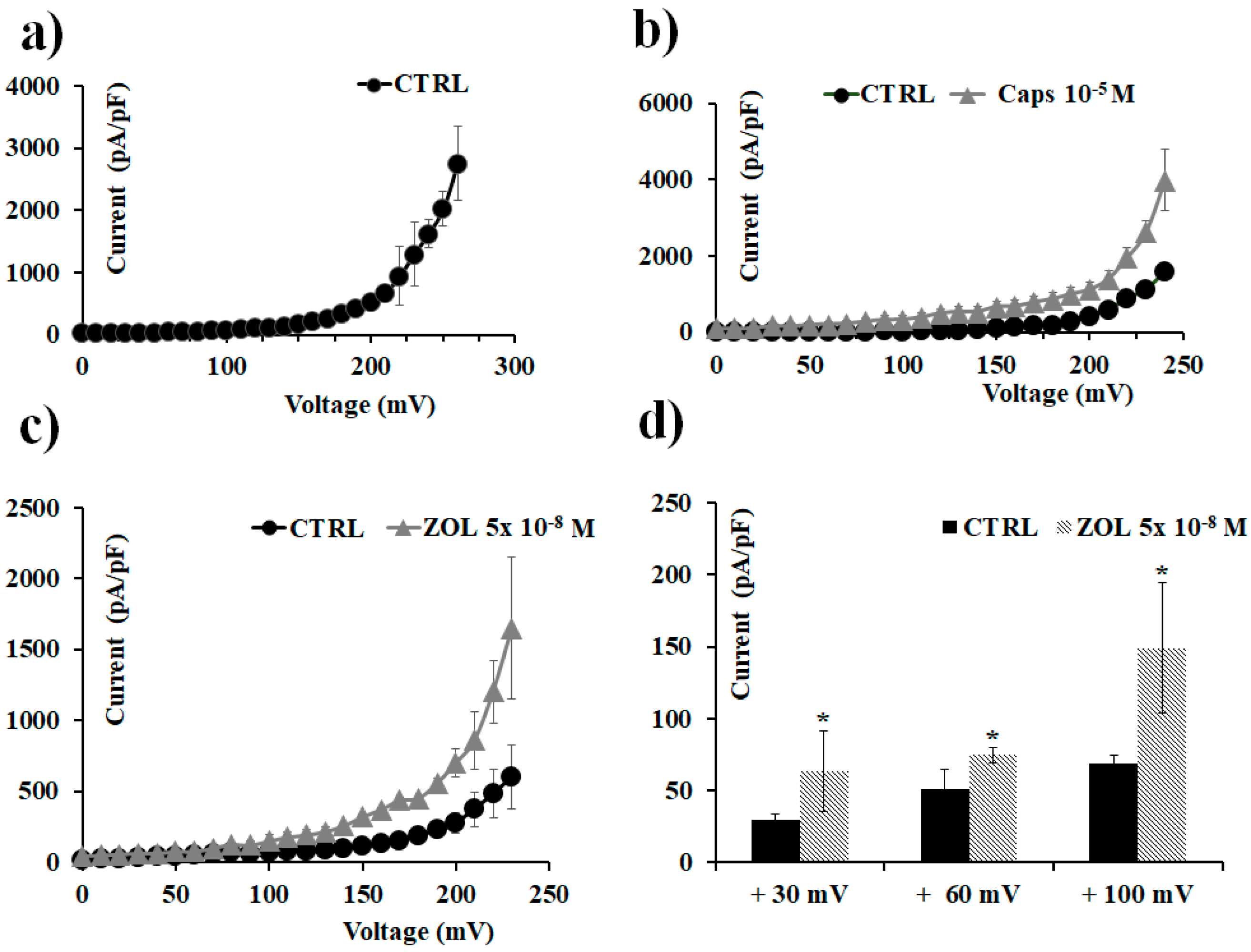
2.2. Characterization of Cation Channel Currents of RAW264.7 and MC3T3-E1 Cell Lines in Controls and in the Presence of ZOL
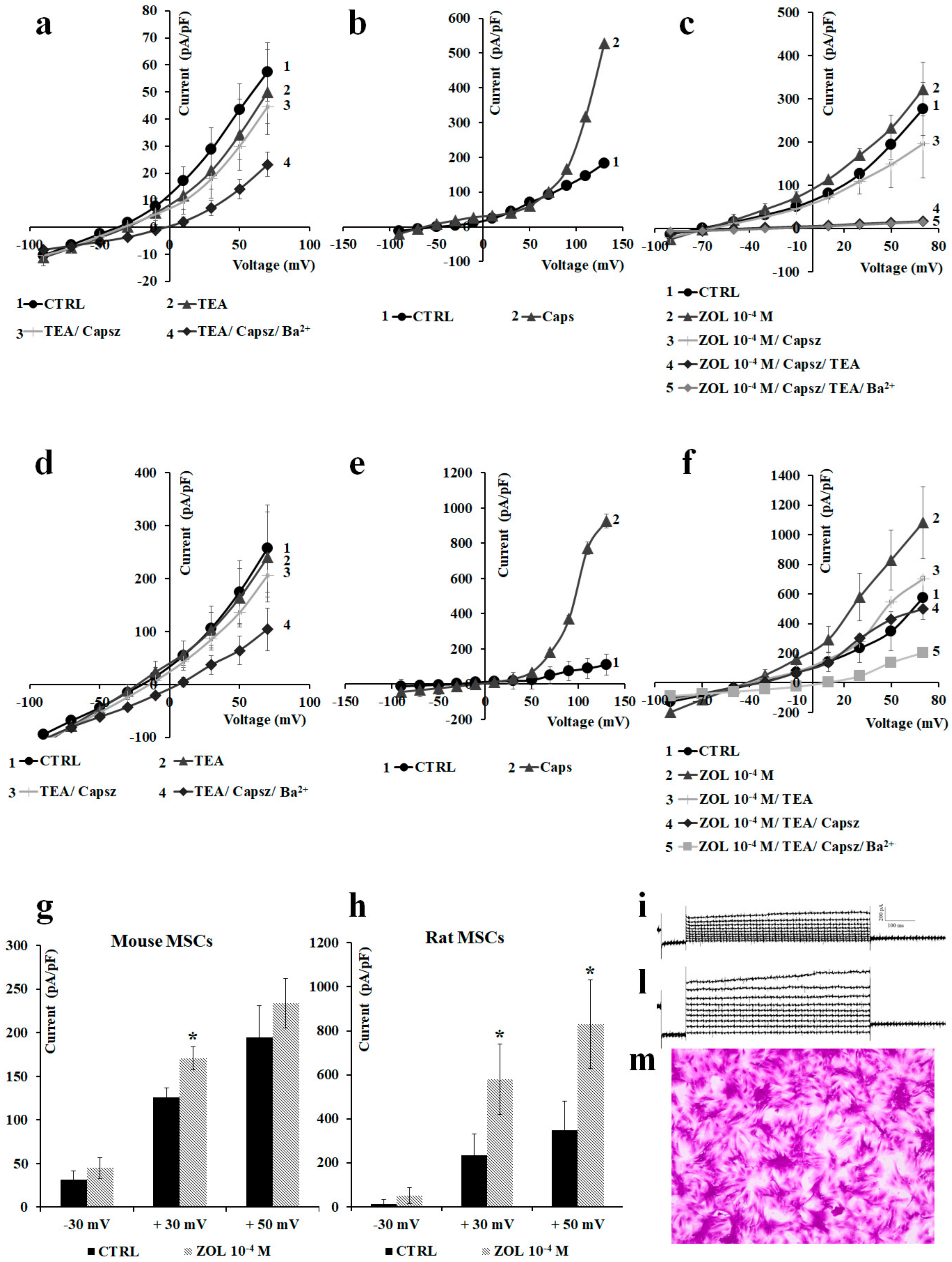
2.3. Pharmacological Characterization of the Whole-Cell Currents of Native Mesenchymal Stem Cells (MSCs) from Bone Marrow of Mouse and Rat
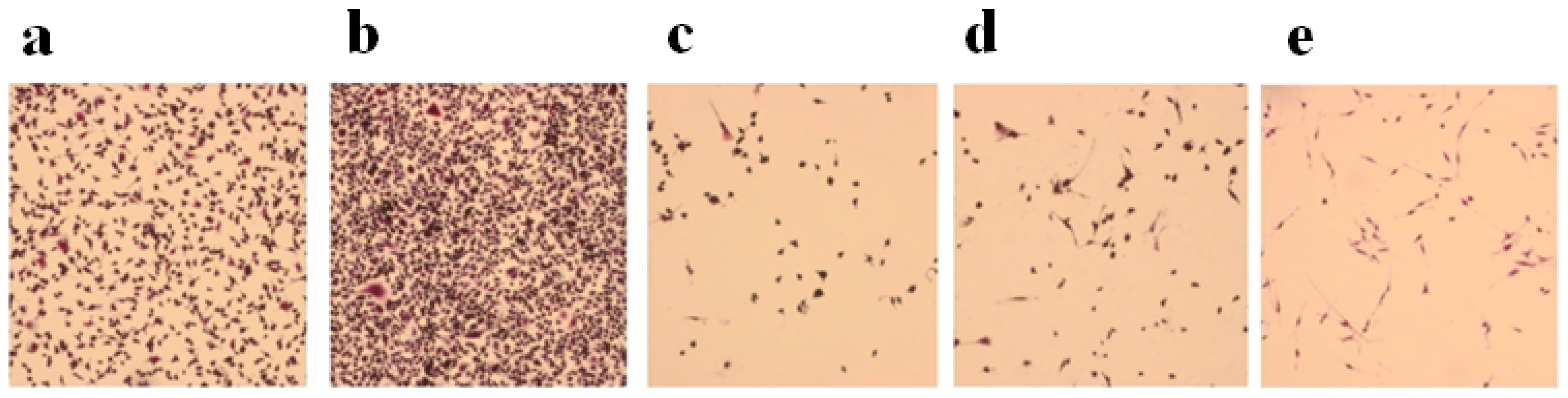
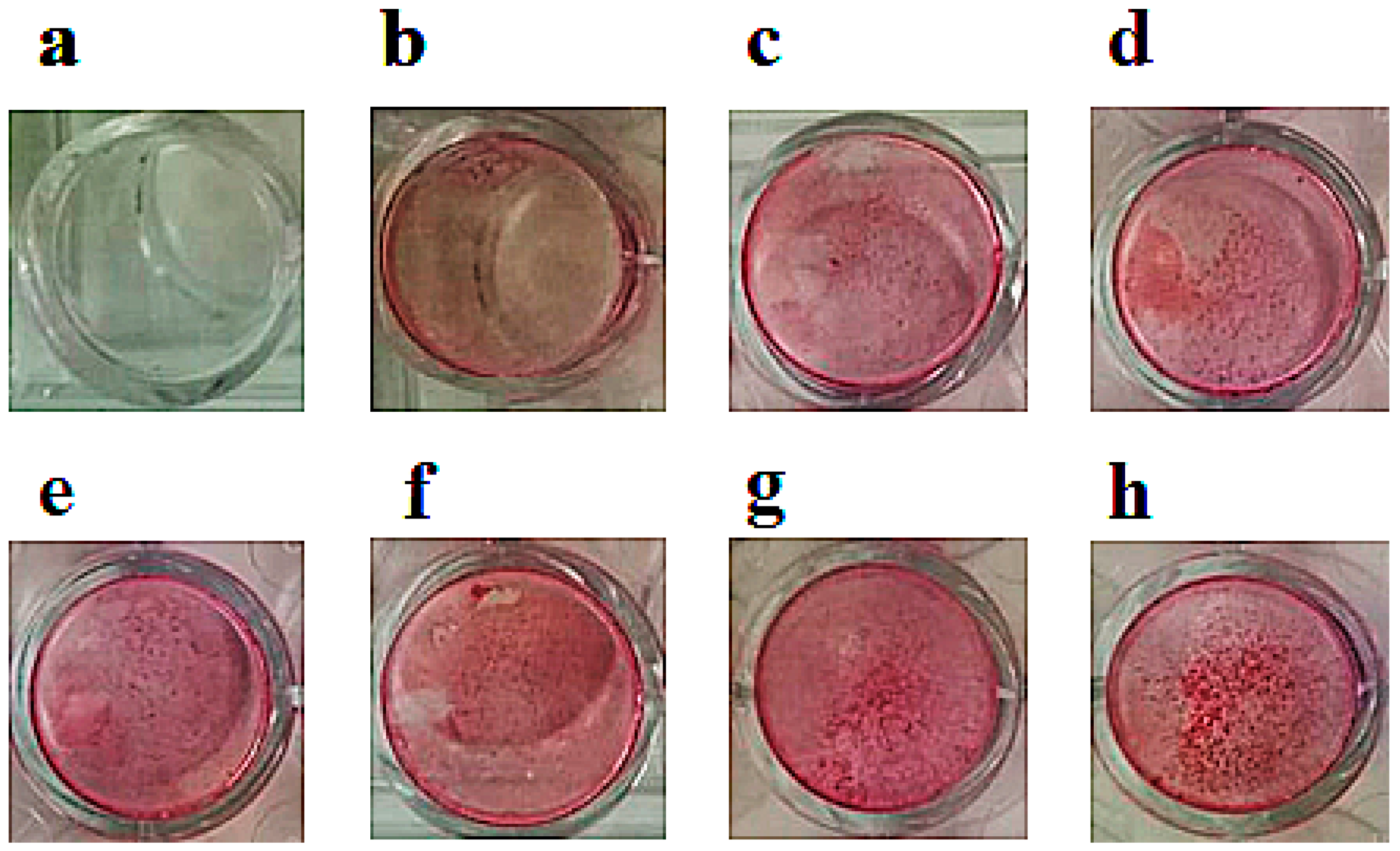
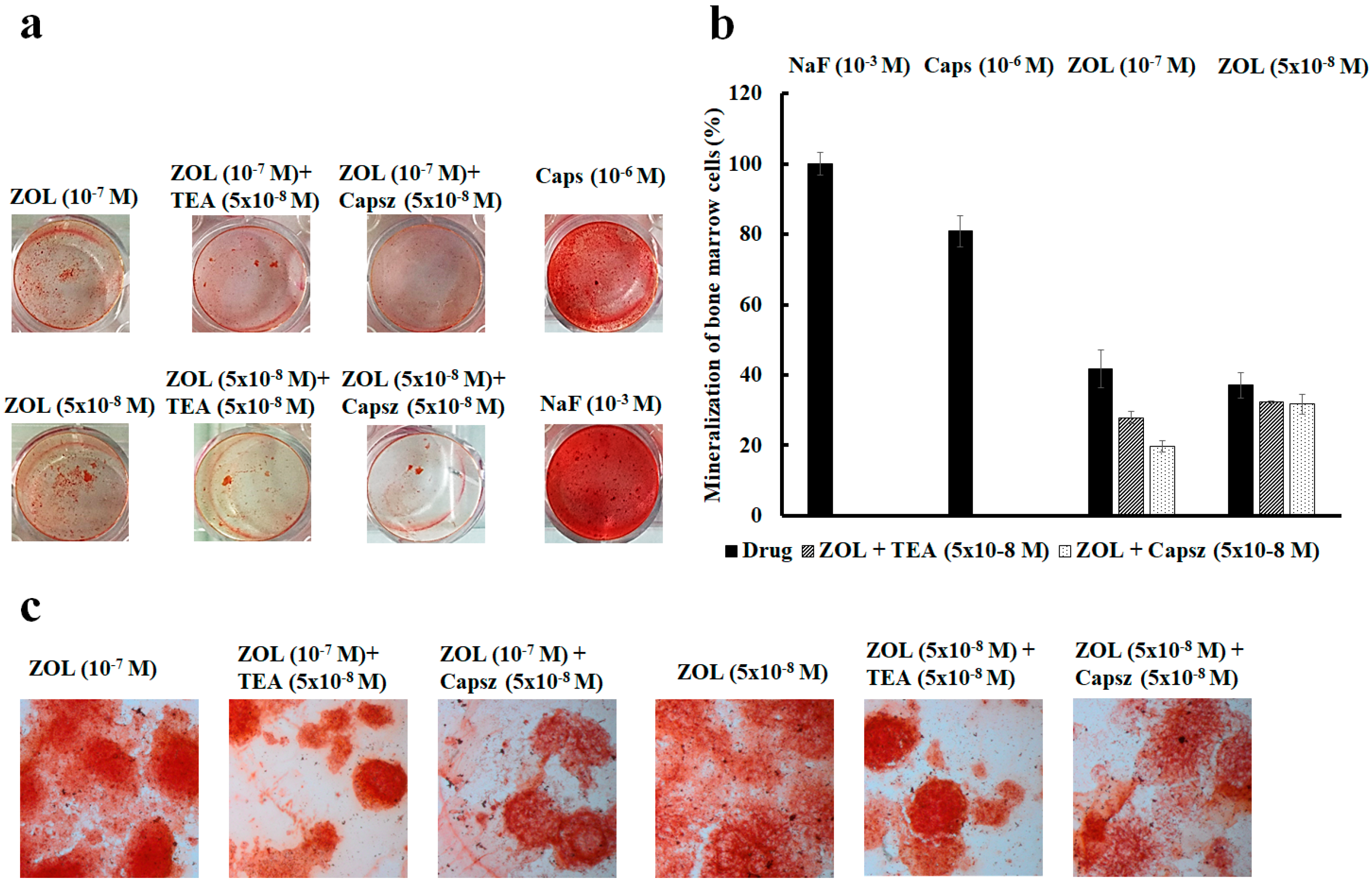
2.4. Effects of Ion Channel Modulators on ZOL-Induced Mineralization on Osteoblasts
2.5. ZOL Effects on Oocytes Transfected with TRPV1 Channel Clone
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Ex-Vivo Culture of Native Mouse and Rat Mesenchymal Stem Cells
4.3. Ethical Statements
4.4. CCK-8 Intracellular Dehydrogenase Assay
4.5. Mineralization Assay
4.6. Osteoclastogenesis Assay
4.7. Drugs and Solutions
4.8. Whole-Cell Recordings in the Cells
4.9. Heterologous Protein Expression Electrophysiology
4.10. Data Analysis and Statistics
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Y.-C.; Sosnoski, D.M.; Mastro, A.M. Breast cancer metastasis to the bone: Mechanisms of bone loss. Breast Cancer Res. 2010, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Waning, D.L.; Guise, T.A. Molecular mechanisms of bone metastasis and associated muscle weakness. Clin. Cancer Res. 2014, 20, 3071–3077. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone metastases: An overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.M.; Zemel, B.S.; Wren, T.A.L.; Leonard, M.B.; Bachrach, L.K.; Rauch, F.; Gilsanz, V.; Rosen, C.J.; Winer, K.K. The Determinants of Peak Bone Mass. J. Pediatr. 2017, 180, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.G.G. Bisphosphonates: Mode of action and pharmacology. Pediatrics 2017, 119, S150–S162. [Google Scholar] [CrossRef] [PubMed]
