The Pivotal Roles of the Epithelial Membrane Protein Family in Cancer Invasiveness and Metastasis
Abstract
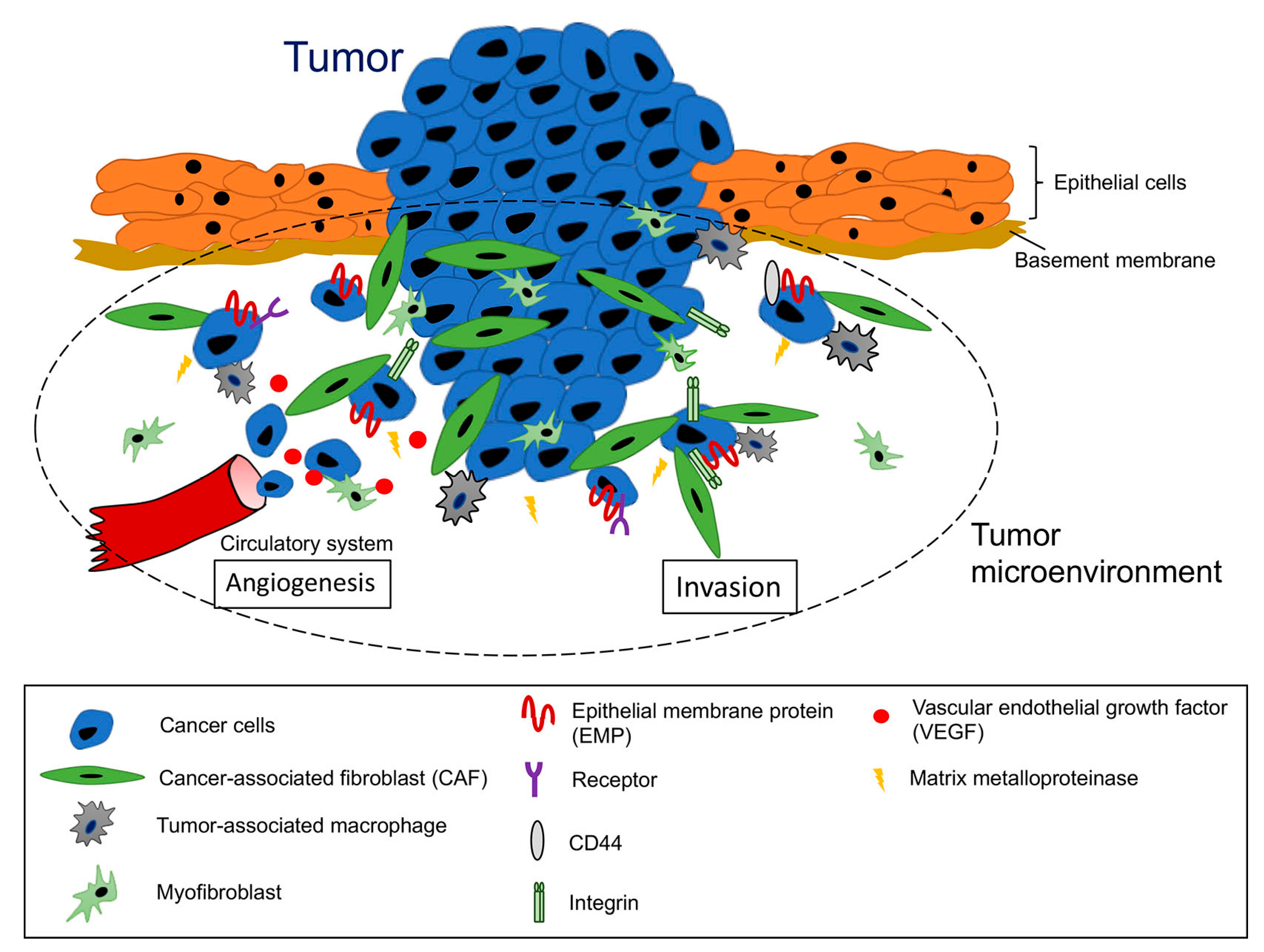
:1. Introduction
2. The Structures, Expressions, and Functions of EMPs
2.1. EMP1
2.2. EMP2
2.3. EMP3
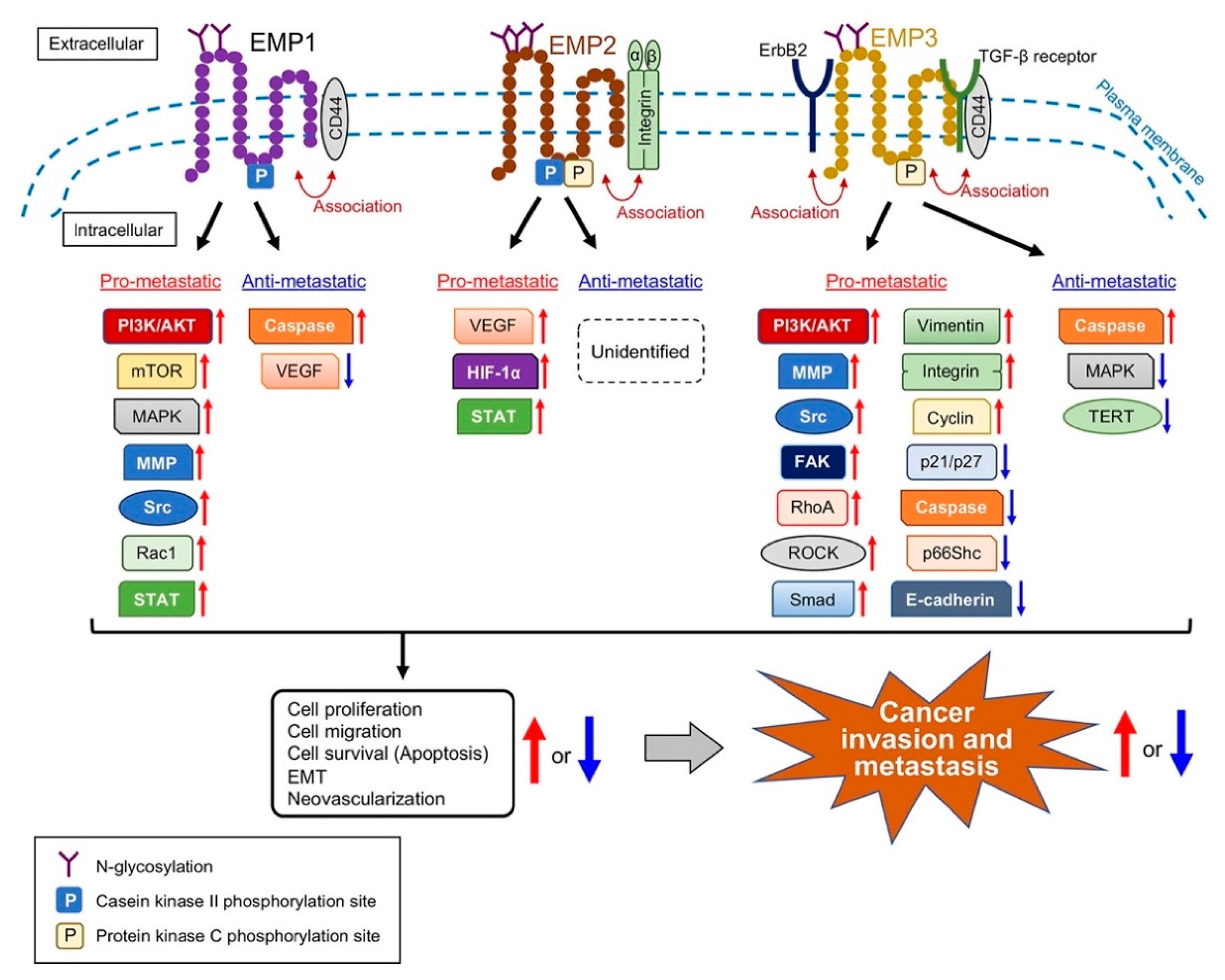
3. Involvement of EMPs in Cancer Metastasis
3.1. EMP1
3.1.1. Pro-Metastatic Roles of EMP1
3.1.2. Anti-Metastatic Roles of EMP1
3.1.3. Opposing Roles of EMP1 in Cancer Invasiveness and Metastasis in the Same Type of Cancer
3.2. EMP2
3.2.1. Pro-Metastatic Roles of EMP2
3.2.2. Anti-Metastatic Roles of EMP2
3.3. EMP3
3.3.1. Pro-Metastatic Roles of EMP3
3.3.2. Anti-Metastatic Roles of EMP3
3.4. PMP22
4. Therapeutic Implications of EMPs
4.1. Development of Monoclonal Antibodies
4.2. Perturbation of Protein−Protein Interactions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Steeg, P.S. Targeting metastasis. Nat. Rev. Cancer 2016, 16, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nat. Rev. Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Bacac, M.; Stamenkovic, I. Metastatic cancer cell. Annu. Rev. Pathol. 2008, 3, 221–247. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; McGowan, P.M.; Gallagher, W.M. Cancer invasion and metastasis: Changing views. J. Pathol. 2008, 214, 283–293. [Google Scholar] [CrossRef]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Andarawewa, K.L.; Motrescu, E.R.; Chenard, M.P.; Gansmuller, A.; Stoll, I.; Tomasetto, C.; Rio, M.C. Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell-adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res. 2005, 65, 10862–10871. [Google Scholar] [CrossRef]
