Tribbles Homolog 3 Involved in Radiation Response of Triple Negative Breast Cancer Cells by Regulating Notch1 Activation
Abstract
1. Introduction
2. Results
2.1. TRIB3 and Notch1 Activation is Upregulated in Radioresistant Triple Negative Breast Cancer Cells
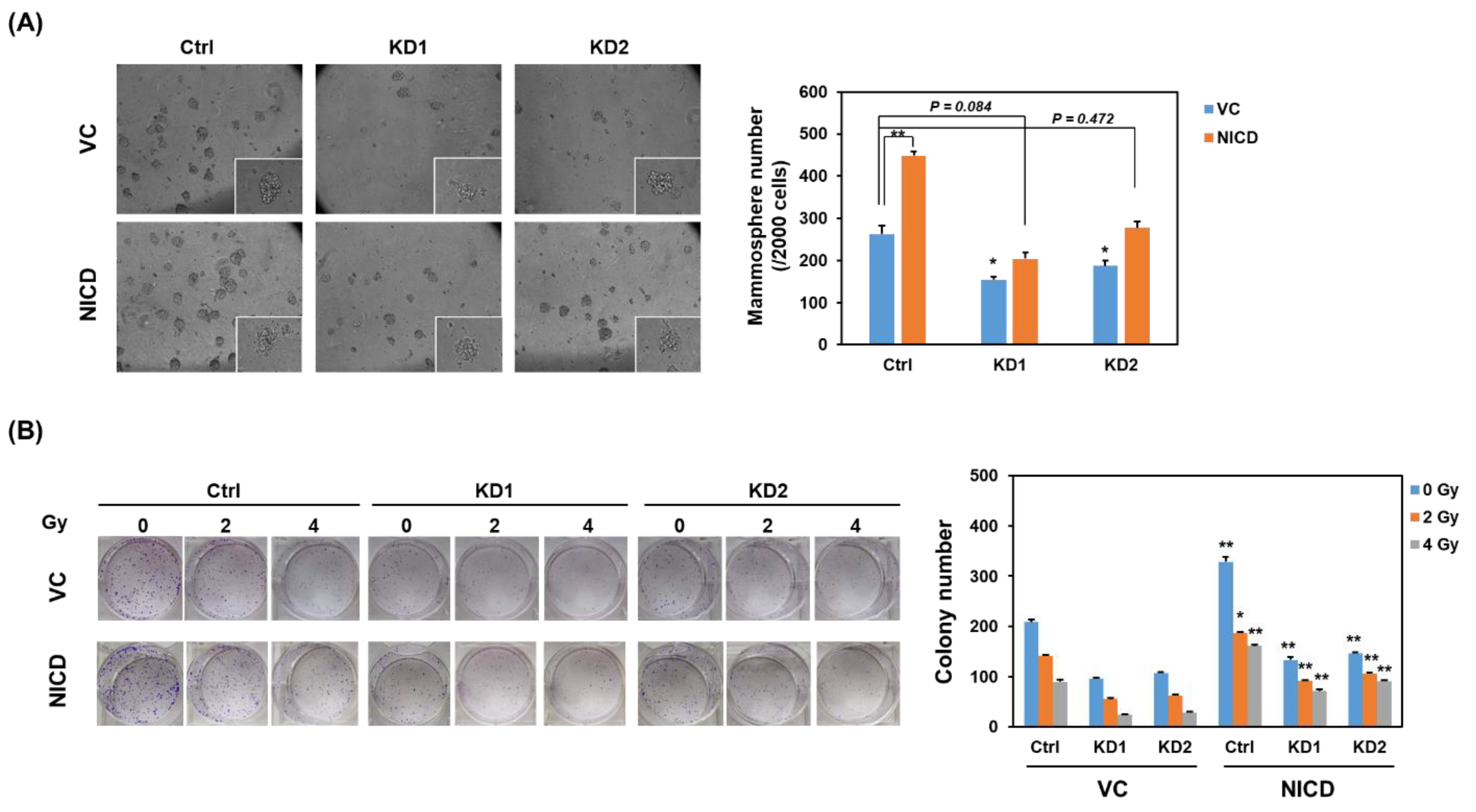
2.2. Knockdown of TRIB3 in Radioresistant MDA-MB-231 Cells Reduced Notch1 Activation and Senisitized Cells Toward Radiation Treatment
2.3. Knockdown of TRIB3 Caused G1 Arrest in Radioresistant MDA-MB-231 Cells
2.4. TRIB3 Iinteracted with BCLAF1, BNIP1, and DDX5 in Radioresistant MDA-MB-231 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Mammosphere Cultivation
4.2. Establishment of Radioresistant Triple Negative Breast Cancer Cells
4.3. Quantitative Real Time-PCR
4.4. Western Blot Analysis
4.5. Lentivirus-Mediated Short Hairpin RNA Delivery
4.6. Transfection of Notch Intracellular Domain Expressing Plasmid
4.7. Cell Growth and Cell Cycle Analysis
4.8. Mass Spectrometry Analysis of TRIB3 Interacting Proteins
4.9. Analysis of the Correlation between TRIB3/BCLAF1/BNIP1/DDX5 and Breast Cancer Patients’ Survival
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Santa-Maria, C.A.; Camp, M.; Cimino-Mathews, A.; Harvey, S.; Wright, J.; Stearns, V. Neoadjuvant therapy for early-stage breast cancer: Current practice, controversies, and future directions. Oncol. (Williston Park) 2015, 29, 828–838. [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative, G.; Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar]
- White, J.; Mamounas, E. Locoregional radiotherapy in patients with breast cancer responding to neoadjuvant chemotherapy: A paradigm for treatment individualization. J. Clin. Oncol. 2014, 32, 494–495. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.C.; Mutter, R.W.; Halyard, M.Y. Benefits, risks, and safety of external beam radiation therapy for breast cancer. Int. J. Womens Health 2015, 7, 449–458. [Google Scholar] [PubMed]
- Langlands, F.E.; Horgan, K.; Dodwell, D.D.; Smith, L. Breast cancer subtypes: Response to radiotherapy and potential radiosensitisation. Br. J. Radiol. 2013, 86, 20120601. [Google Scholar] [CrossRef]
- Du, K.; Herzig, S.; Kulkarni, R.N.; Montminy, M. Trb3: A tribbles homolog that inhibits akt/pkb activation by insulin in liver. Science 2003, 300, 1574–1577. [Google Scholar] [CrossRef]
- Wu, M.; Xu, L.G.; Zhai, Z.; Shu, H.B. Sink is a p65-interacting negative regulator of nf-kappab-dependent transcription. J. Biol. Chem. 2003, 278, 27072–27079. [Google Scholar] [CrossRef]
- Wennemers, M.; Bussink, J.; van den Beucken, T.; Sweep, F.C.; Span, P.N. Regulation of trib3 mRNA and protein in breast cancer. PLoS ONE 2012, 7, e49439. [Google Scholar] [CrossRef]
- Miyoshi, N.; Ishii, H.; Mimori, K.; Takatsuno, Y.; Kim, H.; Hirose, H.; Sekimoto, M.; Doki, Y.; Mori, M. Abnormal expression of trib3 in colorectal cancer: A novel marker for prognosis. Br. J. Cancer 2009, 101, 1664–1670. [Google Scholar] [CrossRef]