- Savino, S.; Toscano, A.; Purgatorio, R.; Profilo, E.; Laghezza, A.; Tortorella, P.; Angelelli, M.; Cellamare, S.; Scala, R.; Tricarico, D.; et al. Novel bisphosphonates with antiresorptive effect in bone mineralization and osteoclastogenesis. Eur. J. Med. Chem. 2018, 158, 184–200. [Google Scholar] [CrossRef]
- Guenther, A.; Gordon, S.; Tiemann, M.; Burger, R.; Bakker, F.; Green, J.R.; Baum, W.; Roelofs, A.J.; Rogers, M.J.; Gramatzki, M. The bisphosphonate zoledronic acid has antimyeloma activity in vivo by inhibition of protein prenylation. Int. J. Cancer 2010, 126, 239–246. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Manolagas, S.C.; Bellido, T. Transduction of cell survival signals by connexin-43 hemichannels. J. Biol. Chem. 2002, 277, 8648–8657. [Google Scholar] [CrossRef]
- Tenenbaum, H.C.; Torontali, M.; Sukhu, B. Effects of bisphosphonates and inorganic pyrophosphate on osteogenesis in vitro. Bone 1992, 13, 249–255. [Google Scholar] [CrossRef]
- Kellinsalmi, M.; Monkkonen, H.; Monkkonen, J.; Leskela, H.-V.; Parikka, V.; Hamalainen, M.; Lehenkari, P. In vitro comparison of clodronate, pamidronate and zoledronic acid effects on rat osteoclasts and human stem cell-derived osteoblasts. Basic Clin. Pharmacol. Toxicol. 2005, 97, 382–391. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, L.I.; Lezcano, V.; Thostenson, J.; Weinstein, R.S.; Manolagas, S.C.; Bellido, T. Connexin 43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo. J. Bone Miner. Res. 2008, 23, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Bilbao, P.S.; Katz, S.; Lezcano, V.; Roldán, E.; Boland, R.; Santillan, G. Protein phosphatases: Possible bisphosphonate binding sites mediating stimulation of osteoblast proliferation. Arch. Biochem. Biophys. 