- Duda, D.G.; Cohen, K.S.; Kozin, S.V.; Perentes, J.Y.; Fukumura, D.; Scadden, D.T.; Jain, R.K. Evidence for incorporation of bone marrow-derived endothelial cells into perfused blood vessels in tumors. Blood 2006, 107, 2774–2776. [Google Scholar] [CrossRef]
- Levin, I. Changes in the tissue surrounding a growing tumor and the significance of the “precancerous state”. J. Exp. Med. 1912, 16, 149–154. [Google Scholar] [CrossRef]
- Lyden, D.; Hattori, K.; Dias, S.; Costa, C.; Blaikie, P.; Butros, L.; Chadburn, A.; Heissig, B.; Marks, W.; Witte, L.; et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 2001, 7, 1194–1201. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Begum, A.; McMillan, R.H.; Chang, Y.T.; Penchev, V.R.; Rajeshkumar, N.V.; Maitra, A.; Goggins, M.G.; Eshelman, J.R.; Wolfgang, C.L.; Rasheed, Z.A.; et al. Direct interactions with cancer-associated fibroblasts lead to enhanced pancreatic cancer stem cell function. Pancreas 2019, 48, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Pantel, K.; Kang, Y. Tumor metastasis: Moving new biological insights into the clinic. Nat. Med. 2013, 19, 1450–1464. [Google Scholar] [CrossRef] [PubMed]
- Malanchi, I.; Santamaria-Martínez, A.; Susanto, E.; Peng, H.; Lehr, H.A.; Delaloye, J.F.; Huelsken, J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 2011, 481, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Oskarsson, T.; Acharyya, S.; Zhang, X.H.; Vanharanta, S.; Tavazoie, S.F.; Morris, P.G.; Downey, R.J.; Manova-Todorova, K.; Brogi, E.; Massagué, J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med. 2011, 17, 867–874. [Google Scholar] [CrossRef] [Green Version]
