Pituitary Adenomas and Invasiveness from Anatomo-Surgical, Radiological, and Histological Perspectives: A Systematic Literature Review
Abstract
1. Introduction
2. Literature Review
2.1. Epidemiology
2.2. Anatomo-Surgical Considerations on Invasiveness
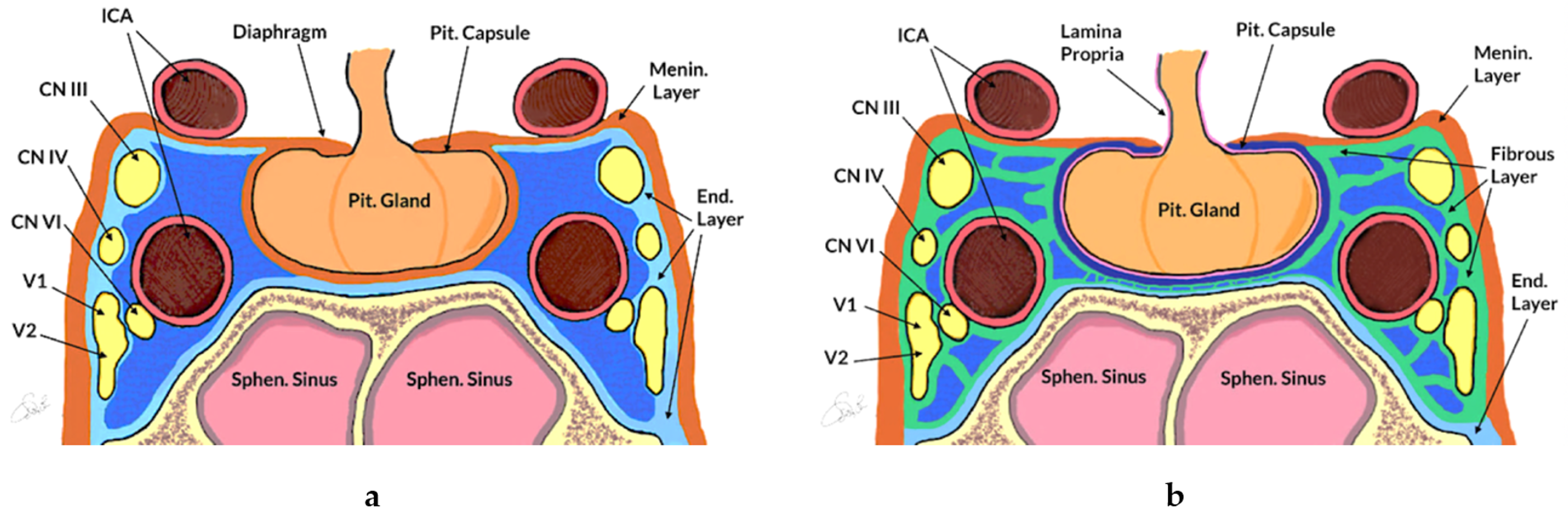
2.2.1. Anatomy of the Sellar and Parasellar Region
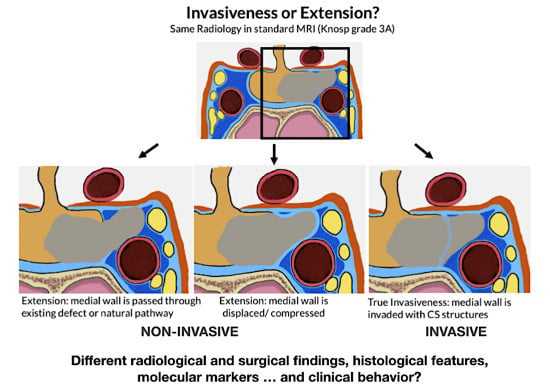
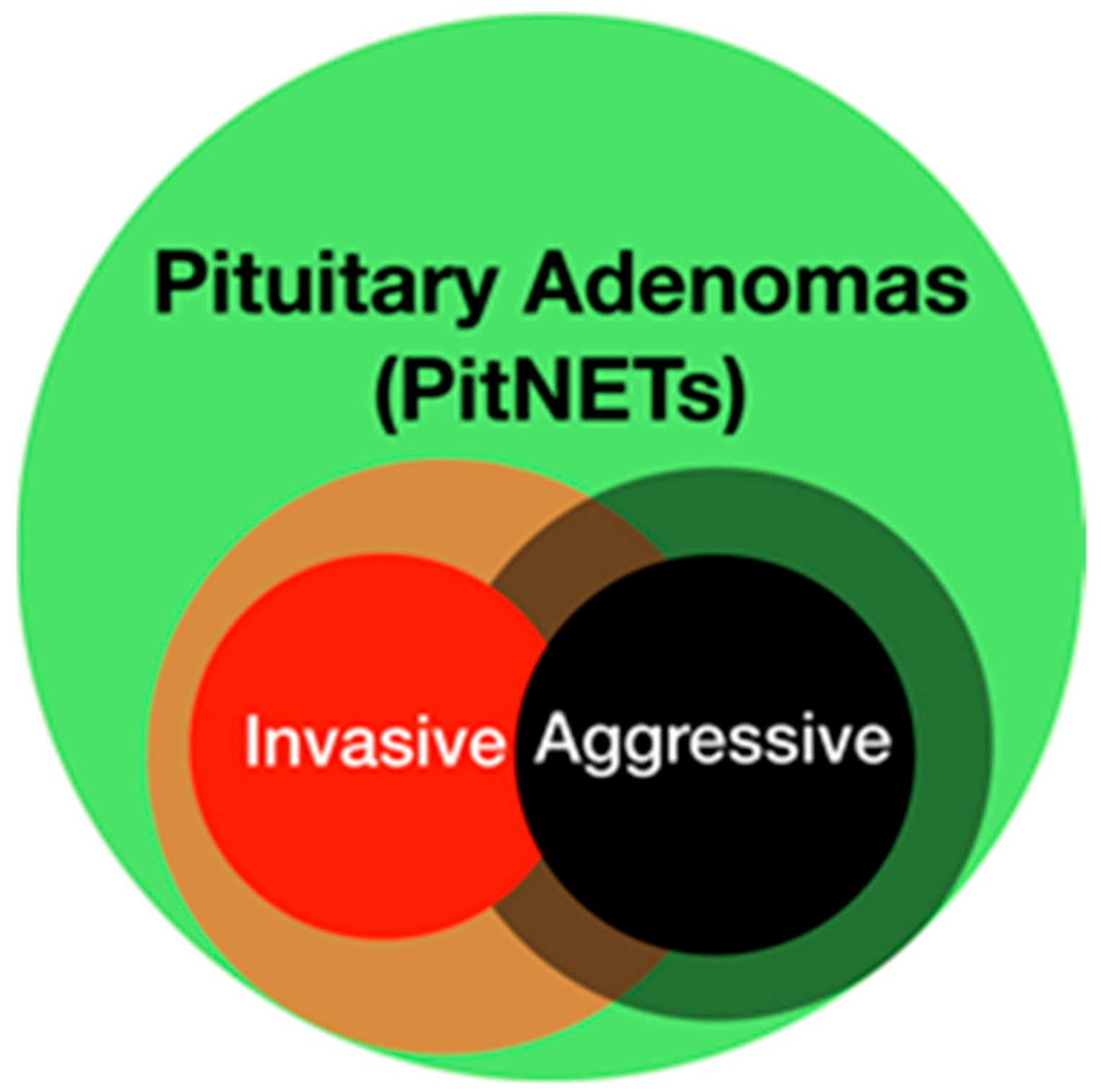
2.2.2. PAs and Surgical Invasiveness
2.3. Radiology and PAs Invasiveness
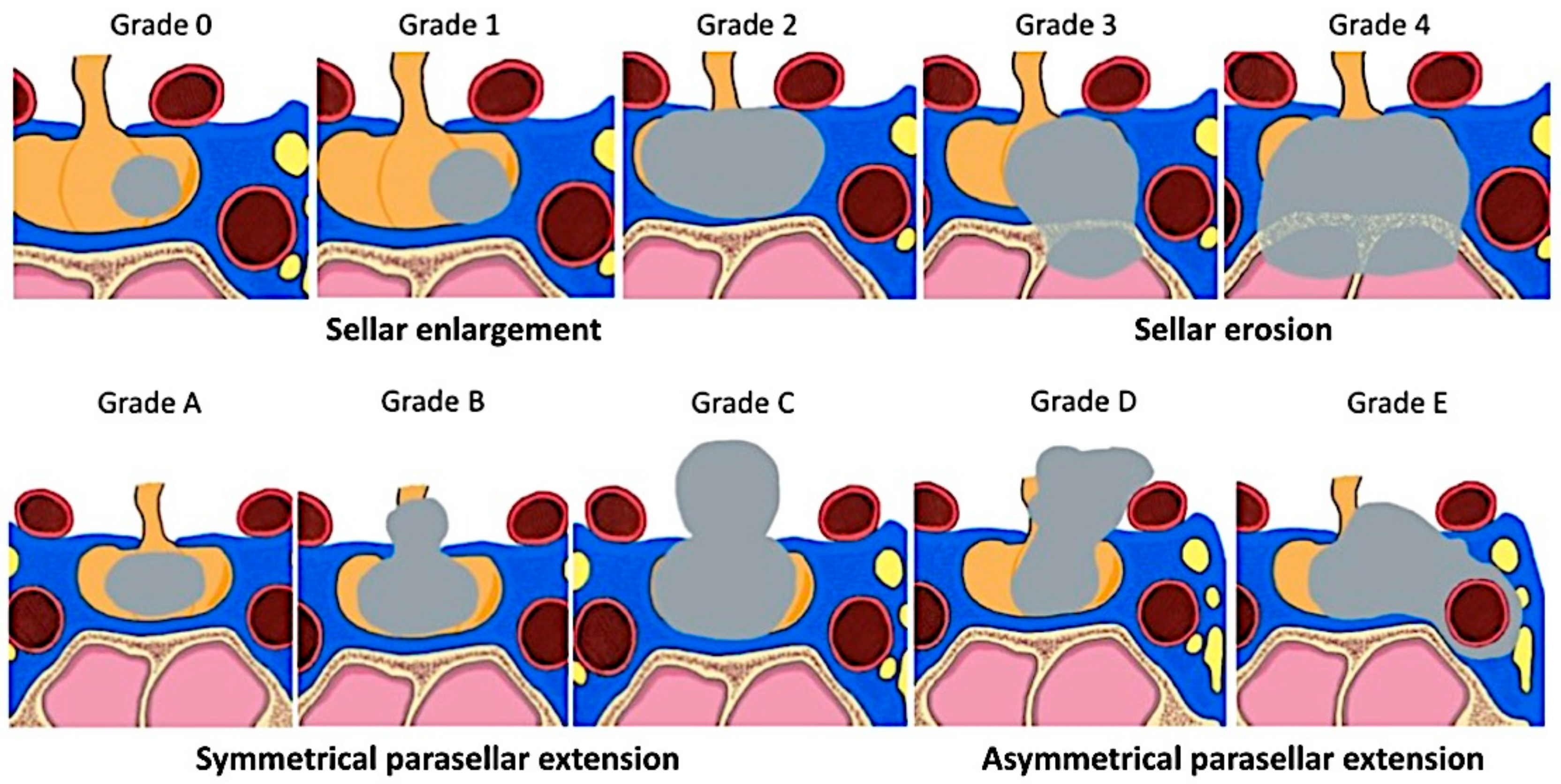
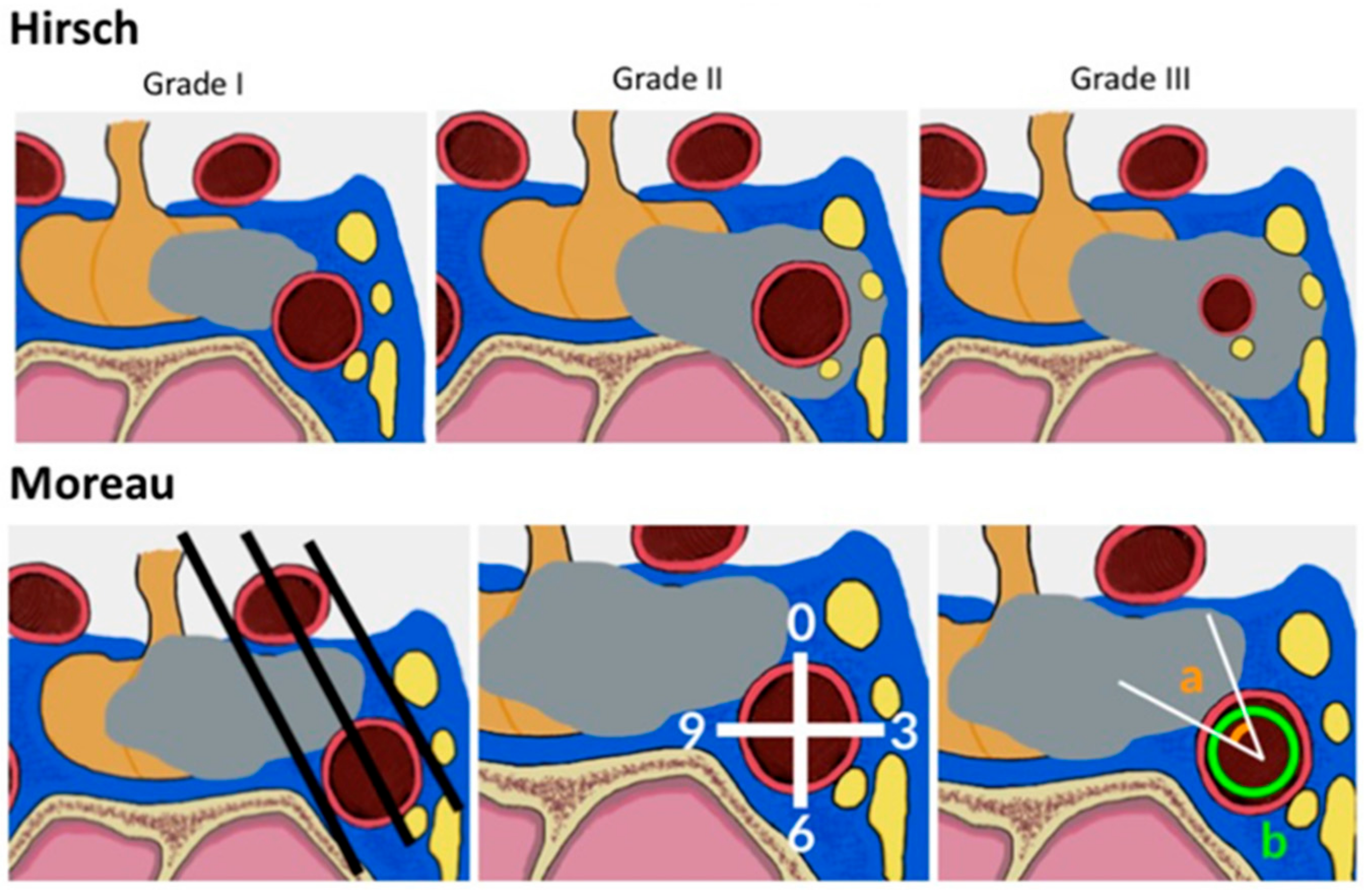
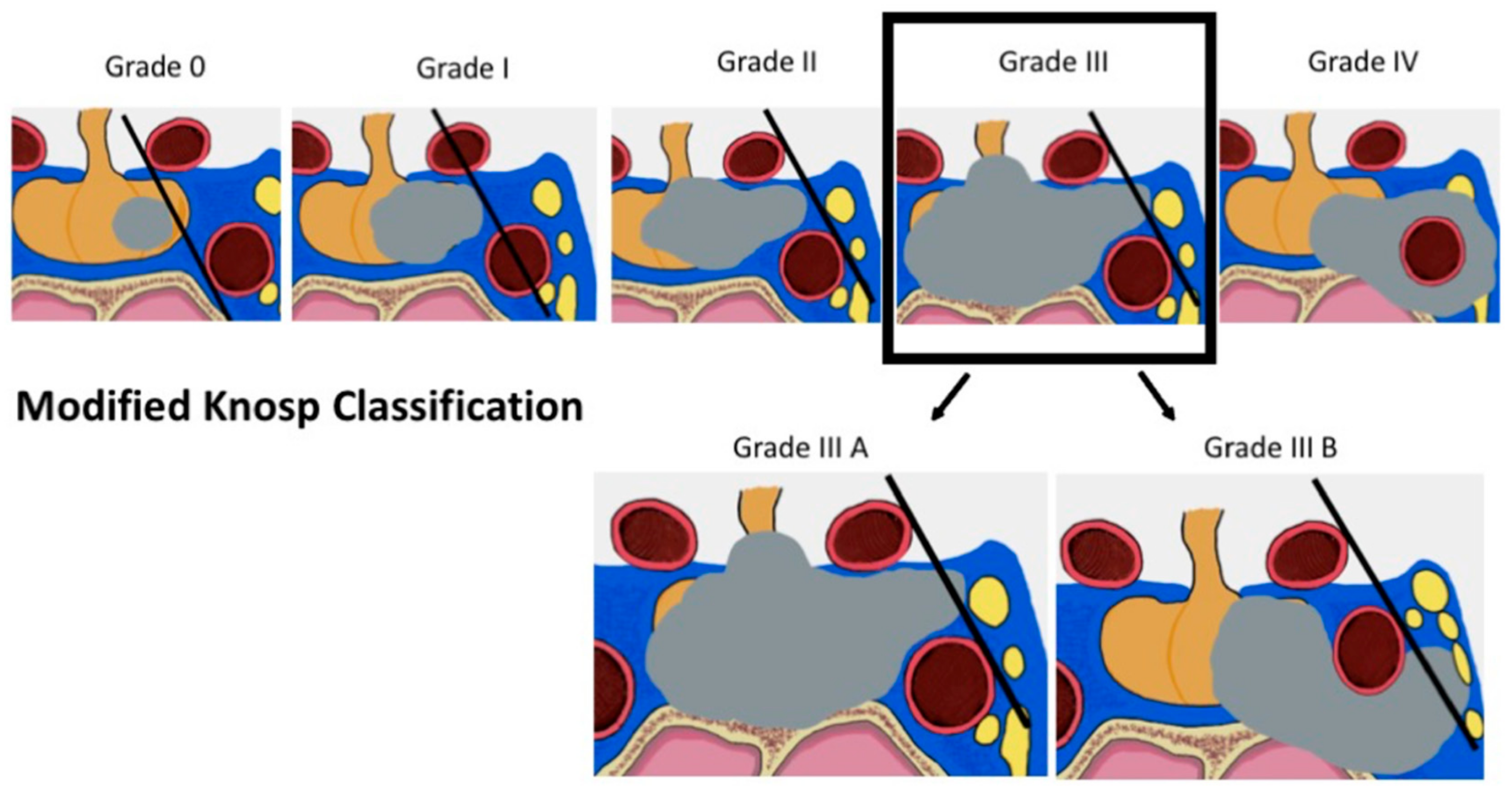
2.3.1. Radiological Criteria and Classifications
2.3.2. Radiological Technical Advancements
2.4. Histological Evidence of Invasiveness
2.5. Pathological and Molecular Considerations
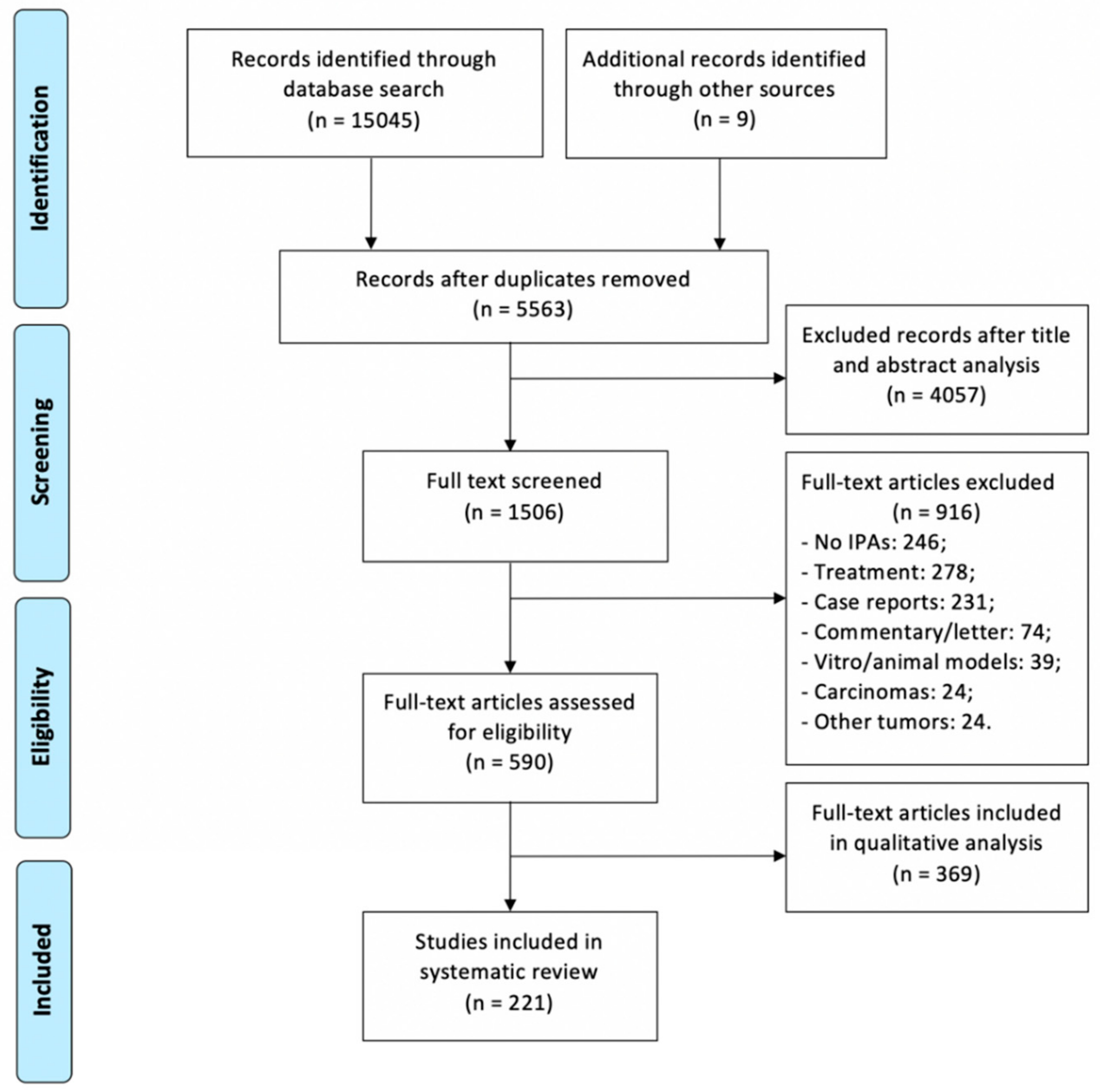
3. Materials and Methods
4. Conclusions
Funding
Conflicts of Interest
References
- Blevins, L.S., Jr.; Verity, D.K.; Allen, G. Aggressive pituitary tumors. Oncology 1998, 12, 1307–1315, Discussions 1315–1318. [Google Scholar] [PubMed]
- Carrasco, C.A.; Gadelha, M.; Manavela, M.; Bruno, O.D. Aggressive tumors and difficult choices in acromegaly. Pituitary 2014, 17, 24–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chatzellis, E.; Alexandraki, K.I.; Androulakis, I.; Kaltsas, G. Aggressive pituitary tumors. Neuroendocrinology 2015, 101, 87–104. [Google Scholar] [CrossRef]
- Dai, C.; Feng, M.; Liu, X.; Ma, S.; Sun, B.; Bao, X.; Yao, Y.; Deng, K.; Wang, Y.; Xing, B.; et al. Refractory pituitary adenoma: A novel classification for pituitary tumors. Oncotarget 2016, 7, 83657–83668. [Google Scholar] [CrossRef]
- Dworakowska, D.; Grossman, A.B. Aggressive and malignant pituitary tumours: State-of-the-art. Endocr. Relat. Cancer 2018, 25, 559–575. [Google Scholar] [CrossRef]
- Nishioka, H.; Inoshita, N. New WHO classification of pituitary adenomas (4th edition): Assessment of pituitary transcription factors and the prognostic histological factors. Brain Tumor Pathol. 2018, 35, 57–61. [Google Scholar] [CrossRef]
- Saeger, W.; Petersenn, S.; Schofl, C.; Knappe, U.J.; Theodoropoulou, M.; Buslei, R.; Honegger, J. Emerging Histopathological and Genetic Parameters of Pituitary Adenomas: Clinical Impact and Recommendation for Future WHO Classification. Endocr. Pathol. 2016, 27, 115–122. [Google Scholar] [CrossRef]
- Berry, R.G.; Caplan, H.J. An overview of pituitary tumors. Ann. Clin. Lab. Sci. 1979, 9, 94–102. [Google Scholar]
- Scheithauer, B.W.; Kovacs, K.T.; Laws, E.R., Jr.; Randall, R.V. Pathology of invasive pituitary tumors with special reference to functional classification. J. Neurosurg. 1986, 65, 733–744. [Google Scholar] [CrossRef]
- Hofstetter, C.P.; Nanaszko, M.J.; Mubita, L.L.; Tsiouris, J.; Anand, V.K.; Schwartz, T.H. Volumetric classification of pituitary macroadenomas predicts outcome and morbidity following endoscopic endonasal transsphenoidal surgery. Pituitary 2012, 15, 450–463. [Google Scholar] [CrossRef]
- Ahmadi, J.; North, C.M.; Segall, H.D.; Zee, C.S.; Weiss, M.H. Cavernous sinus invasion by pituitary adenomas. Am. J. Roentgenol. 1986, 146, 257–262. [Google Scholar] [CrossRef]
- Delgrange, E.; Duprez, T.; Maiter, D. Influence of parasellar extension of macroprolactinomas defined by magnetic resonance imaging on their responsiveness to dopamine agonist therapy. Clin. Endocrinol. 2006, 64, 456–462. [Google Scholar] [CrossRef]
- Cander, S.; Gul, O.O.; Erturk, E.; Tuncel, E.; Ersoy, C. Prolactin levels and gender are associated with tumour behaviour in prolactinomas but Ki-67 index is not. Endokrynol. Pol. 2014, 65, 210–216. [Google Scholar] [CrossRef][Green Version]
- Garibi, J.; Pomposo, I.; Villar, G.; Gaztambide, S. Giant pituitary adenomas: Clinical characteristics and surgical results. Br. J. Neurosurg. 2002, 16, 133–139. [Google Scholar] [CrossRef]
- Potorac, I.; Petrossians, P.; Daly, A.F.; Schillo, F.; Ben Slama, C.; Nagi, S.; Sahnoun, M.; Brue, T.; Girard, N.; Chanson, P.; et al. Pituitary MRI characteristics in 297 acromegaly patients based on T2-weighted sequences. Endocr. Relat. Cancer 2015, 22, 169–177. [Google Scholar] [CrossRef]
- Maira, G.; Anile, C. Pituitary Adenomas in Childhood and Adolescence. Can. J. Neurol. Sci. J. Can. Des Sci. Neurol. 1990, 17, 83–87. [Google Scholar] [CrossRef]
- Mao, J.H.; Guo, H.; Si, N.; Qiu, L.; Guo, L.F.; Sun, Z.S.; Xiang, Y.; Yang, X.H.; Zhao, W.G.; Zhang, W.C. Regulating effect of MMP-9 and TIMP-1 in pituitary adenoma invasion. Genet. Mol. Res. 2015, 14, 17091–17098. [Google Scholar] [CrossRef]
- Mehrazin, M. Pituitary tumors in children: Clinical analysis of 21 cases. Child’s Nerv. Syst. 2007, 23, 391–398. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, P.; Meng, Y.; Ye, F.; Jiang, S. A retrospective review of 34 cases of pediatric pituitary adenoma. Child’s Nerv. Syst. 2017, 33, 1961–1967. [Google Scholar] [CrossRef]
- Rutkowski, M.J.; Alward, R.M.; Chen, R.; Wagner, J.; Jahangiri, A.; Southwell, D.G.; Kunwar, S.; Blevins, L.; Lee, H.; Aghi, M.K. Atypical pituitary adenoma: A clinicopathologic case series. J. Neurosurg. 2018, 128, 1058–1065. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs; WHO: Geneva, Switzerland, 2004; ISBN 978-92-832-4493-6. [Google Scholar]
- Bette, S.; Butenschon, V.M.; Wiestler, B.; von Werder, A.; Schmid, R.M.; Lehmberg, J.; Zimmer, C.; Meyer, B.; Kirschke, J.S.; Gempt, J. MRI criteria of subtypes of adenomas and epithelial cysts of the pituitary gland. Neurosurg. Rev. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ceccato, F.; Regazzo, D.; Barbot, M.; Denaro, L.; Emanuelli, E.; Borsetto, D.; Rolma, G.; Alessio, L.; Gardiman, M.P.; Lombardi, G.; et al. Early recognition of aggressive pituitary adenomas: A single-centre experience. Acta Neurochir. 2018, 160, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Delgrange, E.; Trouillas, J.; Maiter, D.; Donckier, J.; Tourniaire, J. Sex-related difference in the growth of prolactinomas: A clinical and proliferation marker study. J. Clin. Endocrinol. Metab. 1997, 82, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Ayodeji, S.; Effiong, A.; Adefolarin, M. Recent advances in the pathology of pituitary adenomas. J. Morphol. Sci. 2015, 32, 43–52. [Google Scholar] [CrossRef]
