Adjuvant Ipilimumab in High-Risk Uveal Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Patient Monitoring for Adverse Effects and Metastasis
2.3. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Treatment
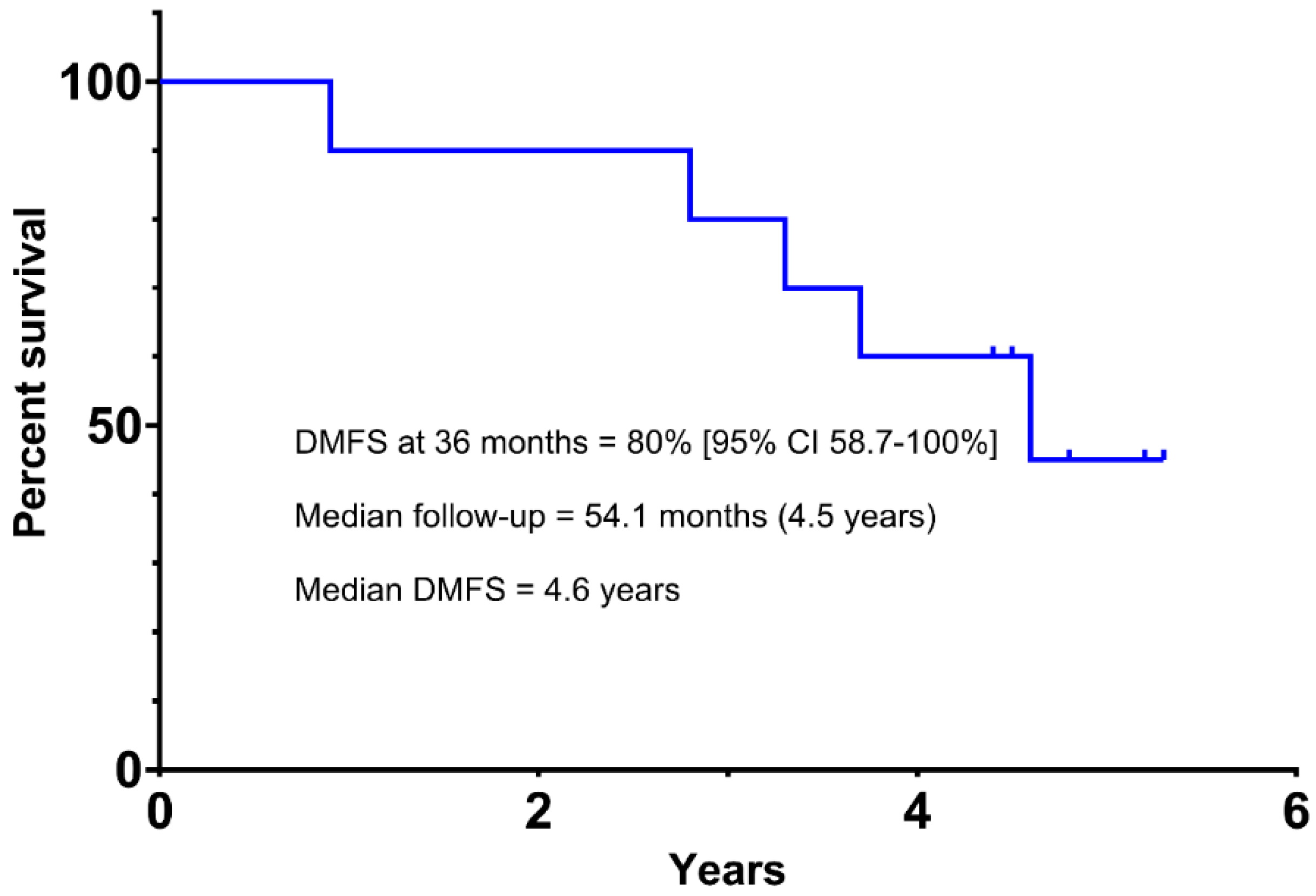
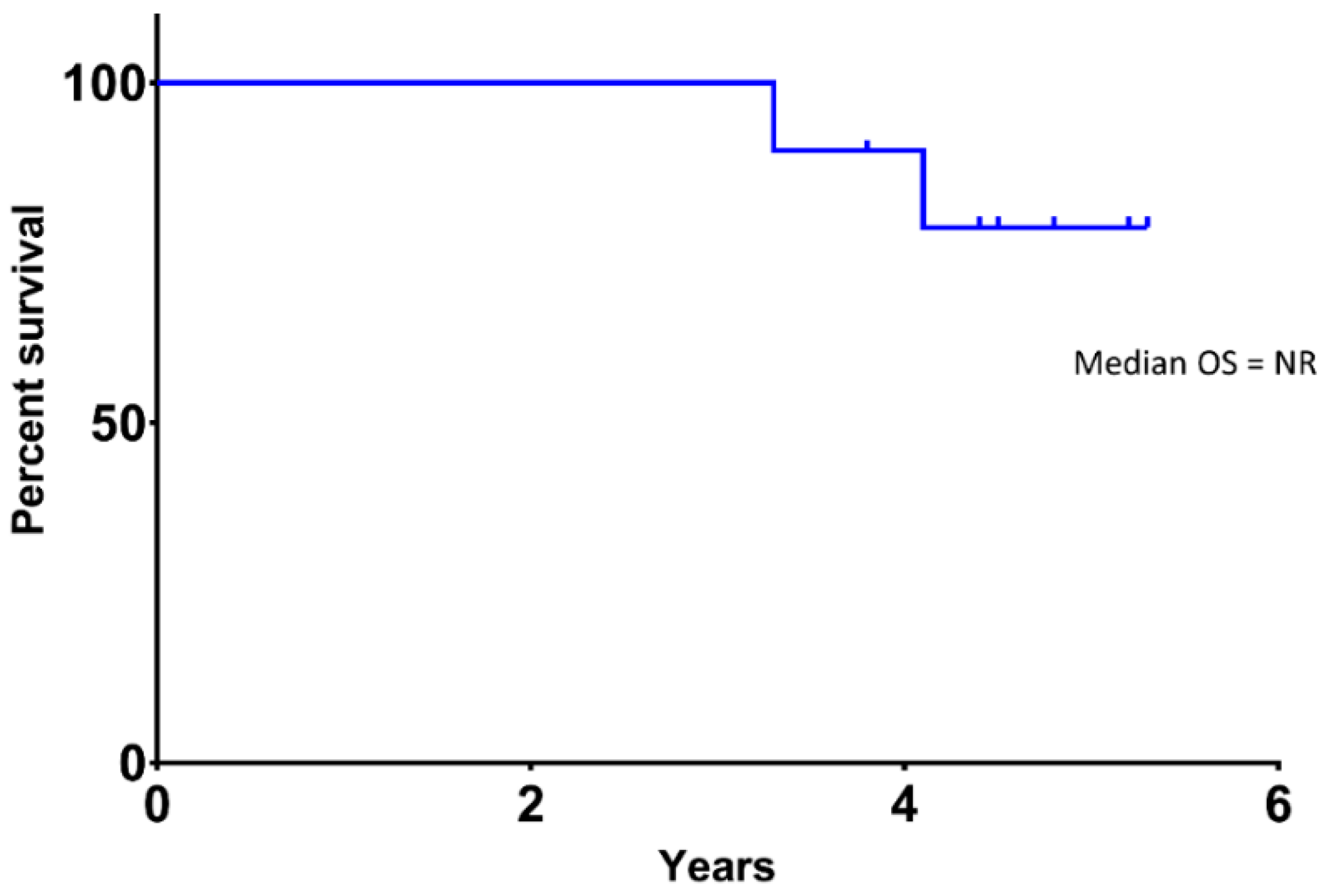
3.3. Efficacy
3.4. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, A.D.; Topham, A. Incidence of uveal melanoma in the United States: 1973–1997. Ophthalmology 2003, 110, 956–961. [Google Scholar] [CrossRef]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, C.; Kim, D.W.; Gombos, D.S.; Oba, J.; Qin, Y.; Williams, M.D.; Esmaeli, B.; Grimm, E.A.; Wargo, J.A.; Woodman, S.E.; et al. Uveal melanoma: From diagnosis to treatment and the science in between. Cancer 2016, 122, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Damato, B. Treatment of primary intraocular melanoma. Expert Rev. Anticancer Ther. 2006, 6, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Kujala, E.; Makitie, T.; Kivela, T. Very long-term prognosis of patients with malignant uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef]
- Augsburger, J.J.; Correa, Z.M.; Shaikh, A.H. Effectiveness of treatments for metastatic uveal melanoma. Am. J. Ophthalmol. 2009, 148, 119–127. [Google Scholar] [CrossRef]
- Sato, T.; Han, F.; Yamamoto, A. The biology and management of uveal melanoma. Curr. Oncol. Rep. 2008, 10, 431–438. [Google Scholar] [CrossRef]
- Singh, A.D.; Borden, E.C. Metastatic uveal melanoma. Ophthalmol. Clin. N. Am. 2005, 18, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Nichols, E.E.; Richmond, A.; Daniels, A.B. Tumor Characteristics, Genetics, Management, and the Risk of Metastasis in Uveal Melanoma. Semin. Ophthalmol. 2016, 31, 304–309. [Google Scholar] [CrossRef]
- Dogrusoz, M.; Bagger, M.; van Duinen, S.G.; Kroes, W.G.; Ruivenkamp, C.A.; Bohringer, S.; Andersen, K.K.; Luyten, G.P.; Kiilgaard, J.F.; Jager, M.J. The Prognostic Value of AJCC Staging in Uveal Melanoma Is Enhanced by Adding Chromosome 3 and 8q Status. Investig. Ophthalmol. Vis. Sci. 2017, 58, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Onken, M.D.; Worley, L.A.; Char, D.H.; Augsburger, J.J.; Correa, Z.M.; Nudleman, E.; Aaberg, T.M., Jr.; Altaweel, M.M.; Bardenstein, D.S.; Finger, P.T.; et