2011, 507, 248–253. [Google Scholar] [CrossRef]
- Henney, N.C.; Li, B.; Elford, C.; Reviriego, P.; Campbell, A.K.; Wann, K.T.; Evans, B.A.J. A large-conductance (BK) potassium channel subtype affects both growth and mineralization of human osteoblasts. Am. J. Physiol. Physiol. 2009, 297, C1397–C1408. [Google Scholar] [CrossRef] [PubMed]
- Yellowley, C.E.; Hancox, J.C.; Skerry, T.M.; Levi, A.J. Whole-cell membrane currents from human osteoblast-like cells. Calcif. Tissue Int. 1998, 62, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Preston, M.R.; El Haj, A.J.; Howl, J.D.; Publicover, S.J. Three types of K(+) currents in murine osteocyte-like cells (MLO-Y4). Bone 2001, 28, 29–37. [Google Scholar] [CrossRef]
- Vigneault, P.; Naud, P.; Qi, X.; Xiao, J.; Villeneuve, L.; Davis, D.R.; Nattel, S. Calcium-dependent potassium channels control proliferation of cardiac progenitor cells and bone marrow-derived mesenchymal stem cells. J. Physiol. 2018, 596, 2359–2379. [Google Scholar] [CrossRef]
- Pini, J.; Giuliano, S.; Matonti, J.; Gannoun, L.; Simkin, D.; Rouleau, M.; Bendahhou, S. Osteogenic and Chondrogenic Master Genes Expression Is Dependent on the Kir2.1 Potassium Channel Through the Bone Morphogenetic Protein Pathway. J. Bone Miner. Res. 2018, 33, 1826–1841. [Google Scholar] [CrossRef]
- Maqoud, F.; Curci, A.; Scala, R.; Pannunzio, A.; Campanella, F.; Coluccia, M.; Passantino, G.; Zizzo, N.; Tricarico, D. Cell Cycle Regulation by Ca(2+)-Activated K(+) (BK) Channels Modulators in SH-SY5Y Neuroblastoma Cells. Int. J. Mol. Sci. 2018, 19, 2442. [Google Scholar] [CrossRef] [PubMed]
