Fibroblasts in Nodular Sclerosing Classical Hodgkin Lymphoma Are Defined by a Specific Phenotype and Protect Tumor Cells from Brentuximab-Vedotin Induced Injury
Abstract
:1. Introduction
2. Results
2.1. Fibroblast Cultures Obtained from Primary Lymph Node Suspensions Show a High Purity
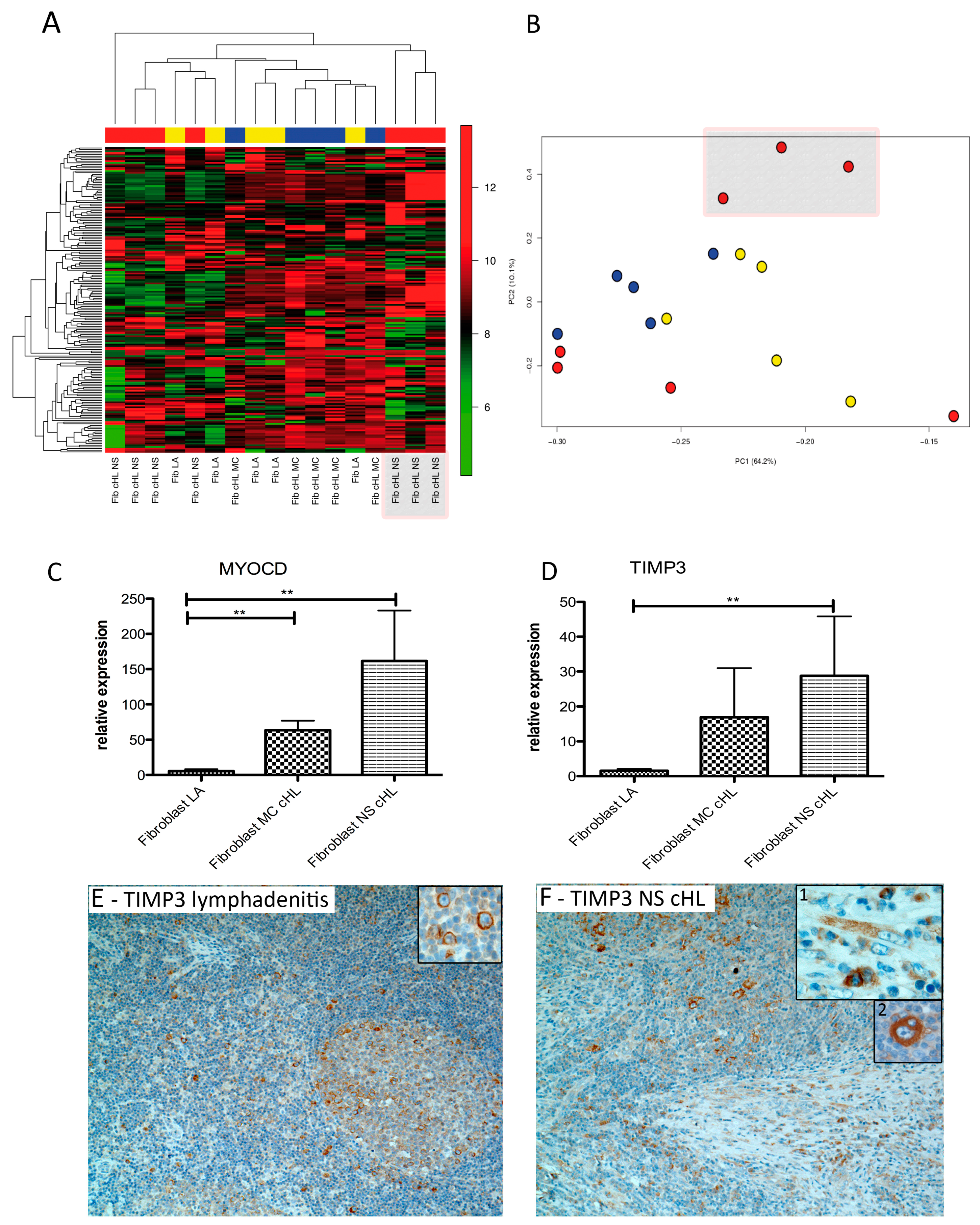
2.2. Fibroblasts Derived from NS cHL Differ in Their Gene Expression Patterns from Fibroblasts Isolated from MC cHL or Reactive Lymph Nodes
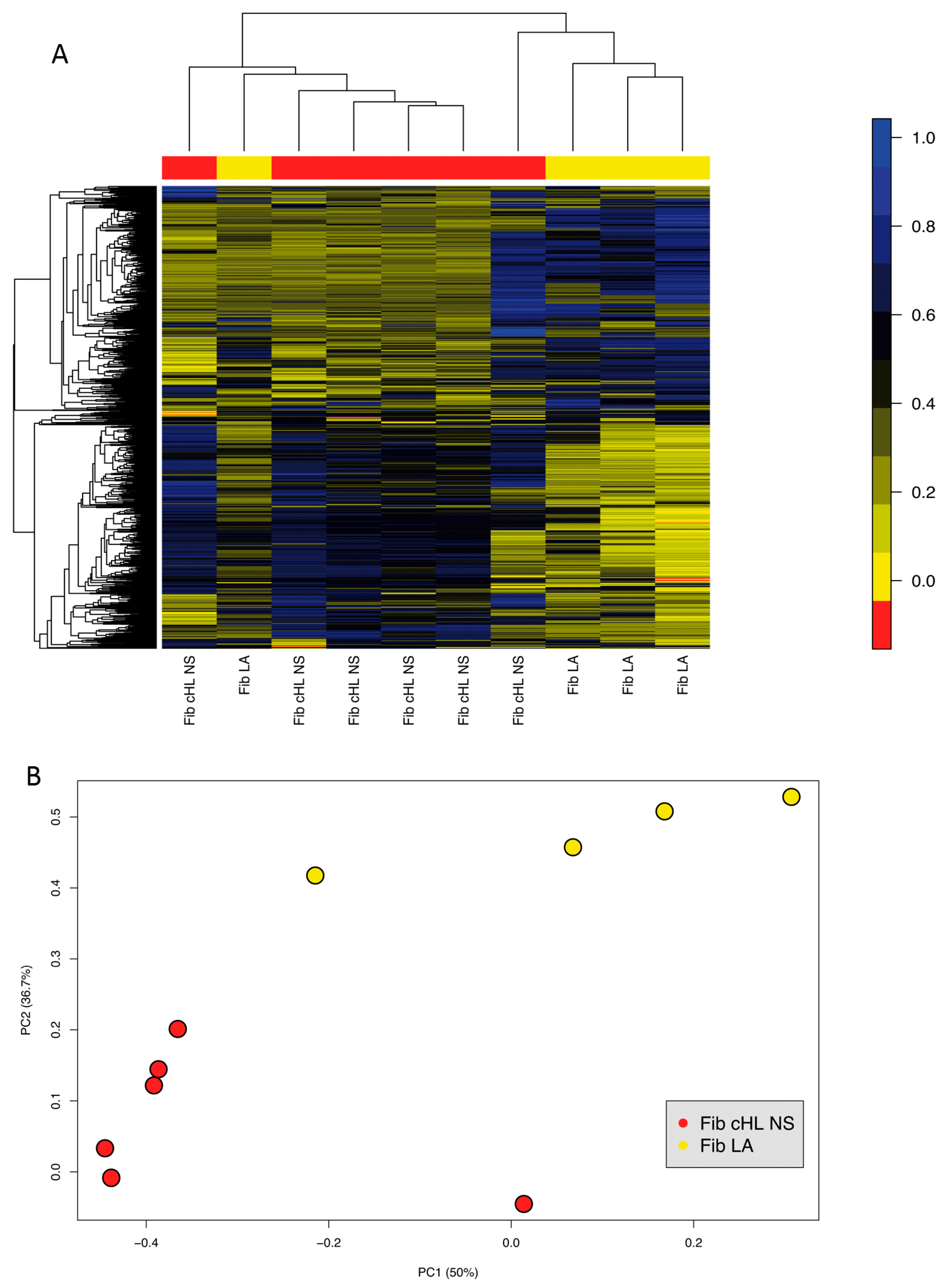
2.3. Fibroblasts Derived from NS cHL Maintain Stable Methylation Profiles in Culture When Compared with Lymphadenitis-Derived Fibroblasts
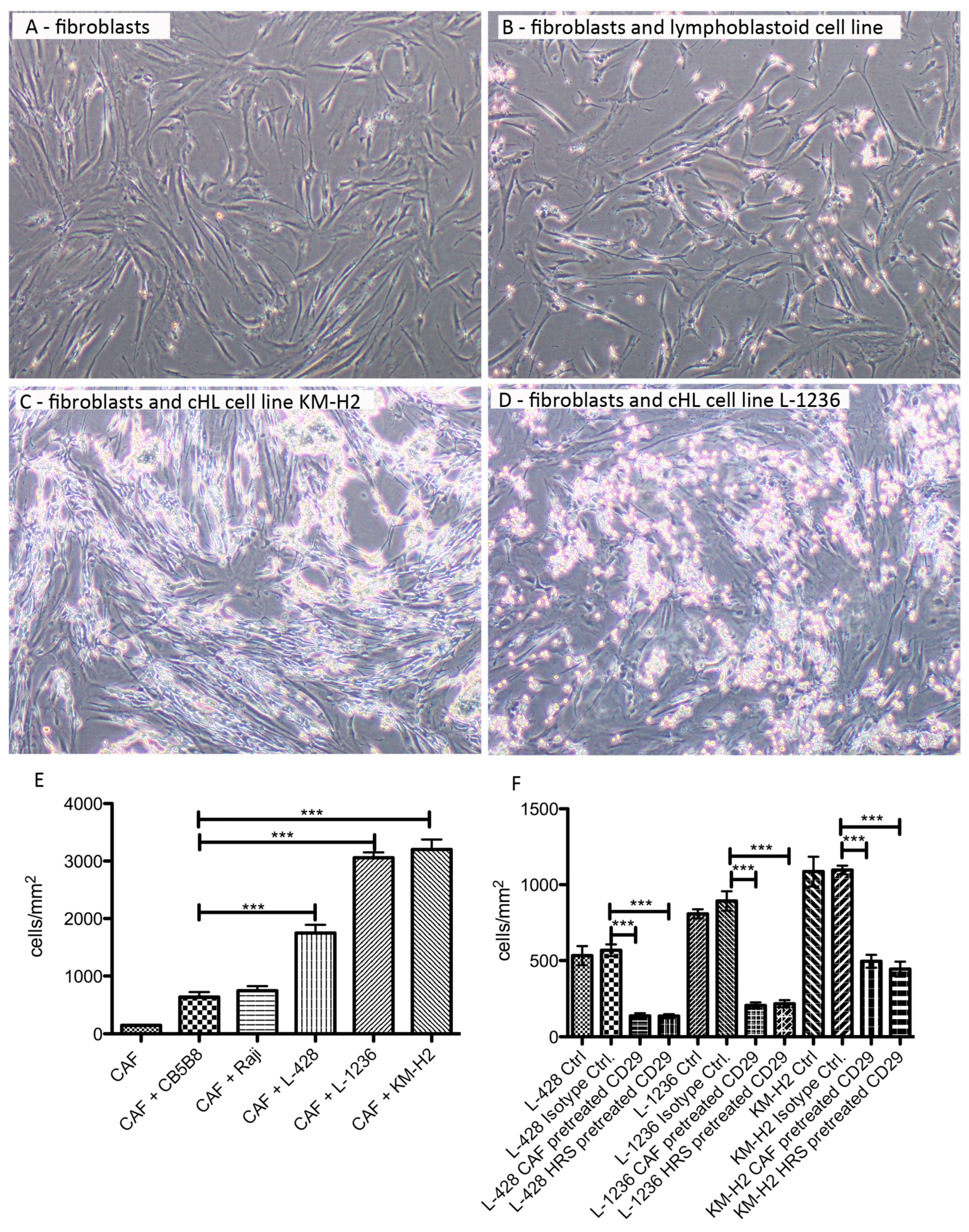
2.4. HRS Cells Show a Strong Adherence to Fibroblasts, Which Is Partly Mediated by CD29
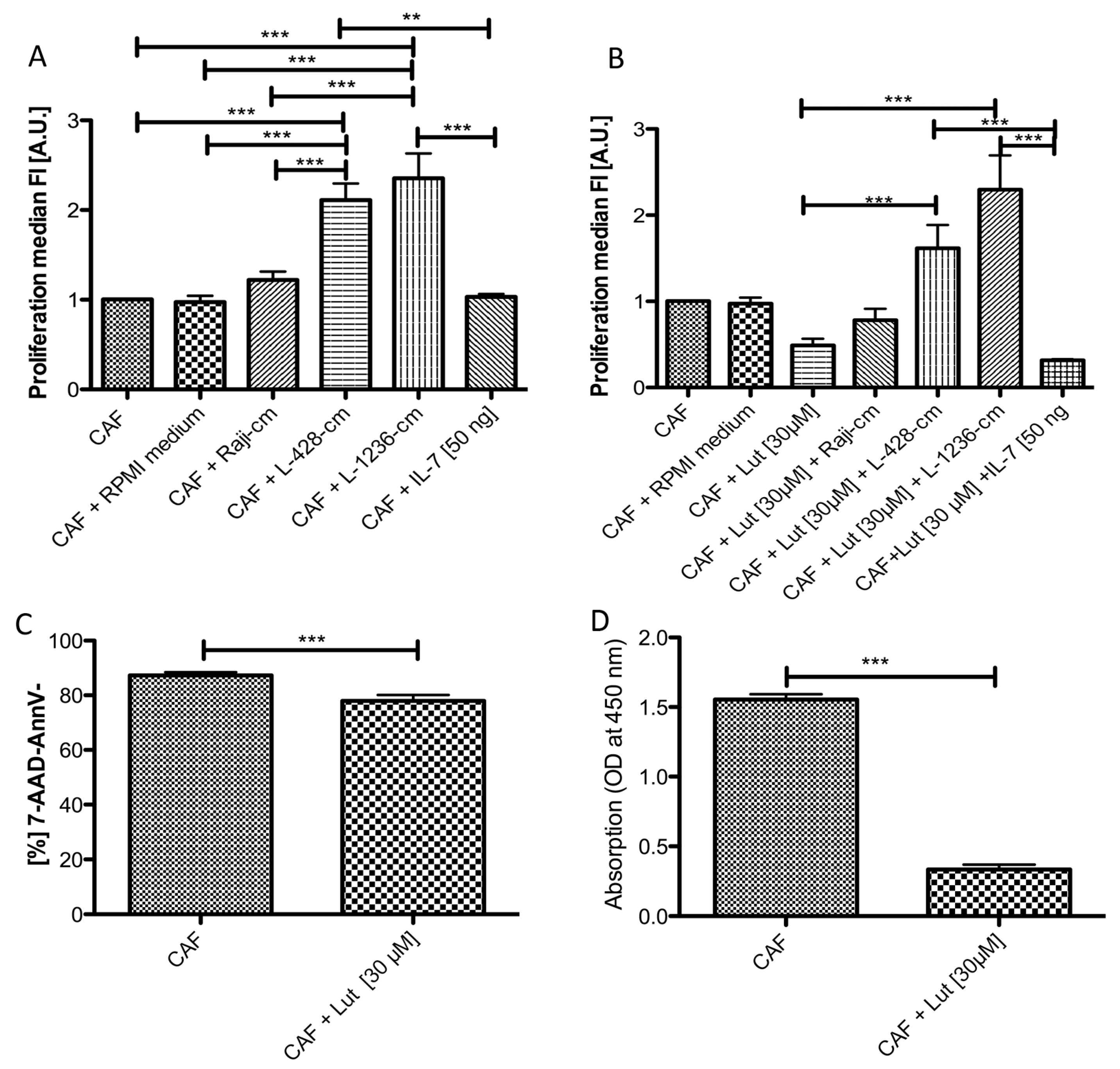
2.5. Conditioned Medium from cHL Cell Lines Has a Growth Promoting Effect on NS cHL Fibroblasts
2.6. Luteolin Has a Growth Inhibitory and Reprogramming Effect on NS cHL Fibroblasts, Which Is Abolished by cHL Conditioned Medium
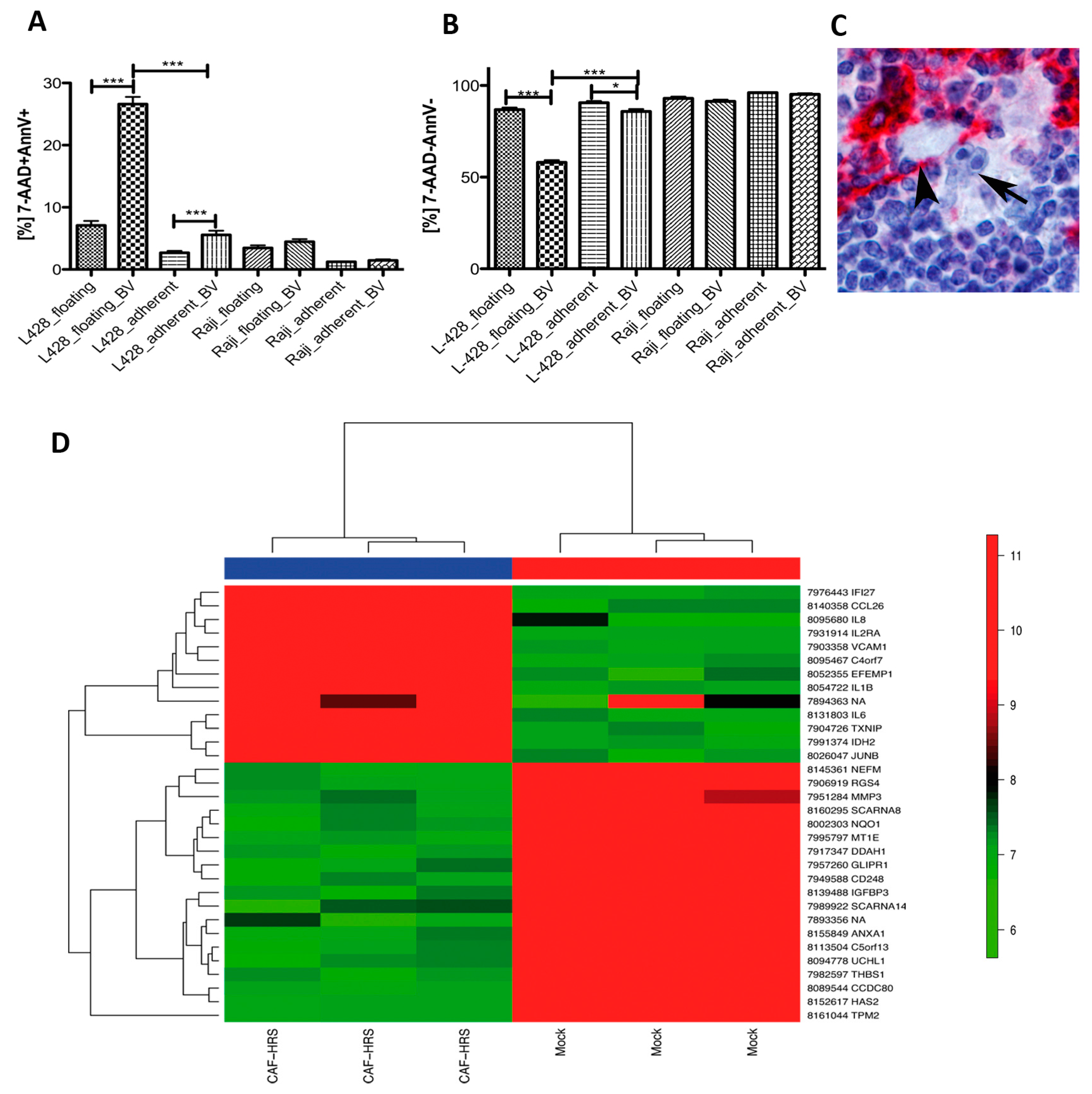
2.7. HRS Cells Require Direct Fibroblast Contact to Gain Protection against Brentuximab-Vedotin
2.8. Coculture of NS cHL Fibroblasts with HRS Cells Induces an Inflammatory Response and a Follicular Dendritic Cell-Like Phenotype
3. Discussion
3.1. cHL Fibroblasts Differ from Lymphadenitis-Derived Fibroblasts
3.2. TIMP3 and MYOCD Are Important for cHL Fibroblasts
3.3. cHL Cell Lines and Fibroblasts Strongly Influence Each Other and Deliver Prosurvival Signals
4. Methods
4.1. Fibroblast Isolation from Primary Lymph Nodes
4.2. Gene Expression Profiling and Methylation Arrays
4.3. Coculture Experiments
4.3.1. Functional Coculture Experiments
4.3.2. Proliferation Assay after Stimulation of Primary Adherent cHL-Derived Fibroblasts and Luteolin Treatment
4.3.3. Apoptosis Assay and Treatment with Brentuximab Vedotin
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmitz, R.; Stanelle, J.; Hansmann, M.L.; Kuppers, R. Pathogenesis of classical and lymphocyte-predominant Hodgkin lymphoma. Annu. Rev. Pathol. 2009, 4, 151–174. [Google Scholar] [CrossRef] [PubMed]