- Liu, W.; Zahr, R.S.; McCartney, S.; Cetas, J.S.; Dogan, A.; Fleseriu, M. Clinical outcomes in male patients with lactotroph adenomas who required pituitary surgery: A retrospective single center study. Pituitary 2018, 21, 454–462. [Google Scholar] [CrossRef]
- Yamaguchi-Okada, M.; Inoshita, N.; Nishioka, H.; Fukuhara, N.; Yamada, S. Clinicopathological analysis of nonfunctioning pituitary adenomas in patients younger than 25 years of age: Clinical article. J. Neurosurg. Pediatr. 2012, 9, 511–516. [Google Scholar] [CrossRef]
- Yamada, S.; Ohyama, K.; Taguchi, M.; Takeshita, A.; Morita, K.; Takano, K.; Sano, T. A study of the correlation between morphological findings and biological activities in clinically nonfunctioning pituitary adenomas. Neurosurgery 2007, 61, 580–584. [Google Scholar] [CrossRef]
- Wu, Z.B.; Yu, C.J.; Su, Z.P.; Zhuge, Q.C.; Wu, J.S.; Zheng, W.M. Bromocriptine treatment of invasive giant prolactinomas involving the cavernous sinus: Results of a long-term follow up. J. Neurosurg. 2006, 104, 54–61. [Google Scholar] [CrossRef]
- Nishioka, H.; Inoshita, N.; Mete, O.; Asa, S.L.; Hayashi, K.; Takeshita, A.; Fukuhara, N.; Yamaguchi-Okada, M.; Takeuchi, Y.; Yamada, S. The Complementary Role of Transcription Factors in the Accurate Diagnosis of Clinically Nonfunctioning Pituitary Adenomas. Endocr. Pathol. 2015, 26, 349–355. [Google Scholar] [CrossRef]
- Marro, B.; Zouaoui, A.; Sahel, M.; Crozat, N.; Gerber, S.; Sourour, N.; Sag, K.; Marsault, C. MRI of pituitary adenomas in acromegaly. Neuroradiology 1997, 39, 394–399. [Google Scholar] [CrossRef]
- Chen, X.; Dai, J.; Ai, L.; Ru, X.; Wang, J.; Li, S.; Young, G.S. Clival invasion on multi-detector CT in 390 pituitary macroadenomas: Correlation with sex, subtype and rates of operative complication and recurrence. Am. J. Neuroradiol. 2011, 32, 785–789. [Google Scholar] [CrossRef]
- Peker, S.; Kurtkaya-Yapicier, O.; Kilic, T.; Pamir, M.N. Microsurgical anatomy of the lateral walls of the pituitary fossa. Acta Neurochir. 2005, 147, 641–648, Discussions 649. [Google Scholar] [CrossRef] [PubMed]
- Chacko, A.G.; Chacko, G.; Seshadri, M.S.; Chandy, M.J. The “capsule” of pituitary macroadenomas represents normal pituitary gland: A histopathological study. Br. J. Neurosurg. 2003, 17, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, S.; Anik, I.; Koc, K.; Kokturk, S.; Cine, N.; Savli, H.; Sirin, G.; Sam, B.; Gazioglu, N. Microsurgical anatomy of membranous layers of the pituitary gland and the expression of extracellular matrix collagenous proteins. Acta Neurochir. 2011, 153, 2435–2443. [Google Scholar] [CrossRef]
- Yilmazlar, S.; Kocaeli, H.; Aydiner, F.; Korfali, E. Medial portion of the cavernous sinus: Quantitative analysis of the medial wall. Clin. Anat. 2005, 18, 416–422. [Google Scholar] [CrossRef]
- Campero, A.; Campero, A.A.; Martins, C.; Yasuda, A.; Rhoton, A.L. Surgical anatomy of the dural walls of the cavernous sinus. J. Clin. Neurosci. 2010, 17, 746–750. [Google Scholar] [CrossRef]
- Yasuda, A.; Campero, A.; Martins, C.; Rhoton, A.L.; Ribas, G.C. The medial wall of the cavernous sinus: Microsurgical anatomy. Neurosurgery 2004, 55, 179–189, Discussion 189–190. [Google Scholar] [CrossRef]
- Songtao, Q.; Yuntao, L.; Jun, P.; Chuanping, H.; Xiaofeng, S. Membranous layers of the pituitary gland: Histological anatomic study and related clinical issues. Neurosurgery 2009, 64, 1–9, Discussions 9–10. [Google Scholar] [CrossRef]
- Destrieux, C.; Kakou, M.K.; Velut, S.; Lefrancq, T.; Jan, M. Microanatomy of the hypophyseal fossa boundaries. J. Neurosurg. 1998, 88, 743–752. [Google Scholar] [CrossRef]
- Knappe, U.J.; Fink, T.; Fisseler-Eckhoff, A.; Schoenmayr, R. Expression of extracellular matrix-proteins in perisellar connective tissue and dura mater. Acta Neurochir. 2010, 152, 345–353. [Google Scholar] [CrossRef]
- Gonçalves, M.B.; de Oliveira, J.G.; Williams, H.A.; Alvarenga, R.M.P.; Landeiro, J.A. Cavernous sinus medial wall: Dural or fibrous layer? Systematic review of the literature. Neurosurg. Rev. 2012, 35, 147–153, Discussion 153–154. [Google Scholar] [CrossRef] [PubMed]
- Campero, A.; Martins, C.; Yasuda, A.; Rhoton, A.L., Jr. Microsurgical anatomy of the diaphragma sellae and its role in directing the pattern of growth of pituitary adenomas. Neurosurgery 2008, 62, 717–723, Discussion 717–723. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Zuccarello, M.; van Loveren, H.R.; Keller, J.T. Expanding the boundaries of the transsphenoidal approach: A microanatomic study. Clin. Anat. 2001, 14, 1–9. [Google Scholar] [CrossRef]
- Yokoyama, S.; Hirano, H.; Moroki, K.; Goto, M.; Imamura, S.; Kuratsu, J.I. Are nonfunctioning pituitary adenomas extending into the cavernous sinus aggressive and/or invasive? Neurosurgery 2001, 49, 857–862, Discussion 862–863. [Google Scholar] [PubMed]
- Kursat, E.; Yilmazlar, S.; Aker, S.; Aksoy, K.; Oygucu, H. Comparison of lateral and superior walls of the pituitary fossa with clinical emphasis on pituitary adenoma extension: Cadaveric-anatomic study. Neurosurg. Rev. 2008, 31, 91–98, Discussion 98–99. [Google Scholar] [CrossRef]
- Knappe, U.J.; Konerding, M.A.; Schoenmayr, R. Medial wall of the cavernous sinus: Microanatomical diaphanoscopic and episcopic investigation. Acta Neurochir. 2009, 151, 961–967, Discussion 967. [Google Scholar] [CrossRef] [PubMed]