al. Collaborative Ocular Oncology Group report number 1: Prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology 2012, 119, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A.V.; Wakade, O.A.; et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch. Ophthalmol. 2009, 127, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Sisley, K.; Rennie, I.G.; Parsons, M.A.; Jacques, R.; Hammond, D.W.; Bell, S.M.; Potter, A.M.; Rees, R.C. Abnormalities of chromosomes 3 and 8 in posterior uveal melanoma correlate with prognosis. Genes Chromosomes Cancer 1997, 19, 22–28. [Google Scholar] [CrossRef]
- Van den Bosch, T.; van Beek, J.G.; Vaarwater, J.; Verdijk, R.M.; Naus, N.C.; Paridaens, D.; de Klein, A.; Kilic, E. Higher percentage of FISH-determined monosomy 3 and 8q amplification in uveal melanoma cells relate to poor patient prognosis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2668–2674. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.M.; Egan, K.M.; Harmon, D.; Holbrook, A.; Munzenrider, J.E.; Gragoudas, E.S. Adjuvant interferon therapy for patients with uveal melanoma at high risk of metastasis. Ophthalmology 2009, 116, 2206–2212. [Google Scholar] [CrossRef] [PubMed]
- McLean, I.W.; Berd, D.; Mastrangelo, M.J.; Shields, J.A.; Davidorf, F.H.; Grever, M.; Makley, T.A.; Gamel, J.W. A randomized study of methanol-extraction residue of bacille Calmette-Guerin as postsurgical adjuvant therapy of uveal melanoma. Am. J. Ophthalmol. 1990, 110, 522–526. [Google Scholar] [CrossRef]
- Valsecchi, M.E.; Orloff, M.; Sato, R.; Chervoneva, I.; Shields, C.L.; Shields, J.A.; Mastrangelo, M.J.; Sato, T. Adjuvant Sunitinib in High-Risk Patients with Uveal Melanoma: Comparison with Institutional Controls. Ophthalmology 2017. [Google Scholar] [CrossRef]
- Voelter, V.; Schalenbourg, A.; Pampallona, S.; Peters, S.; Halkic, N.; Denys, A.; Goitein, G.; Zografos, L.; Leyvraz, S. Adjuvant intra-arterial hepatic fotemustine for high-risk uveal melanoma patients. Melanoma Res. 2008, 18, 220–224. [Google Scholar] [CrossRef]