- Abed, E.; Labelle, D.; Martineau, C.; Loghin, A.; Moreau, R. Expression of transient receptor potential (TRP) channels in human and murine osteoblast-like cells. Mol. Membr. Biol. 2009, 26, 146–158. [Google Scholar] [CrossRef]
- Lieben, L.; Carmeliet, G. The involvement ofTRP channels in bone homeostasis. Front. Endocrinol. (Lausanne) 2012, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- He, L.-H.; Liu, M.; He, Y.; Xiao, E.; Zhao, L.; Zhang, T.; Yang, H.-Q.; Zhang, Y. TRPV1 deletion impaired fracture healing and inhibited osteoclast and osteoblast differentiation. Sci. Rep. 2017, 7, 42385. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.I.; Landao-Bassonga, E.; Ralston, S.H. The TRPV1 ion channel antagonist capsazepine inhibits osteoclast and osteoblast differentiation in vitro and ovariectomy induced bone loss in vivo. Bone 2010, 46, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Mikami, R.; Mizutani, K.; Aoki, A.; Tamura, Y.; Aoki, K.; Izumi, Y. Low-level ultrahigh-frequency and ultrashort-pulse blue laser irradiation enhances osteoblast extracellular calcification by upregulating proliferation and differentiation via transient receptor potential vanilloid 1. Lasers Surg. Med. 2018, 50, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis, T.; van der Eerden, B.C.J.; Hoenderop, J.G.J.; Weinans, H.; van Leeuwen, J.P.T.M.; Bindels, R.J.M. Bone resorption inhibitor alendronate normalizes the reduced bone thickness of TRPV5(-/-) mice. J. Bone Miner. Res. 2008, 23, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Hoenderop, J.G.J.; Nilius, B.; Bindels, R.J.M. Epithelial calcium channels: From identification to function and regulation. Pflugers Arch. 2003, 446, 304–308. [Google Scholar] [CrossRef]