- Kawase, T.; Van Loveren, H.; Keller, J.T.; Tew, J.M. Meningeal architecture of the cavernous sinus: Clinical and surgical implications. Neurosurgery 1996, 39, 527–534, Discussion 534–546. [Google Scholar] [PubMed]
- Hoang, N.; Tran, D.K.; Herde, R.; Couldwell, G.C.; Osborn, A.G.; Couldwell, W.T. Pituitary macroadenomas with oculomotor cistern extension and tracking: Implications for surgical management. J. Neurosurg. 2016, 125, 315–322. [Google Scholar] [CrossRef]
- Taniguchi, M.; Nakai, T.; Kimura, H.; Fujita, Y.; Uozumi, Y.; Kohta, M.; Kohmura, E. Endoscopic endonasal surgery for pituitary adenomas extending to the oculomotor cistern. Head Neck 2018, 40, 536–543. [Google Scholar] [CrossRef]
- Tosaka, M.; Shimizu, T.; Miyagishima, T.; Tanaka, Y.; Osawa, T.; Aihara, M.; Yamaguchi, R.; Yoshimoto, Y. Combined supra-infrasellar approach to pituitary macroadenoma with oculomotor cistern extension: Surgical strategy and experience. Acta Neurochir. 2019, 161, 1025–1031. [Google Scholar] [CrossRef]
- Ferrareze Nunes, C.; Lieber, S.; Truong, H.Q.; Zenonos, G.; Wang, E.W.; Snyderman, C.H.; Gardner, P.A.; Fernandez-Miranda, J.C. Endoscopic endonasal transoculomotor triangle approach for adenomas invading the parapeduncular space: Surgical anatomy, technical nuances, and case series. J. Neurosurg. 2018, 130, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Gu, W.J.; Wang, C.Z.; Ji, X.J.; Mu, Y.M. Matrix metalloproteinase-9 and-2 and tissue inhibitor of matrix metalloproteinase-2 in invasive pituitary adenomas A systematic review and meta-analysis of case-control trials. Medicine 2016, 95, e3904. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Voellger, B.; Benzel, J.; Schlomann, U.; Nimsky, C.; Bartsch, J.W.; Carl, B. Metalloproteinases ADAM12 and MMP-14 are associated with cavernous sinus invasion in pituitary adenomas. Int. J. Cancer 2016, 139, 1327–1339. [Google Scholar] [CrossRef]
- Liu, W.; Kunishio, K.; Matsumoto, Y.; Okada, M.; Nagao, S. Matrix metalloproteinase-2 expression correlates with cavernous sinus invasion in pituitary adenomas. J. Clin. Neurosci. 2005, 12, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, E.; Kachra, Z.; Mousseau, N.; Delbecchi, L.; Hardy, J.; Beliveau, R. Matrix metalloproteinases and their inhibitors in human pituitary tumors. Neurosurgery 1999, 45, 1432–1440, Discussion 1440–1441. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Kawamoto, K.; Mizoue, T.; Uozumi, T.; Arita, K.; Kurisu, K. Matrix metalloproteinase-9 secretion by human pituitary adenomas detected by cell immunoblot analysis. Acta Neurochir. 1996, 138, 1442–1448. [Google Scholar] [CrossRef]
- Ceylan, S.; Anik, I.; Cabuk, B.; Caklili, M.; Anik, Y. Extension Pathways of Pituitary Adenomas with Cavernous Sinus Involvement and Its Surgical Approaches. World Neurosurg. 2019, 986–995. [Google Scholar] [CrossRef]
- Lonser, R.R.; Ksendzovsky, A.; Wind, J.J.; Vortmeyer, A.O.; Oldfield, E.H. Prospective evaluation of the characteristics and incidence of adenoma-associated dural invasion in Cushing disease. J. Neurosurg. 2012, 116, 272–279. [Google Scholar] [CrossRef]
- Micko, A.S.G.; Wöhrer, A.; Wolfsberger, S.; Knosp, E. Invasion of the cavernous sinus space in pituitary adenomas: Endoscopic verification and its correlation with an MRI-based classification. J. Neurosurg. 2015, 122, 803–811. [Google Scholar] [CrossRef]
- Asioli, S.; Righi, A.; Iommi, M.; Baldovini, C.; Ambrosi, F.; Guaraldi, F.; Zoli, M.; Mazzatenta, D.; Faustini-Fustini, M.; Rucci, P.; et al. Validation of a clinicopathological score for the prediction of post-surgical evolution of pituitary adenoma: Retrospective analysis on 566 patients from a tertiary care centre. Eur. J. Endocrinol. 2019, 180, 127–134. [Google Scholar] [CrossRef]
- Frank, G.; Pasquini, E. Endoscopic endonasal cavernous sinus surgery, with special reference to pituitary adenomas. Front. Horm. Res. 2006, 34, 64–82. [Google Scholar] [PubMed]
- Frank, G.; Pasquini, E. Cavernous sinus: Endoscopic endonasal approaches. In Endoscopic Approaches to the Skull Base; Prog Neurol Surg.; Kassam, A.B., Gardner, P.A., Eds.; Karger: Basel, Switzerland, 2012; Volume 26, pp. 119–139. [Google Scholar]
- Ceylan, S.; Anik, I.; Koc, K. A new endoscopic surgical classification and invasion criteria for pituitary adenomas involving the cavernous sinus. Turk. Neurosurg. 2011, 21, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Theodosopoulos, P.V.; Cebula, H.; Kurbanov, A.; Cabero, A.B.; Osorio, J.A.; Zimmer, L.A.; Froelich, S.C.; Keller, J.T. The Medial Extra-Sellar Corridor to the Cavernous Sinus: Anatomic Description and Clinical Correlation. World Neurosurg. 2016, 96, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, Y.; Tachibana, O.; Doai, M.; Akai, T.; Tonami, H.; Iizuka, H. Internal carotid arterial shift after transsphenoidal surgery in pituitary adenomas with cavernous sinus invasion. Pituitary 2013, 16, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Knosp, E.; Steiner, E.; Kitz, K.; Matula, C. Pituitary adenomas with invasion of the cavernous sinus space: A magnetic resonance imaging classification compared with surgical findings. Neurosurgery 1993, 33, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Connor, S.E.; Wilson, F.; Hogarth, K. Magnetic resonance imaging criteria to predict complete excision of parasellar pituitary macroadenoma on postoperative imaging. J. Neurol. Surg. Part B 2014, 75, 41–46. [Google Scholar]
- Fernandez-Miranda, J.C.; Zwagerman, N.T.; Abhinav, K.; Lieber, S.; Wang, E.W.; Snyderman, C.H.; Gardner, P.A. Cavernous sinus compartments from the endoscopic endonasal approach: Anatomical considerations and surgical relevance to adenoma surgery. J. Neurosurg. 2018, 129, 430–441. [Google Scholar] [CrossRef]