- Küppers, R.; Engert, A.; Hansmann, M.L. Hodgkin lymphoma. J. Clin. Invest. 2012, 122, 3439–3447. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; International Agency for Research on Cancer; World Health Organization. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2008; p. 439. [Google Scholar]
- Roozendaal, R.; Mebius, R.E.; Kraal, G. The conduit system of the lymph node. Int. Immunol. 2008, 20, 1483–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohlund, D.; Elyada, E.; Tuveson, D. Fibroblast heterogeneity in the cancer wound. J. Exp. Med. 2014, 211, 1503–1523. [Google Scholar] [CrossRef] [PubMed]
- Mraz, M.; Zent, C.S.; Church, A.K.; Jelinek, D.F.; Wu, X.; Pospisilova, S.; Ansell, S.M.; Novak, A.J.; Kay, N.E.; Witzig, T.E.; et al. Bone marrow stromal cells protect lymphoma B-cells from rituximab-induced apoptosis and targeting integrin alpha-4-beta-1 (VLA-4) with natalizumab can overcome this resistance. Br. J. Haematol. 2011, 155, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Staiger, A.M.; Duppel, J.; Dengler, M.A.; van der Kuip, H.; Vohringer, M.C.; Aulitzky, W.E.; Rosenwald, A.; Ott, G.; Horn, H. An analysis of the role of follicular lymphoma-associated fibroblasts to promote tumor cell viability following drug-induced apoptosis. Leuk. Lymphoma 2017, 58, 1922–1930. [Google Scholar] [CrossRef]
- Celegato, M.; Borghese, C.; Casagrande, N.; Carbone, A.; Colombatti, A.; Aldinucci, D. Bortezomib down-modulates the survival factor interferon regulatory factor 4 in Hodgkin lymphoma cell lines and decreases the protective activity of Hodgkin lymphoma-associated fibroblasts. Leuk. Lymphoma 2014, 55, 149–159. [Google Scholar] [CrossRef]
- Singh, R.R.; Kunkalla, K.; Qu, C.; Schlette, E.; Neelapu, S.S.; Samaniego, F.; Vega, F. ABCG2 is a direct transcriptional target of hedgehog signalling and involved in stroma-induced drug tolerance in diffuse large B-cell lymphoma. Oncogene 2011, 30, 4874–4886. [Google Scholar] [CrossRef]