- Hua, F.; Mu, R.; Liu, J.; Xue, J.; Wang, Z.; Lin, H.; Yang, H.; Chen, X.; Hu, Z. Trb3 interacts with smad3 promoting tumor cell migration and invasion. J. Cell Sci. 2011, 124, 3235–3246. [Google Scholar] [CrossRef]
- Hua, F.; Li, K.; Yu, J.J.; Lv, X.X.; Yan, J.; Zhang, X.W.; Sun, W.; Lin, H.; Shang, S.; Wang, F.; et al. Trb3 links insulin/igf to tumour promotion by interacting with p62 and impeding autophagic/proteasomal degradations. Nat. Commun. 2015, 6, 7951. [Google Scholar] [CrossRef] [PubMed]
- Wennemers, M.; Bussink, J.; Scheijen, B.; Nagtegaal, I.D.; van Laarhoven, H.W.; Raleigh, J.A.; Varia, M.A.; Heuvel, J.J.; Rouschop, K.M.; Sweep, F.C.; et al. Tribbles homolog 3 denotes a poor prognosis in breast cancer and is involved in hypoxia response. Breast Cancer Res. 2011, 13, R82. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Luo, Y.; Chen, J.H.; Hu, J.; Luo, Y.Z.; Wang, W.; Zeng, Y.; Xiao, L. Knockdown of trb3 induces apoptosis in human lung adenocarcinoma cells through regulation of notch 1 expression. Mol. Med. Rep. 2013, 8, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Curigliano, G. Notch inhibitors and their role in the treatment of triple negative breast cancer: Promises and failures. Curr. Opin. Oncol. 2017, 29, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Lagadec, C.; Vlashi, E.; Della Donna, L.; Meng, Y.; Dekmezian, C.; Kim, K.; Pajonk, F. Survival and self-renewing capacity of breast cancer initiating cells during fractionated radiation treatment. Breast Cancer Res. 2010, 12, R13. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.M.; McBride, W.H.; Pajonk, F. The response of cd24(-/low)/cd44+ breast cancer-initiating cells to radiation. J. Natl. Cancer Inst. 2006, 98, 1777–1785. [Google Scholar] [CrossRef]
- Kasof, G.M.; Goyal, L.; White, E. Btf, a novel death-promoting transcriptional repressor that interacts with bcl-2-related proteins. Mol. Cell. Biol. 1999, 19, 4390–4404. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. Ualcan: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Lagadec, C.; Vlashi, E.; Alhiyari, Y.; Phillips, T.M.; Bochkur Dratver, M.; Pajonk, F. Radiation-induced notch signaling in breast cancer stem cells. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 609–618. [Google Scholar] [CrossRef]
- Theys, J.; Yahyanejad, S.; Habets, R.; Span, P.; Dubois, L.; Paesmans, K.; Kattenbeld, B.; Cleutjens, J.; Groot, A.J.; Schuurbiers, O.C.J.; et al. High notch activity induces radiation resistance in non small cell lung cancer. Radiother. Oncol. 2013, 108, 440–445. [Google Scholar] [CrossRef]
- Izrailit, J.; Berman, H.K.; Datti, A.; Wrana, J.L.; Reedijk, M. High throughput kinase inhibitor screens reveal trb3 and mapk-erk/tgfbeta pathways as fundamental notch regulators in breast cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 1714–1719. [Google Scholar] [CrossRef]