- Trevisi, G.; Vigo, V.; Morena, M.G.; Grieco, D.L.; Rigante, M.; Anile, C.; Mangiola, A. Comparison of Endoscopic Versus Microsurgical Resection of Pituitary Adenomas with Parasellar Extension and Evaluation of the Predictive Value of a Simple 4-Quadrant Radiologic Classification. World Neurosurg. 2019, 121, 769–774. [Google Scholar] [CrossRef]
- Cottier, J.P.; Destrieux, C.; Brunereau, L.; Bertrand, P.; Moreau, L.; Jan, M.; Herbreteau, D. Cavernous sinus invasion by pituitary adenoma: MR imaging. Radiology 2000, 215, 463–469. [Google Scholar] [CrossRef]
- Moreau, L.; Cottier, J.P.; Bertrand, P.; Destrieux, C.; Jan, M.; Sonier, C.B.; Herbreteau, D.; Rouleau, P. MRI diagnosis of sinus cavernous invasion by pituitary adenomas. J. Radiol. 1998, 79, 241–246. [Google Scholar] [CrossRef]
- Doglietto, F.; Prevedello, D.M.; Jane, J.A.; Han, J.; Laws, E.R. Brief history of endoscopic transsphenoidal surgery—From Philipp Bozzini to the First World Congress of Endoscopic Skull Base Surgery. Neurosurg. Focus 2005, 19, 1–6. [Google Scholar] [CrossRef]
- Cohen-Cohen, S.; Gardner, P.A.; Alves-Belo, J.T.; Truong, H.Q.; Snyderman, C.H.; Wang, E.W.; Fernandez-Miranda, J.C. The medial wall of the cavernous sinus. Part 2: Selective medial wall resection in 50 pituitary adenoma patients. J. Neurosurg. 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Truong, H.Q.; Lieber, S.; Najera, E.; Alves-Belo, J.T.; Gardner, P.A.; Fernandez-Miranda, J.C. The medial wall of the cavernous sinus. Part 1: Surgical anatomy, ligaments, and surgical technique for its mobilization and/or resection. J. Neurosurg. 2018, 1, 1–9. [Google Scholar] [CrossRef]
- Nagata, Y.; Takeuchi, K.; Yamamoto, T.; Ishikawa, T.; Kawabata, T.; Shimoyama, Y.; Wakabayashi, T. Removal of the Medial Wall of the Cavernous Sinus for Functional Pituitary Adenomas: A Technical Report and Pathologic Significance. World Neurosurg. 2019, 126, 53–58. [Google Scholar] [CrossRef]
- Nishioka, H.; Fukuhara, N.; Horiguchi, K.; Yamada, S. Aggressive transsphenoidal resection of tumors invading the cavernous sinus in patients with acromegaly: Predictive factors, strategies, and outcomes. J. Neurosurg. 2014, 121, 505–510. [Google Scholar] [CrossRef]
- Hlavac, M.; Knoll, A.; Etzrodt-Walter, G.; Sommer, F.; Scheithauer, M.; Coburger, J.; Wirtz, C.R.; Pala, A. Intraoperative MRI in transsphenoidal resection of invasive pituitary macroadenomas. Neurosurg. Rev. 2019, 42, 737–743. [Google Scholar] [CrossRef]
- Pal’a, A.; Knoll, A.; Brand, C.; Etzrodt-Walter, G.; Coburger, J.; Wirtz, C.R.; Hlavac, M. The Value of Intraoperative Magnetic Resonance Imaging in Endoscopic and Microsurgical Transsphenoidal Pituitary Adenoma Resection. World Neurosurg. 2017, 102, 144–150. [Google Scholar] [CrossRef]
- Li, J.; Cong, Z.; Ji, X.; Wang, X.; Hu, Z.; Jia, Y.; Wang, H. Application of intraoperative magnetic resonance imaging in large invasive pituitary adenoma surgery. Asian J. Surg. 2015, 38, 168–173. [Google Scholar] [CrossRef][Green Version]
- Pergolizzi Jr, R.S.; Nabavi, A.; Schwartz, R.B.; Hsu, L.; Wong, T.Z.; Martin, C.; Black, P.M.; Jolesz, F.A. Intra-operative MR guidance during trans-sphenoidal pituitary resection: Preliminary results. J. Magn. Reson. Imaging 2001, 13, 136–141. [Google Scholar] [CrossRef]
- Pergolizzi Jr, R.S.; Schwartz, R.B.; Hsu, L.; Wong, T.Z.; Black, P.M.; Martin, C.; Jolesz, F.A. Transsphenoidal pituitary resection with intraoperative MR guidance: Preliminary results. In Thermal Treatment of Tissue with Image Guidance; International Society for Optics and Photonics: Bellingham, WA, USA, 1999; Volume 3594, pp. 214–220. [Google Scholar]
- Serra, C.; Staartjes, V.E.; Maldaner, N.; Muscas, G.; Akeret, K.; Holzmann, D.; Soyka, M.B.; Schmid, C.; Regli, L. Predicting extent of resection in transsphenoidal surgery for pituitary adenoma. Acta Neurochir. 2018, 160, 2255–2262. [Google Scholar] [CrossRef]
- Li, Y.; Shu, K.; Dong, F.; Wan, F.; Lei, T.; Li, L. Relationship between histopathology and clinical prognosis of invasive pituitary adenoma. Chin. Ger. J. Clin. Oncol. 2005, 4, 179–182. [Google Scholar] [CrossRef]
- Connor, S.E.J.; Penney, C.C. MRI in the differential diagnosis of a sellar mass. Clin. Radiol. 2003, 58, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Luo, C.B.; Teng, M.M.; Guo, W.Y.; Chen, S.S.; Lirng, J.F.; Chang, F.C. Computed tomography and magnetic resonance imaging characteristics of giant pituitary adenomas. J. Formos. Med. Assoc. 2000, 99, 833–838. [Google Scholar] [PubMed]
- Hornyak, M.; Couldwell, W.T. Multimodality treatment for invasive pituitary adenomas. Postgrad. Med. 2009, 121, 168–176. [Google Scholar] [CrossRef]
- Hardy, J.; Vezina, J.L. Transsphenoidal neurosurgery of intracranial neoplasm. Adv. Neurol. 1976, 15, 261–273. [Google Scholar]
- Hardy, J. Transsphenoidal surgery of hypersecreting pituitary tumors. In Diagnosis and Treatment of Pituitary Tumors; Kohler, P.O., Ross, G.T., Eds.; Excerpta Medica: Amsterdam, The Netherlands, 1973; pp. 179–194. [Google Scholar]
- Wilson, C.B. A decade of pituitary microsurgery. The Herbert Olivecrona lecture. J. Neurosurg. 1984, 61, 814–833. [Google Scholar] [CrossRef]
- Davies, B.M.; Carr, E.; Soh, C.; Gnanalingham, K.K. Assessing size of pituitary adenomas: A comparison of qualitative and quantitative methods on MR. Acta Neurochir. 2016, 158, 677–683. [Google Scholar] [CrossRef]
- Mooney, M.A.; Hardesty, D.A.; Sheehy, J.P.; Bird, C.R.; Chapple, K.; White, W.L.; Little, A.S. Rater Reliability of the Hardy Classification for Pituitary Adenomas in the Magnetic Resonance Imaging Era. J. Neurol. Surg. Part B 2017, 78, 413–418. [Google Scholar] [CrossRef]