- Abed, E.; Martineau, C.; Moreau, R. Role of melastatin transient receptor potential 7 channels in the osteoblastic differentiation of murine MC3T3 cells. Calcif. Tissue Int. 2011, 88, 246–253. [Google Scholar] [CrossRef]
- Chen, C.-W.; Lee, S.T.; Wu, W.T.; Fu, W.-M.; Ho, F.-M.; Lin, W.W. Signal transduction for inhibition of inducible nitric oxide synthase and cyclooxygenase-2 induction by capsaicin and related analogs in macrophages. Br. J. Pharmacol. 2003, 140, 1077–1087. [Google Scholar] [CrossRef]
- Yamashiro, K.; Sasano, T.; Tojo, K.; Namekata, I.; Kurokawa, J.; Sawada, N.; Suganami, T.; Kamei, Y.; Tanaka, H.; Tajima, N.; et al. Role of transient receptor potential vanilloid 2 in LPS-induced cytokine production in macrophages. Biochem. Biophys. Res. Commun. 2010, 398, 284–289. [Google Scholar] [CrossRef]
- Tsuji, F.; Murai, M.; Oki, K.; Seki, I.; Ueda, K.; Inoue, H.; Nagelkerken, L.; Sasano, M.; Aono, H. Transient receptor potential vanilloid 1 agonists as candidates for anti-inflammatory and immunomodulatory agents. Eur. J. Pharmacol. 2010, 627, 332–339. [Google Scholar] [CrossRef]
- Sooampon, S.; Manokawinchoke, J.; Pavasant, P. Transient receptor potential vanilloid-1 regulates osteoprotegerin/RANKL homeostasis in human periodontal ligament cells. J. Periodontal Res. 2013, 48, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Watanabe, K.; Yokoyama, S.; Matsumoto, C.; Hirata, M.; Tominari, T.; Inada, M.; Miyaura, C. Capsaicin, a TRPV1 Ligand, Suppresses Bone Resorption by Inhibiting the Prostaglandin E Production of Osteoblasts, and Attenuates the Inflammatory Bone Loss Induced by Lipopolysaccharide. ISRN Pharmacol. 