- Chan, P.Y.; Hall, P.; Hay, G.; Cohen, V.M.L.; Szlosarek, P.W. A major responder to ipilimumab and nivolumab in metastatic uveal melanoma with concomitant autoimmunity. Pigment Cell Melanoma Res. 2017. [Google Scholar] [CrossRef]
- Danielli, R.; Ridolfi, R.; Chiarion-Sileni, V.; Queirolo, P.; Testori, A.; Plummer, R.; Boitano, M.; Calabro, L.; Rossi, C.D.; Giacomo, A.M.; et al. Ipilimumab in pretreated patients with metastatic uveal melanoma: Safety and clinical efficacy. Cancer Immunol. Immunother. 2012, 61, 41–48. [Google Scholar] [CrossRef]
- Karydis, I.; Chan, P.Y.; Wheater, M.; Arriola, E.; Szlosarek, P.W.; Ottensmeier, C.H. Clinical activity and safety of Pembrolizumab in Ipilimumab pre-treated patients with uveal melanoma. Oncoimmunology 2016, 5, e1143997. [Google Scholar] [CrossRef] [PubMed]
- Kelderman, S.; van der Kooij, M.K.; van den Eertwegh, A.J.; Soetekouw, P.M.; Jansen, R.L.; van den Brom, R.R.; Hospers, G.A.; Haanen, J.B.; Kapiteijn, E.; Blank, C.U. Ipilimumab in pretreated metastastic uveal melanoma patients. Results of the Dutch Working group on Immunotherapy of Oncology (WIN-O). Acta Oncol. 2013, 52, 1786–1788. [Google Scholar] [CrossRef]
- Khattak, M.A.; Fisher, R.; Hughes, P.; Gore, M.; Larkin, J. Ipilimumab activity in advanced uveal melanoma. Melanoma Res. 2013, 23, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Kottschade, L.A.; McWilliams, R.R.; Markovic, S.N.; Block, M.S.; Villasboas Bisneto, J.; Pham, A.Q.; Esplin, B.L.; Dronca, R.S. The use of pembrolizumab for the treatment of metastatic uveal melanoma. Melanoma Res. 2016, 26, 300–303. [Google Scholar] [CrossRef]
- Luke, J.J.; Callahan, M.K.; Postow, M.A.; Romano, E.; Ramaiya, N.; Bluth, M.; Giobbie-Hurder, A.; Lawrence, D.P.; Ibrahim, N.; Ott, P.A.; et al. Clinical activity of ipilimumab for metastatic uveal melanoma: A retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer 2013, 119, 3687–3695. [Google Scholar] [CrossRef]
- Maio, M.; Danielli, R.; Chiarion-Sileni, V.; Pigozzo, J.; Parmiani, G.; Ridolfi, R.; De Rosa, F.; Del Vecchio, M.; Di Guardo, L.; Queirolo, P.; et al. Efficacy and safety of ipilimumab in patients with pre-treated, uveal melanoma. Ann. Oncol. 2013, 24, 2911–2915. [Google Scholar] [CrossRef]