- Hirsch, W.L.; Roppolo, H.; Hayman, L. Sella and parasellar regions. Pathology. In MR and CT Imaging of the Head, Neck, and Spine; Latchaw, R., Ed.; Mosby: St. Louis, MO, USA, 1991; pp. 683–747. [Google Scholar]
- Scotti, G.; Yu, C.Y.; Dillon, W.P.; Norman, D.; Colombo, N.; Newton, T.H.; De Groot, J.; Wilson, C.B. MR imaging of cavernous sinus involvement by pituitary adenomas. Am. J. Neuroradiol. 1988, 9, 657–664. [Google Scholar] [CrossRef]
- Vieira, J.O., Jr.; Cukiert, A.; Liberman, B. Evaluation of magnetic resonance imaging criteria for cavernous sinus invasion in patients with pituitary adenomas: Logistic regression analysis and correlation with surgical findings. Surg. Neurol. 2006, 65, 130–135, Discussion 135. [Google Scholar] [CrossRef]
- Vieira, J.O., Jr.; Cukiert, A.; Liberman, B. Magnetic resonance imaging of cavernous sinus invasion by pituitary adenoma diagnostic criteria and surgical findings. Arq. De Neuro Psiquiatr. 2004, 62, 437–443. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonneville, J.F. Magnetic Resonance Imaging of Pituitary Tumors. In Frontiers of Hormone Research; Karger Publishers: Basel, Switzerland, 2016; Volume 45. [Google Scholar]
- Di Maio, S.; Biswas, A.; Vézina, J.L.; Hardy, J.; Mohr, G. Pre-and postoperative magnetic resonance imaging appearance of the normal residual pituitary gland following macroadenoma resection: Clinical implications. Surg. Neurol. Int. 2012, 3, 67. [Google Scholar] [PubMed]
- Chin, B.M.; Orlandi, R.R.; Wiggins, R.H. Evaluation of the Sellar and Parasellar Regions. Magn. Reson. Imaging Clin. N. Am. 2012, 20, 515–543. [Google Scholar] [CrossRef] [PubMed]
- Mastorakos, P.; Taylor, D.G.; Chen, C.J.; Buell, T.; Donahue, J.H.; Jane, J.A. Prediction of cavernous sinus invasion in patients with Cushing’s disease by magnetic resonance imaging. J. Neurosurg. 2019, 130, 1593–1598. [Google Scholar] [CrossRef]
- Cukiert, A.; Andrioli, M.; Goldman, J.; Nery, M.; Salgado, L.; Knoepfelmacher, M.; Pimentel, F.; Liberman, B. Cavernous sinus invasion by pituitary macroadenomas. Neuroradiological, clinical and surgical correlation. Arq. De Neuro Psiquiatr. 1998, 56, 107–110. [Google Scholar] [CrossRef]
- Ajlan, A.; Achrol, A.S.; Albakr, A.; Feroze, A.H.; Westbroek, E.M.; Hwang, P.; Harsh, G.R. Cavernous Sinus Involvement by Pituitary Adenomas: Clinical Implications and Outcomes of Endoscopic Endonasal Resection. J. Neurol. Surg. Part B 2017, 78, 273–282. [Google Scholar] [CrossRef]
- Saeki, N.; Iuchi, T.; Isono, S.; Eda, M.; Yamaura, A. MRI of growth hormone-secreting pituitary adenomas: Factors determining pretreatment hormone levels. Neuroradiology 1999, 41, 765–771. [Google Scholar] [CrossRef]
- Ouyang, T.; Rothfus, W.E.; Ng, J.M.; Challinor, S.M. Imaging of the pituitary. Radiol. Clin. N. Am. 2011, 49, 549–571. [Google Scholar] [CrossRef]
- Sol, Y.L.; Lee, S.K.; Choi, H.S.; Lee, Y.H.; Kim, J.; Kim, S.H. Evaluation of MRI Criteria for Cavernous Sinus Invasion in Pituitary Macroadenoma. J. Neuroimaging 2014, 24, 498–503. [Google Scholar] [CrossRef]
- Ruscalleda, J. Imaging of parasellar lesions. Eur. Radiol. 2005, 15, 549–559. [Google Scholar] [CrossRef]
- Rennert, J.; Doerfler, A. Imaging of sellar and parasellar lesions. Clin. Neurol. Neurosurg. 2007, 109, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E.; Cowen, L.; Stadiem, R.; Uihlein, A.; Naidich, M.; Russell, E. Tumors invading the cavernous sinus that cause internal carotid artery compression are rarely pituitary adenomas. Pituitary 2012, 15, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Micko, A.; Oberndorfer, J.; Weninger, W.J.; Vila, G.; Hoftberger, R.; Wolfsberger, S.; Knosp, E. Challenging Knosp high-grade pituitary adenomas. J. Neurosurg. 2019, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Edal, A.L.; Skjodt, K.; Nepper-Rasmussen, H.J. SIPAP—A new MR classification for pituitary adenomas. Suprasellar, infrasellar, parasellar, anterior and posterior. Acta Radiol. 1997, 38, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Sarlis, N.J.; Gourgiotis, L.; Koch, C.A.; Skarulis, M.C.; Brucker-Davis, F.; Doppman, J.L.; Oldfield, E.H.; Patronas, N.J. MR imaging features of thyrotropin-secreting pituitary adenomas at initial presentation. Am. J. Roentgenol. 2003, 181, 577–582. [Google Scholar] [CrossRef]
- Bourdelot, A.; Coste, J.; Hazebroucq, V.; Gaillard, S.; Cazabat, L.; Bertagna, X.; Bertherat, J. Clinical, hormonal and magnetic resonance imaging (MRI) predictors of transsphenoidal surgery outcome in acromegaly. Eur. J. Endocrinol. 2004, 150, 763–771. [Google Scholar] [CrossRef]
- Daita, G.; Yonemasu, Y.; Nakai, H.; Takei, H.; Ogawa, K. Cavernous Sinus Invasion by Pituitary Adenomas —Relationship between Magnetic Resonance Imaging Findings and Histologically Verified Dural Invasion. Neurol. Med. Chir. 1995, 35, 17–21. [Google Scholar] [CrossRef][Green Version]
- Goel, A.; Nadkarni, T.; Muzumdar, D.; Desai, K.; Phalke, U.; Sharma, P. Giant pituitary tumors: A study based on surgical treatment of 118 cases. Surg. Neurol. 2004, 61, 436–445. [Google Scholar] [CrossRef]
- Hwang, J.; Seol, H.J.; Nam, D.H.; Lee, J.I.; Lee, M.H.; Kong, D.S. Therapeutic Strategy for Cavernous Sinus-Invading Non-Functioning Pituitary Adenomas Based on the Modified Knosp Grading System. Brain Tumor Res. Treat. 2016, 4, 63–69. [Google Scholar] [CrossRef]
- Buliman, A.; Tataranu, L.G.; Ciubotaru, V.; Cazac, T.L.; Dumitrache, C. The multimodal management of GH-secreting pituitary adenomas: Predictive factors, strategies and outcomes. J. Med. Life 2016, 9, 187–192. [Google Scholar]