2012, 2012, 439860. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Nadri, S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc. 2009, 4, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Curci, A.; Maqoud, F.; Mele, A.; Cetrone, M.; Angelelli, M.; Zizzo, N.; Tricarico, D. Antiproliferative effects of neuroprotective drugs targeting big Ca2+-activated K+ (BK) channel in the undifferentiated neuroblastoma cells. Curr. Top. Pharmacol. 2016, 20, 113–131, EID: 2-s2.0-85019677647. [Google Scholar]
- Wu, Y.; Liu, Y.; Hou, P.; Yan, Z.; Kong, W.; Liu, B.; Li, X.; Yao, J.; Zhang, Y.; Qin, F.; et al. TRPV1 Channels Are Functionally Coupled with BK(mSlo1) Channels in Rat Dorsal Root Ganglion (DRG) Neurons. PLoS ONE 2013, 8, e78203. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.J.; Fernandes, M.H. Human bone cell cultures in biocompatibility testing. Part II: Effect of ascorbic acid, beta-glycerophosphate and dexamethasone on osteoblastic differentiation. Biomaterials 2000, 21, 1095–1102. [Google Scholar] [CrossRef]
- Cheng, S.L.; Lai, C.F.; Blystone, S.D.; Avioli, L.V. Bone mineralization and osteoblast differentiation are negatively modulated by integrin alpha(v)beta3. J. Bone Miner. Res. 2001, 16, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Office of Animal Care and Use (OACU), NIH. Guidelines for Egg and Oocyte Harvesting in Xenopus laevis. Animal Research Advisory Committee Guidelines. Approved 06/12/1996, Revised 10/26/2016. Available online: http://oacu.od.nih.gov/ARAC/documents/Oocyte_Harvest.pdf (accessed on 12 July 2017).