- Klein, C.; Dokic, I.; Mairani, A.; Mein, S.; Brons, S.; Haring, P.; Haberer, T.; Jakel, O.; Zimmermann, A.; Zenke, F.; et al. Overcoming hypoxia-induced tumor radioresistance in non-small cell lung cancer by targeting DNA-dependent protein kinase in combination with carbon ion irradiation. Radiat. Oncol. 2017, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Baumann, R.; Depping, R.; Delaperriere, M.; Dunst, J. Targeting hypoxia to overcome radiation resistance in head & neck cancers: Real challenge or clinical fairytale? Expert Rev. Anticancer 2016, 16, 751–758. [Google Scholar]
- Izrailit, J.; Jaiswal, A.; Zheng, W.; Moran, M.F.; Reedijk, M. Cellular stress induces trb3/usp9x-dependent notch activation in cancer. Oncogene 2017, 36, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.J.; Song, T.J.; Zhang, L.W.; Su, Y.; Wang, K.Y.; Sun, Q. Trb3 is elevated in psoriasis vulgaris lesions and mediates hacat cells proliferation in vitro. J. Investig. Med. 2017, 65, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lv, S.; Qin, Y.; Shu, F.; Xu, Y.; Chen, J.; Xu, B.E.; Sun, X.; Wu, J. Trb3 interacts with ctip and is overexpressed in certain cancers. Biochim. Biophys. Acta 2007, 1770, 273–278. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Shi, L.Z.; Zhu, Q.; Wu, P.; Zhang, Y.W.; Basilio, A.; Tonnu, N.; Verma, I.M.; Berns, M.W.; Hunter, T. Ctip links DNA double-strand break sensing to resection. Mol. Cell 2009, 36, 954–969. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Ahmed, S.U.; Carruthers, R.; Gilmour, L.; Yildirim, S.; Watts, C.; Chalmers, A.J. Selective inhibition of parallel DNA damage response pathways optimizes radiosensitization of glioblastoma stem-like cells. Cancer Res. 2015, 75, 4416–4428. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Yu, Y.B.; Gunawardena, H.P.; Xie, L.; Chen, X. Bclaf1 is a radiation-induced H2AX-interacting partner involved in gammaH2AX-mediated regulation of apoptosis and DNA repair. Cell Death Dis. 2012, 3, e359. [Google Scholar] [CrossRef]
- Vohhodina, J.; Barros, E.M.; Savage, A.L.; Liberante, F.G.; Manti, L.; Bankhead, P.; Cosgrove, N.; Madden, A.F.; Harkin, D.P.; Savage, K.I. The RNA processing factors thrap3 and bclaf1 promote the DNA damage response through selective mRNA splicing and nuclear export. Nucleic Acids Res. 2017, 45, 12816–12833. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.I.; Gorski, J.J.; Barros, E.M.; Irwin, G.W.; Manti, L.; Powell, A.J.; Pellagatti, A.; Lukashchuk, N.; McCance, D.J.; McCluggage, W.G.; et al. Identification of a brca1-mRNA splicing complex required for efficient DNA repair and maintenance of genomic stability. Mol. Cell 2014, 54, 445–459. [Google Scholar] [CrossRef]
- Bol, G.M.; Vesuna, F.; Xie, M.; Zeng, J.; Aziz, K.; Gandhi, N.; Levine, A.; Irving, A.; Korz, D.; Tantravedi, S.; et al. Targeting ddx3 with a small molecule inhibitor for lung cancer therapy. EMBO Mol. Med. 2015, 7, 648–669. [Google Scholar] [CrossRef]