- Zimmer, L.; Vaubel, J.; Mohr, P.; Hauschild, A.; Utikal, J.; Simon, J.; Garbe, C.; Herbst, R.; Enk, A.; Kampgen, E.; et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-naive patients with metastatic uveal melanoma. PLoS ONE 2015, 10, e0118564. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015, 16, 522–530. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Suciu, S.; Testori, A. Ipilimumab Adjuvant Therapy in Melanoma. N. Engl. J. Med. 2017, 376, 399. [Google Scholar] [CrossRef]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Onken, M.D.; Worley, L.A.; Ehlers, J.P.; Harbour, J.W. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004, 64, 7205–7209. [Google Scholar] [CrossRef] [PubMed]
- Correa, Z.M.; Augsburger, J.J. Independent Prognostic Significance of Gene Expression Profile Class and Largest Basal Diameter of Posterior Uveal Melanomas. Am. J. Ophthalmol. 2016, 162. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.D.; Chao, D.L.; Feuer, W.; Schiffman, J.; Char, D.H.; Harbour, J.W. Prognostic Implications of Tumor Diameter in Association with Gene Expression Profile for Uveal Melanoma. JAMA Ophthalmol. 2016, 134, 734–740. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Joshua, A.M.; Monzon, J.G.; Mihalcioiu, C.; Hogg, D.; Smylie, M.; Cheng, T. A phase 2 study of tremelimumab in patients with advanced uveal melanoma. Melanoma Res. 2015, 25, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
| Patient ID | Age | Gender | Definitive Treatment Modality | Additional Local Therapy Prior to Enrollment | Size (mm) | T Stage ***** | DecisionDx UM Class | Days Elapsed *** | Number of Cycles of Ipilimumab | Ipilimumab Dose (mg/kg) | Baseline LDH (ULN 618 IU/L) | Reason for Discontinuation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | M | Brachytherapy | Yes, thermotherapy | 16 × 15 × 8 | 3 | 2 | 204 | 7 | 3 | 386 | Completed Therapy |
| 2 | 45 | M | Brachytherapy | - | 12 × 10 × 3 | 1 | 2 | 63 | 5 | 3 | 421 | 4th induction dose and last maintenance dose held due to Grade 3 transaminitis and Grade 2 fatigue and hypothyroidism |
| 3 | 58 | F | Brachytherapy * | Yes, avastin | 15 × 12 × 4 | 2 | 2 | 77 | 6 | 3 | 523 | 3rd induction dose held due to Grade 3 rash |
| 4 | 74 | F | Brachytherapy | - | 13 × 13 × 4.6 | 2 | 2 | 161 | 7 | 10 | 552 | Completed therapy |