- Lau, E.Y.; Lo, J.; Cheng, B.Y.; Ma, M.K.; Lee, J.M.; Ng, J.K.; Chai, S.; Lin, C.H.; Tsang, S.Y.; Ma, S.; et al. Cancer-associated fibroblasts regulate tumor-initiating cell plasticity in hepatocellular carcinoma through c-Met/FRA1/HEY1 signaling. Cell Rep. 2016, 15, 1175–1189. [Google Scholar] [CrossRef]
- Su, S.; Chen, J.; Yao, H.; Liu, J.; Yu, S.; Lao, L.; Wang, M.; Luo, M.; Xing, Y.; Chen, F.; et al. CD10+ GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 2018, 172, 841–856. [Google Scholar] [CrossRef]
- Goulet, C.R.; Champagne, A.; Bernard, G.; Vandal, D.; Chabaud, S.; Pouliot, F.; Bolduc, S. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer 2019, 19, 137. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Sun, X.; Lin, Y.; Chen, W. Cancer-associated fibroblasts induce epithelial-mesenchymal transition through secreted cytokines in endometrial cancer cells. Oncol. Lett. 2018, 15, 5694–5702. [Google Scholar] [CrossRef]
- Yu, Y.; Xiao, C.H.; Tan, L.D.; Wang, Q.S.; Li, X.Q.; Feng, Y.M. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br. J. Cancer 2014, 110, 724–732. [Google Scholar] [CrossRef]
- Zhuang, J.; Lu, Q.; Shen, B.; Huang, X.; Shen, L.; Zheng, X.; Huang, R.; Yan, J.; Guo, H. TGFβ1 secreted by cancer-associated fibroblasts induces epithelial-mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci. Rep. 2015, 5, 11924. [Google Scholar] [CrossRef] [PubMed]
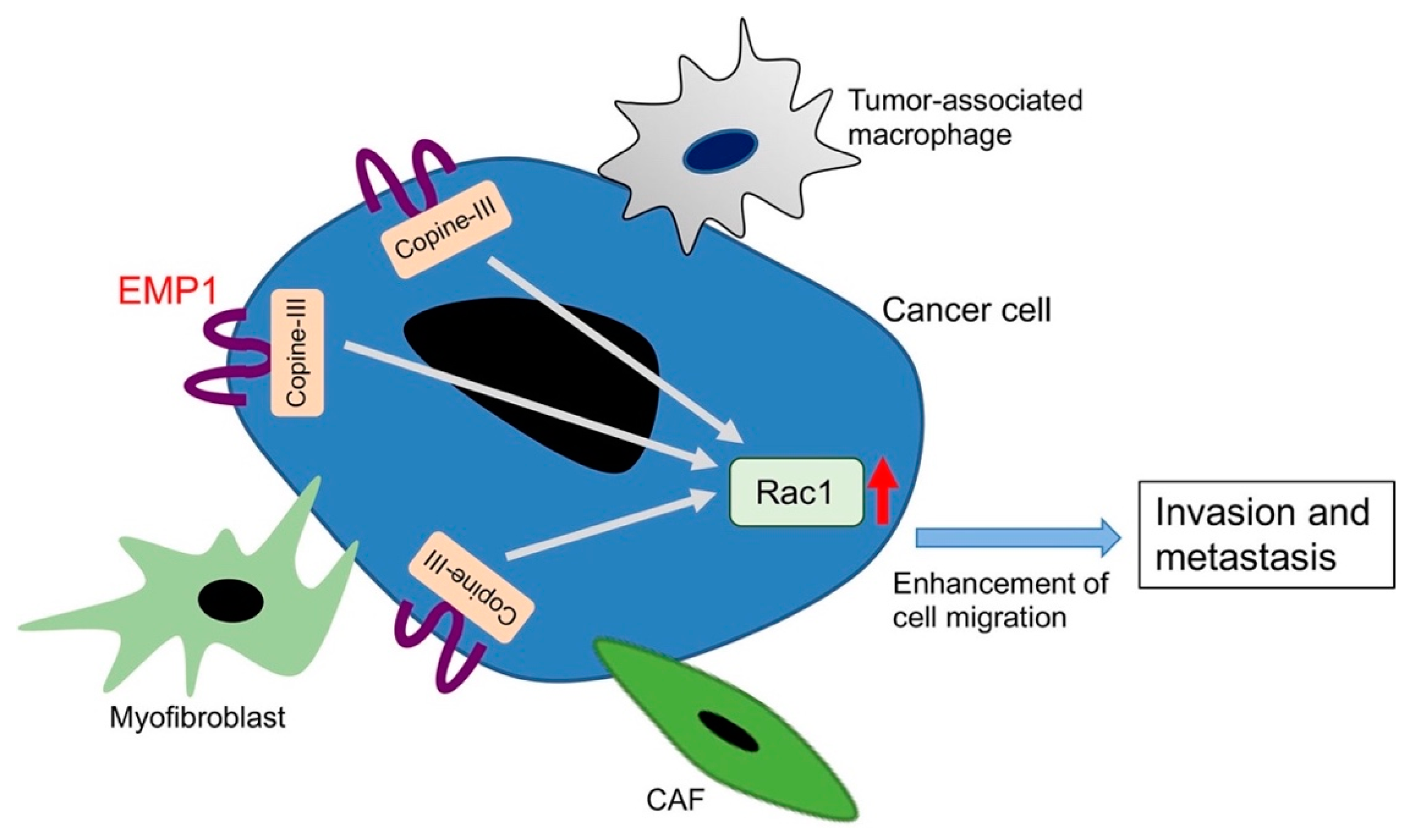
- Ahmat Amin, M.K.B.; Shimizu, A.; Zankov, D.P.; Sato, A.; Kurita, S.; Ito, M.; Maeda, T.; Yoshida, T.; Sakaue, T.; Higashiyama, S.; et al. Epithelial membrane protein 1 promotes tumor metastasis by enhancing cell migration via copine-III and Rac1. Oncogene 2018, 37, 5416–5434. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.D.; Tomlinson, M.G. The ins and outs of the transmembrane 4 superfamily. Immunol. Today 1994, 15, 588–594. [Google Scholar] [CrossRef]