- Wang, D.; Huang, J.; Hu, Z. RNA helicase ddx5 regulates microRNA expression and contributes to cytoskeletal reorganization in basal breast cancer cells. Mol. Cell Proteom. 2012, 11, M111.011932. [Google Scholar] [CrossRef]
- Li, F.; Lv, J.H.; Liang, L.; Wang, J.C.; Li, C.R.; Sun, L.; Li, T. Downregulation of microRNA-21 inhibited radiation-resistance of esophageal squamous cell carcinoma. Cancer Cell Int. 2018, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, A.; Luo, W.; Krasnitz, A.; Hicks, J.; Powers, R.S.; Stillman, B. Ddx5 regulates DNA replication and is required for cell proliferation in a subset of breast cancer cells. Cancer Discov. 2012, 2, 812–825. [Google Scholar] [CrossRef]
- Rose, N.M.; Wu, J.; Yu, L.; Xiao, X.; Zhang, F.M. Roles of ddx5 in the tumorigenesis, proliferation, differentiation, metastasis and pathway regulation of human malignancies. Biochim. Biophys. Acta Rev. Cancer 2018. [Google Scholar]
- Chang, W.W.; Lin, R.J.; Yu, J.; Chang, W.Y.; Fu, C.H.; Lai, A.C.; Yu, J.C.; Yu, A.L. The expression and significance of insulin-like growth factor-1 receptor and its pathway on breast cancer stem/progenitors. Breast Cancer Res. BCR 2013, 15, R39. [Google Scholar] [CrossRef]
- Chang, Y.C.; Tsai, C.H.; Lai, Y.L.; Yu, C.C.; Chi, W.Y.; Li, J.J.; Chang, W.W. Arecoline-induced myofibroblast transdifferentiation from human buccal mucosal fibroblasts is mediated by zeb1. J. Cell. Mol. Med. 2014, 18, 698–708. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. Maxquant enables high peptide identification rates, individualized p.P.B.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-C.; Wang, W.-L.; Chang, W.-C.; Huang, Y.-H.; Hong, G.-C.; Wang, H.-L.; Chou, Y.-H.; Tseng, H.-C.; Lee, H.-T.; Li, S.-T.; et al. Tribbles Homolog 3 Involved in Radiation Response of Triple Negative Breast Cancer Cells by Regulating Notch1 Activation. Cancers 2019, 11, 127. https://doi.org/10.3390/cancers11020127
Lee Y-C, Wang W-L, Chang W-C, Huang Y-H, Hong G-C, Wang H-L, Chou Y-H, Tseng H-C, Lee H-T, Li S-T, et al. Tribbles Homolog 3 Involved in Radiation Response of Triple Negative Breast Cancer Cells by Regulating Notch1 Activation. Cancers. 2019; 11(2):127. https://doi.org/10.3390/cancers11020127
Chicago/Turabian StyleLee, Yueh-Chun, Wen-Ling Wang, Wei-Chao Chang, Yu-Hao Huang, Guan-Ci Hong, Hui-Lin Wang, Ying-Hsiang Chou, Hsien-Chun Tseng, Hsueh-Te Lee, Shao-Ti Li, and et al. 2019. "Tribbles Homolog 3 Involved in Radiation Response of Triple Negative Breast Cancer Cells by Regulating Notch1 Activation" Cancers 11, no. 2: 127. https://doi.org/10.3390/cancers11020127
APA StyleLee, Y.-C., Wang, W.-L., Chang, W.-C., Huang, Y.-H., Hong, G.-C., Wang, H.-L., Chou, Y.-H., Tseng, H.-C., Lee, H.-T., Li, S.-T., Chen, H.-L., Wu, C.-C., Yang, H.-F., Wang, B.-Y., & Chang, W.-W. (2019). Tribbles Homolog 3 Involved in Radiation Response of Triple Negative Breast Cancer Cells by Regulating Notch1 Activation. Cancers, 11(2), 127. https://doi.org/10.3390/cancers11020127