| 5 | 60 | M | Proton beam therapy | - | 21 × 17 × 10.1 | 4 | 2 | 111 | 2 | 10 | 402 | Only 2 doses given due to Grade 4 vasculitis |
| 6 | 59 | F | Brachytherapy | Yes, avastin | 13 × 10 × 4.4 | 2 | 2 | 317 | 2 | 10 | 428 | Only 2 doses given due to Grade 3 diarrhea, Transaminitis |
| 7 | 65 | M | Enucleation | - | 14 × 12 × 11 | 3 | 2 | 83 | 7 | 10 | 357 | Completed therapy |
| 8 | 50 | F | Brachytherapy | - | 6 × 5 | N/A | 2 | 179 | 6 | 10 | 287 | 3rd induction dose held secondary to Grade 3 hypophysitis |
| 9 | 57 | M | Brachytherapy | ** | 8 × 8.1 × 2.7 | 1 | 2 | 67 | 5 | 10 | 417 | 4th induction dose held secondary to Grade 2 hypophysitis; last maintenance dose held due to Grade 3 transaminitis |
| 10 | 58 | M | Enucleation | - | 15 × 14 × 9 | 3 | 2 | 153 | 7 | 10 | 592 **** | Completed therapy |
| Adverse Events | Grade 1–2 (n/10) | Grade 3–4 (n/10) |
|---|---|---|
| Constitutional | 7 | 0 |
| Endocrine | 4 | 1 |
| Gastrointestinal | 5 | 2 |
| Hematologic | 0 | 0 |
| Hepatic | 6 | 3 |
| Infectious | 1 | 0 |
| Musculoskeletal | 1 | 0 |
| Neurological | 2 | 0 |
| Pain | 0 | 0 |
| Psychiatric | 0 | 0 |
| Pulmonary | 1 | 0 |
| Renal | 0 | 2 |
| Skin | 8 | 2 |
| Vascular | 0 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fountain, E.; Bassett, R.L.; Cain, S.; Posada, L.; Gombos, D.S.; Hwu, P.; Bedikian, A.; Patel, S.P. Adjuvant Ipilimumab in High-Risk Uveal Melanoma. Cancers 2019, 11, 152. https://doi.org/10.3390/cancers11020152
Fountain E, Bassett RL, Cain S, Posada L, Gombos DS, Hwu P, Bedikian A, Patel SP. Adjuvant Ipilimumab in High-Risk Uveal Melanoma. Cancers. 2019; 11(2):152. https://doi.org/10.3390/cancers11020152
Chicago/Turabian StyleFountain, Eric, Roland L. Bassett, Suzanne Cain, Liberty Posada, Dan S. Gombos, Patrick Hwu, Agop Bedikian, and Sapna P. Patel. 2019. "Adjuvant Ipilimumab in High-Risk Uveal Melanoma" Cancers 11, no. 2: 152. https://doi.org/10.3390/cancers11020152
APA StyleFountain, E., Bassett, R. L., Cain, S., Posada, L., Gombos, D. S., Hwu, P., Bedikian, A., & Patel, S. P. (2019). Adjuvant Ipilimumab in High-Risk Uveal Melanoma. Cancers, 11(2), 152. https://doi.org/10.3390/cancers11020152