- Tanei, T.; Nagatani, T.; Nakahara, N.; Watanabe, T.; Nishihata, T.; Nielsen, M.L.; Takebayashi, S.; Hirano, M.; Wakabayashi, T. Use of high-field intraoperative magnetic resonance imaging during endoscopic transsphenoidal surgery for functioning pituitary microadenomas and small adenomas located in the intrasellar region. Neurol. Med. Chir. 2013, 53, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Sanmillan, J.L.; Torres-Diaz, A.; Sanchez-Fernandez, J.J.; Lau, R.; Ciller, C.; Puyalto, P.; Gabarros, A. Radiologic Predictors for Extent of Resection in Pituitary Adenoma Surgery. A Single-Center Study. World Neurosurg. 2017, 108, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Ramm-Pettersen, J.; Berg-Johnsen, J.; Hol, P.K.; Roy, S.; Bollerslev, J.; Schreiner, T.; Helseth, E. Intra-operative MRI facilitates tumour resection during trans-sphenoidal surgery for pituitary adenomas. Acta Neurochir. 2011, 153, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Pamir, M.N.; Kilic, T.; Özek, M.M.; Özduman, K.; Türe, U. Non-meningeal tumours of the cavernous sinus: A surgical analysis. J. Clin. Neurosci. 2006, 13, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Chohan, M.O.; Levin, A.M.; Singh, R.; Zhou, Z.; Green, C.L.; Kazam, J.J.; Tsiouris, A.J.; Anand, V.K.; Schwartz, T.H. Three-dimensional volumetric measurements in defining endoscope-guided giant adenoma surgery outcomes. Pituitary 2016, 19, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Wolfsberger, S.; Ba-Ssalamah, A.; Pinker, K.; Mlynarik, V.; Czech, T.; Knosp, E.; Trattnig, S. Application of three-tesla magnetic resonance imaging for diagnosis and surgery of sellar lesions. J. Neurosurg. 2004, 100, 278–286. [Google Scholar] [CrossRef]
- Cao, L.; Chen, H.; Hong, J.; Ma, M.; Zhong, Q.; Wang, S. Magnetic resonance imaging appearance of the medial wall of the cavernous sinus for the assessment of cavernous sinus invasion by pituitary adenomas. J. Neuroradiol. 2013, 40, 245–251. [Google Scholar] [CrossRef]
- Davis, M.A.; Castillo, M. Evaluation of the pituitary gland using magnetic resonance imaging: T1-weighted vs. VIBE imaging. Neuroradiol. J. 2013, 26, 297–300. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Yao, Z.; Yang, Z.; Ma, Z.; Wang, Y. Effective performance of contrast enhanced SPACE imaging in clearly depicting the margin of pituitary adenoma. Pituitary 2015, 18, 480–486. [Google Scholar] [CrossRef]
- Lien, R.J.; Corcuera-Solano, I.; Pawha, P.S.; Naidich, T.P.; Tanenbaum, L.N. Three-tesla imaging of the pituitary and parasellar region: T1-weighted 3-dimensional fast spin echo cube outperforms conventional 2-dimensional magnetic resonance imaging. J. Comput. Assist. Tomogr. 2015, 39, 329–333. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, S.; Ma, S.; Diao, J.; Zhou, W.; Tian, J.; Zang, Y.; Jia, W. Preoperative prediction of cavernous sinus invasion by pituitary adenomas using a radiomics method based on magnetic resonance images. Eur. Radiol. 2019, 29, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Yoneoka, Y.; Watanabe, N.; Matsuzawa, H.; Tsumanuma, I.; Ueki, S.; Nakada, T.; Fujii, Y. Preoperative depiction of cavernous sinus invasion by pituitary macroadenoma using three-dimensional anisotropy contrast periodically rotated overlapping parallel lines with enhanced reconstruction imaging on a 3-tesla system. J. Neurosurg. 2008, 108, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Linn, J.; Peters, F.; Lummel, N.; Schankin, C.; Rachinger, W.; Brueckmann, H.; Yousry, I. Detailed imaging of the normal anatomy and pathologic conditions of the cavernous region at 3 Tesla using a contrast-enhanced MR angiography. Neuroradiology 2011, 53, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, S.; Aoki, S.; Ohtomo, K. Cranial nerve assessment in cavernous sinus tumors with contrast-enhanced 3D fast-imaging employing steady-state acquisition MR imaging. Neuroradiology 2009, 51, 467–470. [Google Scholar] [CrossRef]
- Selman, W.R.; Laws, E.R., Jr.; Scheithauer, B.W.; Carpenter, S.M. The occurrence of dural invasion in pituitary adenomas. J. Neurosurg. 1986, 64, 402–407. [Google Scholar] [CrossRef]
- Meij, B.P.; Lopes, M.B.; Ellegala, D.B.; Alden, T.D.; Laws, E.R., Jr. The long-term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated with transsphenoidal surgery. J. Neurosurg. 2002, 96, 195–208. [Google Scholar] [CrossRef]
- Daita, G.; Yonemasu, Y. Dural invasion and proliferative potential of pituitary adenomas. Neurol. Med. Chir. 1996, 36, 211–214. [Google Scholar] [CrossRef]
- Landolt, A.M.; Shibata, T.; Kleihues, P. Growth rate of human pituitary adenomas. J. Neurosurg. 1987, 67, 803–806. [Google Scholar] [CrossRef]
- Miermeister, C.P.; Petersenn, S.; Buchfelder, M.; Fahlbusch, R.; Ludecke, D.K.; Holsken, A.; Bergmann, M.; Knappe, H.U.; Hans, V.H.; Flitsch, J.; et al. Histological criteria for atypical pituitary adenomas - data from the German pituitary adenoma registry suggests modifications. Acta Neuropathol. Commun. 2015, 3, 50. [Google Scholar] [CrossRef]
- Asa, S.L.; Casar-Borota, O.; Chanson, P.; Delgrange, E.; Earls, P.; Ezzat, S.; Grossman, A.; Ikeda, H.; Inoshita, N.; Karavitaki, N.; et al. From pituitary adenoma to pituitary neuroendocrine tumor (pitnet): An international pituitary pathology club proposal. Endocr. Relat. Cancer 2017, 24, C5–C8. [Google Scholar] [CrossRef]