- Seigneuret, M.; Delaguillaumie, A.; Lagaudrière-Gesbert, C.; Conjeaud, H. Structure of the tetraspanin main extracellular domain. A partially conserved fold with a structurally variable domain insertion. J. Biol. Chem. 2001, 276, 40055–40064. [Google Scholar] [CrossRef] [PubMed]
- Stipp, C.S.; Kolesnikova, T.V.; Hemler, M.E. Functional domains in tetraspanin proteins. Trends Biochem. Sci. 2003, 28, 106–112. [Google Scholar] [CrossRef]
- Jetten, A.M.; Suter, U. The peripheral myelin protein 22 and epithelial membrane protein family. Prog. Nucleic Acid Res. Mol. Biol. 2000, 64, 97–129. [Google Scholar] [PubMed] [Green Version]
- Suter, U.; Snipes, G.J. Peripheral myelin protein 22: Facts and hypotheses. J. Neurosci. Res. 1995, 40, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Fabbretti, E.; Edomi, P.; Brancolini, C.; Schneider, C. Apoptotic phenotype induced by overexpression of wild-type gas3/PMP22: Its relation to the demyelinating peripheral neuropathy CMT1A. Genes Dev. 1995, 9, 1846–1856. [Google Scholar] [CrossRef]
- Zoidl, G.; Blass-Kampmann, S.; D’Urso, D.; Schmalenbach, C.; Müller, H.W. Retroviral-mediated gene transfer of the peripheral myelin protein PMP22 in Schwann cells: Modulation of cell growth. EMBO J. 1995, 14, 1122–1128. [Google Scholar] [CrossRef]