- Aldinucci, D.; Celegato, M.; Casagrande, N. Microenvironmental interactions in classical Hodgkin lymphoma and their role in promoting tumor growth, immune escape and drug resistance. Cancer Lett. 2016, 380, 243–252. [Google Scholar] [CrossRef]
- Aldinucci, D.; Gloghini, A.; Pinto, A.; De Filippi, R.; Carbone, A. The classical Hodgkin’s lymphoma microenvironment and its role in promoting tumour growth and immune escape. J. Pathol. 2010, 221, 248–263. [Google Scholar] [CrossRef]
- Aldinucci, D.; Gloghini, A.; Pinto, A.; Colombatti, A.; Carbone, A. The role of CD40/CD40L and interferon regulatory factor 4 in Hodgkin lymphoma microenvironment. Leuk. Lymphoma 2012, 53, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Aldinucci, D.; Celegato, M.; Borghese, C.; Colombatti, A.; Carbone, A. IRF4 silencing inhibits Hodgkin lymphoma cell proliferation, survival and CCL5 secretion. Br. J. Haematol. 2011, 152, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Aldinucci, D.; Lorenzon, D.; Cattaruzza, L.; Pinto, A.; Gloghini, A.; Carbone, A.; Colombatti, A. Expression of CCR5 receptors on Reed-Sternberg cells and Hodgkin lymphoma cell lines: Involvement of CCL5/Rantes in tumor cell growth and microenvironmental interactions. Int. J. Cancer 2008, 122, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Cattaruzza, L.; Gloghini, A.; Olivo, K.; Di Francia, R.; Lorenzon, D.; De Filippi, R.; Carbone, A.; Colombatti, A.; Pinto, A.; Aldinucci, D. Functional coexpression of Interleukin (IL)-7 and its receptor (IL-7R) on Hodgkin and Reed-Sternberg cells: Involvement of IL-7 in tumor cell growth and microenvironmental interactions of Hodgkin’s lymphoma. Int. J. Cancer 2009, 125, 1092–1101. [Google Scholar] [CrossRef]
- Aldinucci, D.; Colombatti, A. The inflammatory chemokine CCL5 and cancer progression. Mediat. Inflamm. 2014, 2014, 292376. [Google Scholar] [CrossRef]
- Tesch, H.; Jucker, M.; Klein, S.; Abts, H.; Gunther, A.; Krueger, G.R.; Diehl, V. Hodgkin and Reed-Sternberg cells express interleukin 6 and interleukin 6 receptors. Leuk. Lymphoma 1992, 7, 297–303. [Google Scholar] [CrossRef]