- Trouillas, J.; Roy, P.; Sturm, N.; Dantony, E.; Cortet-Rudelli, C.; Viennet, G.; Bonneville, J.F.; Assaker, R.; Auger, C.; Brue, T.; et al. A new prognostic clinicopathological classification of pituitary adenomas: A multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 2013, 126, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Raverot, G.; Jouanneau, E.; Trouillas, J. Management of endocrine disease: Clinicopathological classification and molecular markers of pituitary tumours for personalized therapeutic strategies. Eur. J. Endocrinol. 2014, 170, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Vasiljevic, A.; Jaffrain-Rea, M.L.; Ansorge, O.; Asioli, S.; Barresi, V.; Chinezu, L.; Gardiman, M.P.; Lania, A.; Lapshina, A.M.; et al. A standardised diagnostic approach to pituitary neuroendocrine tumours (PitNETs): A European Pituitary Pathology Group (EPPG) proposal. Virchows Arch. 2019, 475, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Chacko, A.G.; Chacko, G. Clinicopathological correlates of extrasellar growth patterns in pituitary adenomas. J. Clin. Neurosci. 2015, 22, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.C.; Marroni, C.P.; Pizarro, C.B.; Pereira-Lima, J.F.; Barbosa-Coutinho, L.M.; Ferreira, N.P. Expression of p53 protein in pituitary adenomas. Braz. J. Med. Biol. Res. 2002, 35, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, A.; Bayram, F.; Ozturk, F.; Tumturk, A.; Diri, H.; Oral, S.; Tucer, B.; Durak, A.C.; Kurtsoy, A. Evaluation of Aggressive Behavior and Invasive Features of Pituitary Adenomas Using Radiological, Surgical, Clinical and Histopathological Markers. Turk. Neurosurg. 2016, 26, 671–677. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saeger, W. Pituitary tumors: Prognostic indicators. Endocrine 2005, 28, 57–66. [Google Scholar] [CrossRef]
- Schreiber, S.; Saeger, W.; Ludecke, D.K. Proliferation markers in different types of clinically non-secreting pituitary adenomas. Pituitary 1999, 1, 213–220. [Google Scholar] [CrossRef]
- Wierzbicka-Tutka, I.; Sokołowski, G.; Bałdys-Waligórska, A.; Adamek, D.; Radwańska, E.; Gołkowski, F. PTTG and Ki-67 expression in pituitary adenomas. Przeglad Lekarski 2016, 73, 53–58. [Google Scholar]
- Wolfsberger, S.; Kitz, K.; Wunderer, J.; Czech, T.; Boecher-Schwarz, H.G.; Hainfellner, J.A.; Knosp, E. Multiregional sampling reveals a homogenous distribution of Ki-67 proliferation rate in pituitary adenomas. Acta Neurochir. 2004, 146, 1323–1327, Discussion 1327–1328. [Google Scholar] [CrossRef]
- Glebauskiene, B.; Liutkeviciene, R.; Vilkeviciute, A.; Gudinaviciene, I.; Rocyte, A.; Simonaviciute, D.; Mazetyte, R.; Kriauciuniene, L.; Zaliuniene, D. Association of Ki-67 Labelling Index and IL-17A with Pituitary Adenoma. Biomed. Res. Int. 2018, 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Chacko, G.; Chacko, A.G.; Lombardero, M.; Mani, S.; Seshadri, M.S.; Kovacs, K.; Scheithauer, B.W. Clinicopathologic correlates of giant pituitary adenomas. J. Clin. Neurosci. 2009, 16, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Paek, K.I.; Kim, S.H.; Song, S.H.; Choi, S.W.; Koh, H.S.; Youm, J.Y.; Kim, Y. Clinical significance of Ki-67 labeling index in pituitary macroadenoma. J. Korean Med. Sci. 2005, 20, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Pang, B.; Wang, Q.; Yang, S.; Gao, T.; Ding, Q.; Liu, H.; Yang, Y.; Fan, H.; Zhang, R.; et al. EZH2 upregulation correlates with tumor invasiveness, proliferation, and angiogenesis in human pituitary adenomas. Hum. Pathol. 2017, 66, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Matsumoto, Y.; Okada, M.; Miyake, K.; Kunishio, K.; Kawai, N.; Tamiya, T.; Nagao, S. Matrix metalloproteinase 2 and 9 expression correlated with cavernous sinus invasion of pituitary adenomas. J. Med. Investig. 2005, 52, 151–158. [Google Scholar] [CrossRef][Green Version]
- Pan, Y.; Han, C.; Wang, C.; Hu, G.; Luo, C.; Gan, X.; Zhang, F.; Lu, Y.; Ding, X. ADAM10 promotes pituitary adenoma cell migration by regulating cleavage of CD44 and L1. J. Mol. Endocrinol. 2012, 49, 21–33. [Google Scholar] [CrossRef]
- Huang, J.; Pan, Y.; Hu, G.; Sun, W.; Jiang, L.; Wang, P.; Ding, X. SRC fine-tunes ADAM10 shedding activity to promote pituitary adenoma cell progression. FEBS J. 2019. [Google Scholar] [CrossRef]
- Sun, B.; Liu, X.; Yang, Y.; Dai, C.; Li, Y.; Jiao, Y.; Wei, Z.; Yao, Y.; Feng, M.; Bao, X.; et al. The Clinical Utility of TIMP3 Expression in ACTH-Secreting Pituitary Tumor. J. Mol. Neurosci. 2016, 58, 137–144. [Google Scholar] [CrossRef]
- Ozkaya, H.M.; Comunoglu, N.; Keskin, F.E.; Oz, B.; Haliloglu, O.A.; Tanriover, N.; Gazioglu, N.; Kadioglu, P. Locally produced estrogen through aromatization might enhance tissue expression of pituitary tumor transforming gene and fibroblast growth factor 2 in growth hormone-secreting adenomas. Endocrine 2016, 52, 632–640. [Google Scholar] [CrossRef]
- Zhu, X.; Asa, S.L.; Ezzat, S. Fibroblast growth factor 2 and estrogen control the balance of histone 3 modifications targeting MAGE-A3 in pituitary neoplasia. Clin. Cancer Res. 2008, 14, 1984–1996. [Google Scholar] [CrossRef]
- Ezzat, S.; Zheng, L.; Asa, S.L. Pituitary tumor-derived fibroblast growth factor receptor 4 isoform disrupts neural cell-adhesion molecule/N-cadherin signaling to diminish cell adhesiveness: A mechanism underlying pituitary neoplasia. Mol. Endocrinol. 2004, 18, 2543–2552. [Google Scholar] [CrossRef]
- Trouillas, J.; Daniel, L.; Guigard, M.P.; Tong, S.; Gouvernet, J.; Jouanneau, E.; Jan, M.; Perrin, G.; Fischer, G.; Tabarin, A.; et al. Polysialylated neural cell adhesion molecules expressed in human pituitary tumors and related to extrasellar invasion. J. Neurosurg. 2003, 98, 1084–1093. [Google Scholar] [CrossRef]
- Heath, V. Pituitary: Nuclear translocation of E-cadherin in invasive pituitary adenomas. Nat. Rev. Endocrinol. 2009, 5, 235. [Google Scholar] [CrossRef]
- Elston, M.S.; Gill, A.J.; Conaglen, J.V.; Clarkson, A.; Cook, R.J.; Little, N.S.; Robinson, B.G.; Clifton-Bligh, R.J.; McDonald, K.L. Nuclear accumulation of E-Cadherin correlates with loss of cytoplasmic membrane staining and invasion in pituitary adenomas. J. Clin. Endocrinol. Metab. 2009, 94, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Mallea-Gil, M.S.; Cristina, C.; Perez-Millan, M.I.; Villafane, A.M.; Ballarino, C.; Stalldecker, G.; Becu-Villalobos, D. Invasive giant prolactinoma with loss of therapeutic response to cabergoline: Expression of angiogenic markers. Endocr. Pathol. 2009, 20, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Turner, H.E.; Harris, A.L.; Melmed, S.; Wass, J.A. Angiogenesis in endocrine tumors. Endocr. Rev. 2003, 24, 600–632. [Google Scholar] [CrossRef]