- Cai, W.; Chen, G.; Luo, Q.; Liu, J.; Guo, X.; Zhang, T.; Ma, F.; Yuan, L.; Li, B.; Cai, J. PMP22 regulates self-renewal and chemoresistance of gastric cancer cells. Mol. Cancer Ther. 2017, 16, 1187–1198. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Z. The functional role of PMP22 gene in the proliferation and invasion of osteosarcoma. Med. Sci. Monit. 2015, 21, 1976–1982. [Google Scholar] [PubMed]
- Tong, D.; Heinze, G.; Pils, D.; Wolf, A.; Singer, C.F.; Concin, N.; Hofstetter, G.; Schiebel, I.; Rudas, M.; Zeillinger, R. Gene expression of PMP22 is an independent prognostic factor for disease-free and overall survival in breast cancer patients. BMC Cancer 2010, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- EMP1 in The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000134531-EMP1/tissue (accessed on 8 October 2019).
- Taylor, V.; Welcher, A.A.; Program, A.E.; Suter, U. Epithelial membrane protein-1, peripheral myelin protein 22, and lens membrane protein 20 define a novel gene family. J. Biol. Chem. 1995, 270, 28824–28833. [Google Scholar] [CrossRef] [PubMed]
- Wulf, P.; Suter, U. Embryonic expression of epithelial membrane protein 1 in early neurons. Brain Res. Dev. Brain Res. 1999, 116, 169–180. [Google Scholar] [CrossRef]
- Gnirke, A.U.; Weidle, U.H. Investigation of prevalence and regulation of expression of progression associated protein (PAP). Anticancer Res. 1998, 18, 4363–4369. [Google Scholar] [PubMed]
- Bangsow, T.; Baumann, E.; Bangsow, C.; Jaeger, M.H.; Pelzer, B.; Gruhn, P.; Wolf, S.; von Melchner, H.; Stanimirovic, D.B. The epithelial membrane protein 1 is a novel tight junction protein of the blood-brain barrier. J. Cereb. Blood Flow Metab. 2008, 28, 1249–1260. [Google Scholar] [CrossRef]
- EMP2 in The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000213853-EMP2/tissue (accessed on 8 October 2019).