- Hsu, S.M.; Waldron, J.; Xie, S.S.; Hsu, P.L. Hodgkin’s Disease and Anaplastic Large Cell Lymphoma Revisited. 1. unique cytokine and cytokine receptor profile distinguished from that of non-hodgkin’s lymphomas. J. Biomed. Sci. 1995, 2, 302–313. [Google Scholar] [CrossRef]
- Fischer, M.; Juremalm, M.; Olsson, N.; Backlin, C.; Sundstrom, C.; Nilsson, K.; Enblad, G.; Nilsson, G. Expression of CCL5/RANTES by Hodgkin and Reed-Sternberg cells and its possible role in the recruitment of mast cells into lymphomatous tissue. Int. J. Cancer 2003, 107, 197–201. [Google Scholar] [CrossRef]
- Ma, Y.; Visser, L.; Roelofsen, H.; de Vries, M.; Diepstra, A.; van Imhoff, G.; van der Wal, T.; Luinge, M.; Alvarez-Llamas, G.; Vos, H.; et al. Proteomics analysis of Hodgkin lymphoma: Identification of new players involved in the cross-talk between HRS cells and infiltrating lymphocytes. Blood 2008, 111, 2339–2346. [Google Scholar] [CrossRef]
- Ohshima, K.; Akaiwa, M.; Umeshita, R.; Suzumiya, J.; Izuhara, K.; Kikuchi, M. Interleukin-13 and interleukin-13 receptor in Hodgkin’s disease: Possible autocrine mechanism and involvement in fibrosis. Histopathology 2001, 38, 368–375. [Google Scholar] [CrossRef]
- Kadin, M.; Butmarc, J.; Elovic, A.; Wong, D. Eosinophils are the major source of transforming growth factor-beta 1 in nodular sclerosing Hodgkin’s disease. Am. J. Pathol. 1993, 142, 11–16. [Google Scholar] [PubMed]
- Karai, L.J.; Kadin, M.E.; Hsi, E.D.; Sluzevich, J.C.; Ketterling, R.P.; Knudson, R.A.; Feldman, A.L. Chromosomal rearrangements of 6p25.3 define a new subtype of lymphomatoid papulosis. Am. J. Surg. Pathol. 2013, 37, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Puri, R.K. Suppression of an IL-13 autocrine growth loop in a human Hodgkin/Reed-Sternberg tumor cell line by a novel IL-13 antagonist. Cell Immunol. 2001, 211, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.L.; Rusch, E.; Kaupp, M.; Nieselt, K.; Aicher, W.K. Expression of Desmoglein 2, Desmocollin 3 and Plakophilin 2 in Placenta and Bone Marrow-Derived Mesenchymal Stromal Cells. Stem Cell Rev. 2017, 13, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Jackson, H.W.; Defamie, V.; Waterhouse, P.; Khokha, R. TIMPs: Versatile extracellular regulators in cancer. Nat. Rev. Cancer 2017, 17, 38–53. [Google Scholar] [CrossRef]