- Dinardo, M.M.; Camerino, G.M.; Mele, A.; Latorre, R.; Conte Camerino, D.; Tricarico, D. Splicing of the rSlo gene affects the molecular composition and drug response of Ca2+-activated K+ channels in skeletal muscle. PLoS ONE 2012, 7, e40235. [Google Scholar] [CrossRef]
- Mele, A.; Buttiglione, M.; Cannone, G.; Vitiello, F.; Camerino, D.C.; Tricarico, D. Opening/blocking actions of pyruvate kinase antibodies on neuronal and muscular KATP channels. Pharmacol. Res. 2012, 66, 401–408. [Google Scholar] [CrossRef]
- Tricarico, D.; Mele, A.; Calzolaro, S.; Cannone, G.; Camerino, G.M.; Dinardo, M.M.; Latorre, R.; Conte Camerino, D. Emerging Role of Calcium-Activated Potassium Channel in the Regulation of Cell Viability Following Potassium Ions Challenge in HEK293 Cells and Pharmacological Modulation. PLoS ONE 2013, 8, e69551. [Google Scholar] [CrossRef]
- Tricarico, D.; Mele, A.; Conte Camerino, D. Carbonic anhydrase inhibitors ameliorate the symptoms of hypokalaemic periodic paralysis in rats by opening the muscular Ca2+-activated-K+ channels. Neuromuscul. Disord. 2006, 16, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, D.; Lovaglio, S.; Mele, A.; Rotondo, G.; Mancinelli, E.; Meola, G.; Conte Camerino, D. Acetazolamide prevents vacuolar myopathy in skeletal muscle of K+-depleted rats. Br. J. Pharmacol. 2008, 154, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Poblete, H.; Oyarzún, I.; Olivero, P.; Comer, J.; Zuñiga, M.; Sepulveda, R.V.; Báez-Nieto, D.; Leon, C.G.; González-Nilo, F.; Latorre, R. Molecular determinants of phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) binding to transient receptor potential V1 (TRPV1) channels. J. Biol. Chem. 2015, 290, 2086–2098. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, D.; Capriulo, R.; Conte Camerino, D. Involvement of KCa2+ channels in the local abnormalities and hyperkalemia following the ischemia-reperfusion injury of rat skeletal muscle. Neuromuscul. Disord. 2002, 12, 258–265. [Google Scholar] [CrossRef]
- Tricarico, D.; Barbieri, M.; Antonio, L.; Tortorella, P.; Loiodice, F.; Conte Camerino, D. Dualistic actions of cromakalim and new potent 2H-1,4-benzoxazine derivatives on the native skeletal muscle K ATP channel. Br. J. Pharmacol. 2003, 139, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, D.; Montanari, L.; Conte Camerino, D. Involvement of 3Na+/2K+ ATP-ase and Pi-3 kinase in the response of skeletal muscle ATP-sensitive K+ channels to insulin. Neuromuscul. Disord. 2003, 13, 712–719. [Google Scholar] [CrossRef]
- Castillo, J.P.; Sanchez-Rodriguez, J.E.; Hyde, H.C.; Zaelzer, C.A.; Aguayo, D.; Sepulveda, R.V.; Luk, L.Y.P.; Kent, S.B.H.; Gonzalez-Nilo, F.D.; Bezanilla, F.; et al. beta1-subunit-induced structural rearrangements of the Ca2+- and voltage-activated K+ (BK) channel. Proc. Natl. Acad. Sci. USA 2016, 113, E3231–E3239. [Google Scholar] [CrossRef]