- Wadehra, M.; Sulur, G.G.; Braun, J.; Gordon, L.K.; Goodglick, L. Epithelial membrane protein-2 is expressed in discrete anatomical regions of the eye. Exp. Mol. Pathol. 2003, 74, 106–112. [Google Scholar] [CrossRef]
- Taylor, V.; Suter, U. Epithelial membrane protein-2 and epithelial membrane protein-3: Two novel members of the peripheral myelin protein 22 gene family. Gene 1996, 175, 115–120. [Google Scholar] [CrossRef]
- Wadehra, M.; Iyer, R.; Goodglick, L.; Braun, J. The tetraspan protein epithelial membrane protein-2 interacts with β1 integrins and regulates adhesion. J. Biol. Chem. 2002, 277, 41094–41100. [Google Scholar] [CrossRef]
- Wadehra, M.; Su, H.; Gordon, L.K.; Goodglick, L.; Braun, J. The tetraspan protein EMP2 increases surface expression of class I major histocompatibility complex proteins and susceptibility to CTL-mediated cell death. Clin. Immunol. 2003, 107, 129–136. [Google Scholar] [CrossRef]
- Wadehra, M.; Goodglick, L.; Braun, J. The tetraspan protein EMP2 modulates the surface expression of caveolins and glycosylphosphatidyl inositol-linked proteins. Mol. Biol. Cell 2004, 15, 2073–2083. [Google Scholar] [CrossRef] [PubMed]
- Wadehra, M.; Forbes, A.; Pushkarna, N.; Goodglick, L.; Gordon, L.K.; Williams, C.J.; Braun, J. Epithelial membrane protein-2 regulates surface expression of αvβ3 integrin in the endometrium. Dev. Biol. 2005, 287, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Wadehra, M.; Dayal, M.; Mainigi, M.; Ord, T.; Iyer, R.; Braun, J.; Williams, C.J. Knockdown of the tetraspan protein epithelial membrane protein-2 inhibits implantation in the mouse. Dev. Biol. 2006, 292, 430–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, S.A.; Mareninov, S.; Wadehra, M.; Zhang, L.; Goodglick, L.; Braun, J.; Gordon, L.K. FAK activation and the role of epithelial membrane protein 2 (EMP2) in collagen gel contraction. Investig. Ophthalmol. Vis. Sci. 2009, 50, 462–469. [Google Scholar] [CrossRef]
- Parsons, J.T.; Martin, K.H.; Slack, J.K.; Taylor, J.M.; Weed, S.A. Focal adhesion kinase: A regulator of focal adhesion dynamics and cell movement. Oncogene 2000, 19, 5606–5613. [Google Scholar] [CrossRef]
- Schlaepfer, D.D.; Hauck, C.R.; Sieg, D.J. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 1999, 71, 435–478. [Google Scholar] [CrossRef] [Green Version]
- EMP3 in The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000142227-EMP3/tissue (accessed on 8 October 2019).
- Kusumoto, Y.; Okuyama, H.; Shibata, T.; Konno, K.; Takemoto, Y.; Maekawa, D.; Kononaga, T.; Ishii, T.; Akashi-Takamura, S.; Saitoh, S.I.; et al. Epithelial membrane protein 3 (Emp3) downregulates induction and function of cytotoxic T lymphocytes by macrophages via TNF-α production. Cell. Immunol. 2018, 324, 33–41. [Google Scholar] [CrossRef]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T cell activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef]
- Hotta, H.; Ross, A.H.; Huebner, K.; Isobe, M.; Wendeborn, S.; Chao, M.V.; Ricciardi, R.P.; Tsujimoto, Y.; Croce, C.M.; Koprowski, H. Molecular cloning and characterization of an antigen associated with early stages of melanoma tumor progression. Cancer Res. 1988, 48, 2955–2962. [Google Scholar]
- Oren, R.; Takahashi, S.; Doss, C.; Levy, R.; Levy, S. TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol. Cell. Biol. 1990, 10, 4007–4015. [Google Scholar] [CrossRef]
- Wright, M.D.; Henkle, K.J.; Mitchell, G.F. An immunogenic Mr 23,000 integral membrane protein of Schistosoma mansoni worms that closely resembles a human tumor-associated antigen. J. Immunol. 1990, 144, 3195–3200. [Google Scholar] [PubMed]
- Cabodi, S.; Di Stefano, P.; Leal, M.P.; Tinnirello, A.; Bisaro, B.; Morello, V.; Damiano, L.; Aramu, S.; Repetto, D.; Tornillo, G.; et al. Integrins and signal transduction. Adv. Exp. Med. Biol. 2010, 674, 43–54. [Google Scholar] [PubMed]