- Miano, J.M. Myocardin in biology and disease. J. Biomed. Res. 2015, 29, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Leyva-Lopez, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef]
- Gray, A.L.; Stephens, C.A.; Bigelow, R.L.; Coleman, D.T.; Cardelli, J.A. The polyphenols (-)-epigallocatechin-3-gallate and luteolin synergistically inhibit TGF-beta-induced myofibroblast phenotypes through RhoA and ERK inhibition. PLoS ONE 2014, 9, e109208. [Google Scholar] [CrossRef]
- Bhowmick, N.A.; Neilson, E.G.; Moses, H.L. Stromal fibroblasts in cancer initiation and progression. Nature 2004, 432, 332–337. [Google Scholar] [CrossRef]
- Rengstl, B.; Kim, S.; Doring, C.; Weiser, C.; Bein, J.; Bankov, K.; Herling, M.; Newrzela, S.; Hansmann, M.L.; Hartmann, S. Small and big Hodgkin-Reed-Sternberg cells of Hodgkin lymphoma cell lines L-428 and L-1236 lack consistent differences in gene expression profiles and are capable to reconstitute each other. PLoS ONE 2017, 12, e0177378. [Google Scholar] [CrossRef]
- Lorenzi, L.; Doring, C.; Rausch, T.; Benes, V.; Lonardi, S.; Bugatti, M.; Campo, E.; Cabecadas, J.; Simonitsch-Klupp, I.; Borges, A.; et al. Identification of novel follicular dendritic cell sarcoma markers, FDCSP and SRGN, by whole transcriptome sequencing. Oncotarget 2017, 8, 16463–16472. [Google Scholar] [CrossRef] [PubMed]
- Petrasch, S.; Brittinger, G.; Wacker, H.H.; Schmitz, J.; Kosco-Vilbois, M. Follicular dendritic cells in non-Hodgkin’s lymphomas. Leuk. Lymphoma 1994, 15, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Raphel, L.; Talasila, A.; Cheung, C.; Sinha, S. Myocardin overexpression is sufficient for promoting the development of a mature smooth muscle cell-like phenotype from human embryonic stem cells. PLoS ONE 2012, 7, e44052. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Rockey, D.C. Upregulation of the actin cytoskeleton via myocardin leads to increased expression of type 1 collagen. Lab. Invest. 