- Castillo, K.; Contreras, G.F.; Pupo, A.; Torres, Y.P.; Neely, A.; González, C.; Latorre, R. Molecular mechanism underlying β1 regulation in voltage- and calcium-activated potassium (BK) channels. Proc. Natl. Acad. Sci. USA 2015, 112, 4809–4814. [Google Scholar] [CrossRef]
- Armstrong, C.M.; Bezanilla, F. Currents related to movement of the gating particles of the sodium channels. Nature 1973, 242, 459–461. [Google Scholar] [CrossRef]
- Ito, N.; Ruegg, U.T.; Kudo, A.; Miyagoe-Suzuki, Y.; Takeda, S. Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat. Med. 2013, 19, 101–106. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, L.; Xu, Y.; Yang, K.; Luo, L.; Wang, L.; Li, Y.; Wang, J.; Shu, G.; Wang, S.; et al. Diversity effect of capsaicin on different types of skeletal muscle. Mol. Cell. Biochem. 2018, 443, 11–23. [Google Scholar] [CrossRef] [PubMed]
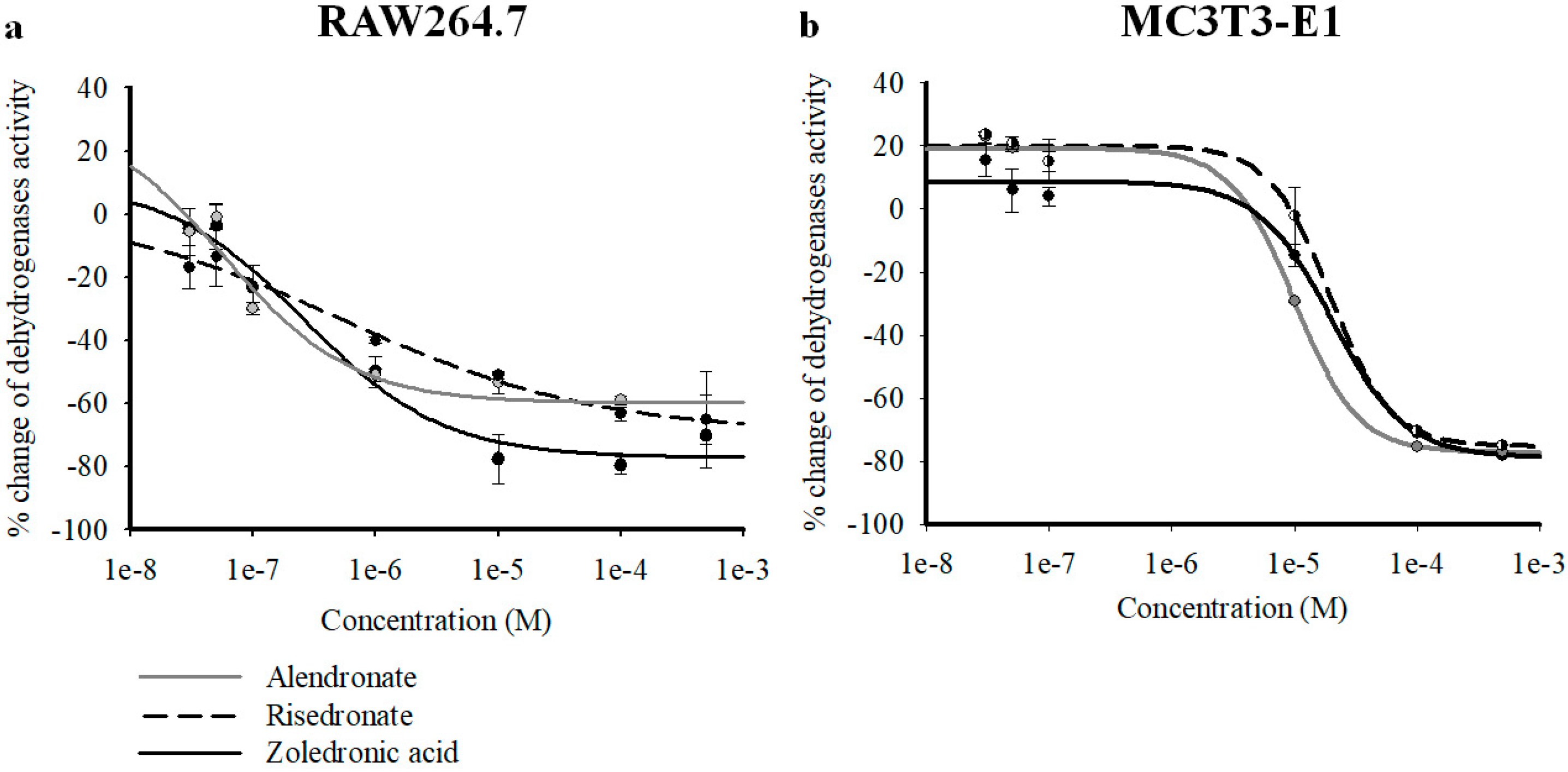
| Drugs | RAW264.7 Emax (%) | RAW264.7 IC50 (M) | RAW264.7 Hill Slope | MC3T3-E1 Emax (%) | MC3T3-E1 IC50 (M) | MC3T3-E1 Hill Slope |
|---|---|---|---|---|---|---|
| ZOL | −77.07 ± 5.63 | 2.62 × 10−7 ± 3.21 × 10−8 | 0.77 ± 0.1 | −78.88 ± 7.54 | 2.02 × 10−5 ± 7.70 × 10−6 | 1.44 ± 0.19 |
| ALE | −59.77 ± 5.64 * | 5.87 × 10−8 ± 1.64 × 10−9 | 0.82 ± 0.1 | −77.28 ± 3.49 | 9.98 × 10−6 ± 1.07 × 10−7 | 1.67 ± 0.11 |
| RIS | −69.25 ± 9.57 | 5.35 × 10−7 ± 1.08 × 10−8 | 0.41 ± 0.08 | −75.33 ± 5 | 1.97 × 10−5 ± 5.45 × 10−7 | 1.75 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scala, R.; Maqoud, F.; Angelelli, M.; Latorre, R.; Perrone, M.G.; Scilimati, A.; Tricarico, D. Zoledronic Acid Modulation of TRPV1 Channel Currents in Osteoblast Cell Line and Native Rat and Mouse Bone Marrow-Derived Osteoblasts: Cell Proliferation and Mineralization Effect. Cancers 2019, 11, 206. https://doi.org/10.3390/cancers11020206
Scala R, Maqoud F, Angelelli M, Latorre R, Perrone MG, Scilimati A, Tricarico D. Zoledronic Acid Modulation of TRPV1 Channel Currents in Osteoblast Cell Line and Native Rat and Mouse Bone Marrow-Derived Osteoblasts: Cell Proliferation and Mineralization Effect. Cancers. 2019; 11(2):206. https://doi.org/10.3390/cancers11020206
Chicago/Turabian StyleScala, Rosa, Fatima Maqoud, Mariacristina Angelelli, Ramon Latorre, Maria Grazia Perrone, Antonio Scilimati, and Domenico Tricarico. 2019. "Zoledronic Acid Modulation of TRPV1 Channel Currents in Osteoblast Cell Line and Native Rat and Mouse Bone Marrow-Derived Osteoblasts: Cell Proliferation and Mineralization Effect" Cancers 11, no. 2: 206. https://doi.org/10.3390/cancers11020206
APA StyleScala, R., Maqoud, F., Angelelli, M., Latorre, R., Perrone, M. G., Scilimati, A., & Tricarico, D. (2019). Zoledronic Acid Modulation of TRPV1 Channel Currents in Osteoblast Cell Line and Native Rat and Mouse Bone Marrow-Derived Osteoblasts: Cell Proliferation and Mineralization Effect. Cancers, 11(2), 206. https://doi.org/10.3390/cancers11020206