- Basak, S.; Dhar, R.; Das, C. Steroids modulate the expression of α4 integrin in mouse blastocysts and uterus during implantation. Biol. Reprod. 2002, 66, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Lessey, B.A.; Castelbaum, A.J.; Wolf, L.; Greene, W.; Paulson, M.; Meyer, W.R.; Fritz, M.A. Use of integrins to date the endometrium. Fertil. Steril. 2000, 73, 779–787. [Google Scholar] [CrossRef]
- Lessey, B.A.; Gui, Y.; Apparao, K.B.; Young, S.L.; Mulholland, J. Regulated expression of heparin-binding EGF-like growth factor (HB-EGF) in the human endometrium: A potential paracrine role during implantation. Mol. Reprod. Dev. 2002, 62, 446–455. [Google Scholar] [CrossRef]
- Miao, L.; Jiang, Z.; Wang, J.; Yang, N.; Qi, Q.; Zhou, W.; Feng, Z.; Li, W.; Zhang, Q.; Huang, B.; et al. Epithelial membrane protein 1 promotes glioblastoma progression through the PI3K/AKT/mTOR signaling pathway. Oncol. Rep. 2019, 42, 605–614. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Wu, H.; Wang, H.; Yao, L.; Deng, Z.; Zhou, Y. EMP1 regulates cell proliferation, migration, and stemness in gliomas through PI3K-AKT signaling and CD44. J. Cell. Biochem. 2019, 120, 17142–17150. [Google Scholar] [CrossRef]
- Wang, H.H.; Liao, C.C.; Chow, N.H.; Huang, L.L.; Chuang, J.I.; Wei, K.C.; Shin, J.W. Whether CD44 is an applicable marker for glioma stem cells. Am. J. Transl. Res. 2017, 9, 4785–4806. [Google Scholar]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018, 20, iv1–iv86. [Google Scholar] [CrossRef]
- Onken, M.D.; Ehlers, J.P.; Worley, L.A.; Makita, J.; Yokota, Y.; Harbour, J.W. Functional gene expression analysis uncovers phenotypic switch in aggressive uveal melanomas. Cancer Res. 2006, 66, 4602–4609. [Google Scholar] [CrossRef]
- Emami, K.H.; Nguyen, C.; Ma, H.; Kim, D.H.; Jeong, K.W.; Eguchi, M.; Moon, R.T.; Teo, J.L.; Oh, S.W.; Kim, H.Y.; et al. A small molecule inhibitor of β-catenin/CREB-binding protein transcription [corrected]. Proc. Natl. Acad. Sci. USA 2004, 101, 12682–12687. [Google Scholar] [CrossRef] [PubMed]
- Kaochar, S.; Dong, J.; Torres, M.; Rajapakshe, K.; Nikolos, F.; Davis, C.M.; Ehli, E.A.; Coarfa, C.; Mitsiades, N.; Poulaki, V. ICG-001 exerts potent anticancer activity against uveal melanoma cells. Investig. Ophthalmol. Vis. Sci. 2018, 59, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Wang, G.; Cao, X.; Li, Z.; Hu, J.; Wang, J. EMP-1 promotes tumorigenesis of NSCLC through PI3K/AKT pathway. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2012, 32, 834–838. [Google Scholar] [CrossRef] [PubMed]
- De Marco, C.; Laudanna, C.; Rinaldo, N.; Oliveira, D.M.; Ravo, M.; Weisz, A.; Ceccarelli, M.; Caira, E.; Rizzuto, A.; Zoppoli, P.; et al. Specific gene expression signatures induced by the multiple oncogenic alterations that occur within the PTEN/PI3K/AKT pathway in lung cancer. PLoS ONE 2017, 12, e0178865. [Google Scholar] [CrossRef]
- Jain, A.; Tindell, C.A.; Laux, I.; Hunter, J.B.; Curran, J.; Galkin, A.; Afar, D.E.; Aronson, N.; Shak, S.; Natale, R.B.; et al. Epithelial membrane protein-1 is a biomarker of gefitinib resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 11858–11863. [Google Scholar] [CrossRef] [Green Version]
- Ariës, I.M.; Jerchel, I.S.; van den Dungen, R.E.; van den Berk, L.C.; Boer, J.M.; Horstmann, M.A.; Escherich, G.; Pieters, R.; den Boer, M.L. EMP1, a novel poor prognostic factor in pediatric leukemia regulates prednisolone resistance, cell proliferation, migration and adhesion. Leukemia 2014, 28, 1828–1837. [Google Scholar] [CrossRef]
- Sun, G.G.; Lu, Y.F.; Fu, Z.Z.; Cheng, Y.J.; Hu, W.N. EMP1 inhibits nasopharyngeal cancer cell growth and metastasis through induction apoptosis and angiogenesis. Tumour Biol. 2014, 35, 3185–3193. [Google Scholar] [CrossRef]
- Wang, H.T.; Kong, J.P.; Ding, F.; Wang, X.Q.; Wang, M.R.; Liu, L.X.; Wu, M.; Liu, Z.H. Analysis of gene expression profile induced by EMP-1 in esophageal cancer cells using cDNA Microarray. World J. Gastroenterol. 2003, 9, 392–398. [Google Scholar] [CrossRef]