- Cohen, A.B.; Lessell, S. Angiogenesis and pituitary tumors. Semin. Ophthalmol. 2009, 24, 185–189. [Google Scholar] [CrossRef]
- Cristina, C.; Luque, G.M.; Demarchi, G.; Lopez Vicchi, F.; Zubeldia-Brenner, L.; Perez Millan, M.I.; Perrone, S.; Ornstein, A.M.; Lacau-Mengido, I.M.; Berner, S.I.; et al. Angiogenesis in pituitary adenomas: Human studies and new mutant mouse models. Int. J. Endocrinol. 2014, 2014. [Google Scholar] [CrossRef]
- Ortiz, L.D.; Syro, L.V.; Scheithauer, B.W.; Ersen, A.; Uribe, H.; Fadul, C.E.; Rotondo, F.; Horvath, E.; Kovacs, K. Anti-VEGF therapy in pituitary carcinoma. Pituitary 2012, 15, 445–449. [Google Scholar] [CrossRef]
- Yang, Q.; Li, X. Molecular network basis of invasive pituitary adenoma: A review. Front. Endocrinol. 2019, 10, 7. [Google Scholar] [CrossRef]
- Zhou, W.; Ma, C.-X.; Xing, Y.-Z.; Yan, Z.-Y. Identification of candidate target genes of pituitary adenomas based on the DNA microarray. Mol. Med. Rep. 2016, 13, 2182–2186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, W.; Xu, Z.; Fu, L.; Liu, W.; Li, X. Pathogenesis analysis of pituitary adenoma based on gene expression profiling. Oncol. Lett. 2014, 8, 2423–2430. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seltzer, J.; Scotton, T.C.; Kang, K.; Zada, G.; Carmichael, J.D. Gene expression in prolactinomas: A systematic review. Pituitary 2016, 19, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yu, S.Y.; Li, C.Z.; Li, Z.Y.; Zhang, Y.Z. Integrative proteomics and transcriptomics revealed that activation of the IL-6R/JAK2/STAT3/MMP9 signaling pathway is correlated with invasion of pituitary null cell adenomas. Mol. Cell. Endocrinol. 2016, 436, 195–203. [Google Scholar] [CrossRef]
- Cao, C.; Wang, W.; Ma, C.; Jiang, P. Computational analysis identifies invasion-associated genes in pituitary adenomas. Mol. Med. Rep. 2015, 12, 1977–1982. [Google Scholar] [CrossRef]
- Joshi, H.; Vastrad, B.; Vastrad, C. Identification of Important Invasion-Related Genes in Non-functional Pituitary Adenomas. J. Mol. Neurosci. 2019, 68, 565–589. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Y.; Wu, L.; Cheng, R.; Li, C.; Qu, C.; Ji, H. Immunohistochemical Study of NR2C2, BTG2, TBX19, and CDK2 Expression in 31 Paired Primary/Recurrent Nonfunctioning Pituitary Adenomas. Int. J. Endocrinol. 2019, 2019, 8. [Google Scholar] [CrossRef]
- Wierinckx, A.; Roche, M.; Legras-Lachuer, C.; Trouillas, J.; Raverot, G.; Lachuer, J. MicroRNAs in pituitary tumors. Mol. Cell. Endocrinol. 2017, 456, 51–61. [Google Scholar] [CrossRef]
- Liang, H.Q.; Wang, R.J.; Diao, C.F.; Li, J.W.; Su, J.L.; Zhang, S. The PTTG1-targeting miRNAs miR-329, miR-300, miR-381, and miR-655 inhibit pituitary tumor cell tumorigenesis and are involved in a p53/PTTG1 regulation feedback loop. Oncotarget 2015, 6, 29413–29427. [Google Scholar] [CrossRef]
- Yu, G.; Wang, H.; Yu, S.; Li, C.; Bai, J.; Gui, S.; Zhang, Y.; Zhao, P. Study on miRNAs’ expression for the invasion of pituitary adenomas. Turk. Neurosurg. 2017. [Google Scholar] [CrossRef]
- Manoranjan, B.; Mahendram, S.; Almenawer, S.A.; Venugopal, C.; McFarlane, N.; Hallett, R.; Vijayakumar, T.; Algird, A.; Murty, N.K.; Sommer, D.D.; et al. The identification of human pituitary adenoma-initiating cells. Acta Neuropathol. Commun. 2016, 4, 125. [Google Scholar] [CrossRef]
- Oriola, J.; Lucas, T.; Halperin, I.; Mora, M.; Perales, M.J.; Alvarez-Escolá, C.; De Miguel-Novoa, P.; Soto, G.D.; Salinas, I.; Julián, M.T.; et al. Germline mutations of AIP gene in somatotropinomas resistant to somatostatin analogues. Eur. J. Endocrinol. 2013, 168, 9–13. [Google Scholar] [CrossRef]
- Barry, S.; Carlsen, E.; Marques, P.; Stiles, C.E.; Gadaleta, E.; Berney, D.M.; Roncaroli, F.; Chelala, C.; Solomou, A.; Herincs, M.; et al. Tumor microenvironment defines the invasive phenotype of AIP-mutation-positive pituitary tumors. Oncogene 2019, 38, 5381–5395. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serioli, S.; Doglietto, F.; Fiorindi, A.; Biroli, A.; Mattavelli, D.; Buffoli, B.; Ferrari, M.; Cornali, C.; Rodella, L.; Maroldi, R.; et al. Pituitary Adenomas and Invasiveness from Anatomo-Surgical, Radiological, and Histological Perspectives: A Systematic Literature Review. Cancers 2019, 11, 1936. https://doi.org/10.3390/cancers11121936
Serioli S, Doglietto F, Fiorindi A, Biroli A, Mattavelli D, Buffoli B, Ferrari M, Cornali C, Rodella L, Maroldi R, et al. Pituitary Adenomas and Invasiveness from Anatomo-Surgical, Radiological, and Histological Perspectives: A Systematic Literature Review. Cancers. 2019; 11(12):1936. https://doi.org/10.3390/cancers11121936
Chicago/Turabian StyleSerioli, Simona, Francesco Doglietto, Alessandro Fiorindi, Antonio Biroli, Davide Mattavelli, Barbara Buffoli, Marco Ferrari, Claudio Cornali, Luigi Rodella, Roberto Maroldi, and et al. 2019. "Pituitary Adenomas and Invasiveness from Anatomo-Surgical, Radiological, and Histological Perspectives: A Systematic Literature Review" Cancers 11, no. 12: 1936. https://doi.org/10.3390/cancers11121936
APA StyleSerioli, S., Doglietto, F., Fiorindi, A., Biroli, A., Mattavelli, D., Buffoli, B., Ferrari, M., Cornali, C., Rodella, L., Maroldi, R., Gasparotti, R., Nicolai, P., Fontanella, M. M., & Poliani, P. L. (2019). Pituitary Adenomas and Invasiveness from Anatomo-Surgical, Radiological, and Histological Perspectives: A Systematic Literature Review. Cancers, 11(12), 1936. https://doi.org/10.3390/cancers11121936