2017, 97, 1412–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.H.; Peng, K.L.; Kang, M.L.; Chen, Y.R.; Yang, Y.C.; Tsai, C.H.; Chu, C.S.; Jeng, Y.M.; Chen, Y.T.; Lin, F.M.; et al. TET1 suppresses cancer invasion by activating the tissue inhibitors of metalloproteinases. Cell Rep. 2012, 2, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Zhang, J.; Song, S.; Dai, D. Promoter methylation and expression of TIMP3 gene in gastric cancer. Diagn. Pathol. 2013, 8, 110. [Google Scholar] [CrossRef]
- Catasus, L.; Pons, C.; Munoz, J.; Espinosa, I.; Prat, J. Promoter hypermethylation contributes to TIMP3 down-regulation in high stage endometrioid endometrial carcinomas. Histopathology 2013, 62, 632–641. [Google Scholar] [CrossRef]
- Shimoda, M.; Principe, S.; Jackson, H.W.; Luga, V.; Fang, H.; Molyneux, S.D.; Shao, Y.W.; Aiken, A.; Waterhouse, P.D.; Karamboulas, C.; et al. Loss of the Timp gene family is sufficient for the acquisition of the CAF-like cell state. Nat. Cell Biol. 2014, 16, 889–901. [Google Scholar] [CrossRef]
- Hansen, H.P.; Engels, H.M.; Dams, M.; Paes Leme, A.F.; Pauletti, B.A.; Simhadri, V.L.; Durkop, H.; Reiners, K.S.; Barnert, S.; Engert, A.; et al. Protrusion-guided extracellular vesicles mediate CD30 trans-signalling in the microenvironment of Hodgkin’s lymphoma. J. Pathol. 2014, 232, 405–414. [Google Scholar] [CrossRef]
- Sivina, M.; Hartmann, E.; Vasyutina, E.; Boucas, J.M.; Breuer, A.; Keating, M.J.; Wierda, W.G.; Rosenwald, A.; Herling, M.; Burger, J.A. Stromal cells modulate TCL1 expression, interacting AP-1 components and TCL1-targeting micro-RNAs in chronic lymphocytic leukemia. Leukemia 2012, 26, 1812–1820. [Google Scholar] [CrossRef] [Green Version]
- von Einem, J.C.; Guenther, C.; Volk, H.D.; Grutz, G.; Hirsch, D.; Salat, C.; Stoetzer, O.; Nelson, P.J.; Michl, M.; Modest, D.P.; et al. Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells: Results from the phase 1/2 TREAT-ME-1 trial. Int. J. Cancer 2019, 145, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Schuhmacher, B.; Rengstl, B.; Doring, C.; Bein, J.; Newrzela, S.; Brunnberg, U.; Kvasnicka, H.M.; Vornanen, M.; Kuppers, R.; Hansmann, M.L.; et al. A strong host response and lack of MYC expression are characteristic for diffuse large B cell lymphoma transformed from nodular lymphocyte predominant Hodgkin lymphoma. Oncotarget 2016, 7, 72197–72210. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.P.; Triche, T.J., Jr.; Hansen, K.D. Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics 2017, 33, 558–560. [Google Scholar] [CrossRef] [PubMed]
| Fold Change Fibroblasts cHL/LA | p-Value | False Fiscovery Rate (FDR) | Gene Symbol | Gene Description |
|---|---|---|---|---|
| 4.2 | 0.006 | 0.281 | TIMP3 | TIMP metallopeptidase inhibitor 3 |
| 3.9 | 0.00006 | 0.138 | MYOCD | myocardin |
| 2.0 | 0.004 | 0.278 | RGS4 | regulator of G-protein signalling 4 |
| 1.9 | 0.006 | 0.281 | IER3 | immediate early response 3 |
| 1.7 | 0.001 | 0.187 | ENO2 | enolase 2 (gamma, neuronal) |
| 1.6 | 0.005 | 0.278 | SERPINE1 | serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1), member 1 |
| 1.6 | 0.004 | 0.278 | PMS2L2 | postmeiotic segregation increased 2-like 2 pseudogene |
| 1.6 | 0.002 | 0.215 | GPX7 | glutathione peroxidase 7 |
| 1.5 | 0.005 | 0.278 | CT47A10 | cancer/testis antigen family 47, member A10 |
| −1.5 | 0.004 | 0.278 | TSHZ2 | teashirt zinc finger homeobox 2 |
| −1.5 | 0.0004 | 0.152 | KIAA1598 | KIAA1598 |
| −1.6 | 0.005 | 0.278 | BMP2K | BMP2 inducible kinase |
| −1.6 | 0.004 | 0.278 | CD14 | CD14 molecule |
| −1.6 | 0.006 | 0.281 | OSBPL8 | oxysterol binding protein-like 8 |
| −1.6 | 0.0002 | 0.138 | PDE4DIP | phosphodiesterase 4D interacting protein |
| −1.7 | 0.004 | 0.278 | CCNL1 | cyclin L1 |
| −1.7 | 0.004 | 0.278 | SMG1 | SMG1 phosphatidylinositol 3-kinase-related kinase |
| −1.7 | 0.002 | 0.215 | MOCOS | molybdenum cofactor sulfurase |
| −1.7 | 0.005 | 0.281 | SMG1 | SMG1 phosphatidylinositol 3-kinase-related kinase |
| −1.7 | 0.0002 | 0.138 | RNA5SP187 | RNA, 5S ribosomal pseudogene 187 |
| −1.8 | 0.005 | 0.281 | DSEL | dermatan sulfate epimerase-like |
| −1.8 | 0.002 | 0.230 | SCARNA9 | small Cajal body-specific RNA 9 |
| −1.8 | 0.004 | 0.278 | ALPK2 | alpha-kinase 2 |
| −1.9 | 0.005 | 0.281 | TFAP2A | transcription factor AP-2 alpha (activating enhancer binding protein 2 alpha) |
| −2.0 | 0.001 | 0.206 | RNA5SP129 | RNA, 5S ribosomal pseudogene 129 |
| −2.1 | 0.003 | 0.247 | PRKG2 | protein kinase, cGMP-dependent, type II |
| −2.2 | 0.0002 | 0.138 | VIT | vitrin |
| −2.4 | 0.0001 | 0.138 | MT-TA | mitochondrially encoded tRNA alanine |
| −2.7 | 0.0003 | 0.138 | GPNMB | glycoprotein (transmembrane) nmb |
| −2.9 | 0.002 | 0.215 | HTR2B | 5-hydroxytryptamine (serotonin) receptor 2B, G protein-coupled |
| −4.0 | 0.001 | 0.161 | DSC3 | desmocollin 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bankov, K.; Döring, C.; Ustaszewski, A.; Giefing, M.; Herling, M.; Cencioni, C.; Spallotta, F.; Gaetano, C.; Küppers, R.; Hansmann, M.-L.; et al. Fibroblasts in Nodular Sclerosing Classical Hodgkin Lymphoma Are Defined by a Specific Phenotype and Protect Tumor Cells from Brentuximab-Vedotin Induced Injury. Cancers 2019, 11, 1687. https://doi.org/10.3390/cancers11111687
Bankov K, Döring C, Ustaszewski A, Giefing M, Herling M, Cencioni C, Spallotta F, Gaetano C, Küppers R, Hansmann M-L, et al. Fibroblasts in Nodular Sclerosing Classical Hodgkin Lymphoma Are Defined by a Specific Phenotype and Protect Tumor Cells from Brentuximab-Vedotin Induced Injury. Cancers. 2019; 11(11):1687. https://doi.org/10.3390/cancers11111687
Chicago/Turabian StyleBankov, Katrin, Claudia Döring, Adam Ustaszewski, Maciej Giefing, Marco Herling, Chiara Cencioni, Francesco Spallotta, Carlo Gaetano, Ralf Küppers, Martin-Leo Hansmann, and et al. 2019. "Fibroblasts in Nodular Sclerosing Classical Hodgkin Lymphoma Are Defined by a Specific Phenotype and Protect Tumor Cells from Brentuximab-Vedotin Induced Injury" Cancers 11, no. 11: 1687. https://doi.org/10.3390/cancers11111687
APA StyleBankov, K., Döring, C., Ustaszewski, A., Giefing, M., Herling, M., Cencioni, C., Spallotta, F., Gaetano, C., Küppers, R., Hansmann, M.-L., & Hartmann, S. (2019). Fibroblasts in Nodular Sclerosing Classical Hodgkin Lymphoma Are Defined by a Specific Phenotype and Protect Tumor Cells from Brentuximab-Vedotin Induced Injury. Cancers, 11(11), 1687. https://doi.org/10.3390/cancers11111687