- Sun, G.; Zhao, G.; Lu, Y.; Wang, Y.; Yang, C. Association of EMP1 with gastric carcinoma invasion, survival and prognosis. Int. J. Oncol. 2014, 45, 1091–1098. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.G.; Wang, Y.D.; Cui, D.W.; Cheng, Y.J.; Hu, W.N. Epithelial membrane protein 1 negatively regulates cell growth and metastasis in colorectal carcinoma. World J. Gastroenterol. 2014, 20, 4001–4010. [Google Scholar] [CrossRef]
- Demirag, G.G.; Kefeli, M.; Kemal, Y.; Yucel, I. Epithelial membrane protein 1 expression in ovarian serous tumors. Oncol. Lett. 2016, 11, 2140–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turashvili, G.; Bouchal, J.; Baumforth, K.; Wei, W.; Dziechciarkova, M.; Ehrmann, J.; Klein, J.; Fridman, E.; Skarda, J.; Srovnal, J.; et al. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer 2007, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.G.; Wang, Y.D.; Lu, Y.F.; Hu, W.N. EMP1, a member of a new family of antiproliferative genes in breast carcinoma. Tumour Biol. 2014, 35, 3347–3354. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.G.; Wang, Y.D.; Cui, D.W.; Cheng, Y.J.; Hu, W.N. EMP1 regulates caspase-9 and VEGFC expression and suppresses prostate cancer cell proliferation and invasion. Tumour Biol. 2014, 35, 3455–3462. [Google Scholar] [CrossRef]
- Newman, L.A.; Buzdar, A.U.; Singletary, S.E.; Kuerer, H.M.; Buchholz, T.; Ames, F.C.; Ross, M.I.; Hunt, K.K. A prospective trial of preoperative chemotherapy in resectable breast cancer: Predictors of breast-conservation therapy feasibility. Ann. Surg. Oncol. 2002, 9, 228–234. [Google Scholar] [CrossRef]
- Arpino, G.; Bardou, V.J.; Clark, G.M.; Elledge, R.M. Infiltrating lobular carcinoma of the breast: Tumor characteristics and clinical outcome. Breast Cancer Res. 2004, 6, R149–R156. [Google Scholar] [CrossRef]
- Mersin, H.; Yildirim, E.; Gülben, K.; Berberoğlu, U. Is invasive lobular carcinoma different from invasive ductal carcinoma. Eur. J. Surg. Oncol. 2003, 29, 390–395. [Google Scholar] [CrossRef]
- Chung, L.K.; Pelargos, P.E.; Chan, A.M.; Demos, J.V.; Lagman, C.; Sheppard, J.P.; Nguyen, T.; Chang, Y.L.; Hojat, S.A.; Prins, R.M.; et al. Tissue microarray analysis for epithelial membrane protein-2 as a novel biomarker for gliomas. Brain Tumor Pathol. 2018, 35, 1–9. [Google Scholar] [CrossRef]
- Freije, W.A.; Castro-Vargas, F.E.; Fang, Z.; Horvath, S.; Cloughesy, T.; Liau, L.M.; Mischel, P.S.; Nelson, S.F. Gene expression profiling of gliomas strongly predicts survival. Cancer Res. 2004, 64, 6503–6510. [Google Scholar] [CrossRef]
- Qin, Y.; Fu, M.; Takahashi, M.; Iwanami, A.; Kuga, D.; Rao, R.G.; Sudhakar, D.; Huang, T.; Kiyohara, M.; Torres, K.; et al. Epithelial membrane protein-2 (EMP2) activates Src protein and is a novel therapeutic target for glioblastoma. J. Biol. Chem. 2014, 289, 13974–13985. [Google Scholar] [CrossRef]
- Qin, Y.; Takahashi, M.; Sheets, K.; Soto, H.; Tsui, J.; Pelargos, P.; Antonios, J.P.; Kasahara, N.; Yang, I.; Prins, R.M.; et al. Epithelial membrane protein-2 (EMP2) promotes angiogenesis in glioblastoma multiforme. J. Neurooncol. 2017, 134, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, D.; Fan, M.; Yang, C.H.; Zbytek, B.; Finkelstein, D.; Roussel, M.F.; Pfeffer, L.M. The critical role that STAT3 plays in glioma-initiating cells: STAT3 addiction in glioma. Oncotarget 2018, 9, 22095–22112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, J.; Salari, K.; Bocanegra, M.; Choi, Y.L.; Girard, L.; Gandhi, J.; Kwei, K.A.; Hernandez-Boussard, T.; Wang, P.; Gazdar, A.F.; et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS ONE 2009, 4, e6146. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y. Bioinformatics analysis of gene expression data for the identification of critical genes in breast invasive carcinoma. Mol. Med. Rep. 2017, 16, 8657–8664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.J.; Dahiya, S.; Richardson, E.; Erlander, M.; Sgroi, D.C. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009, 11, R7. [Google Scholar] [CrossRef]
- Watson, M.A.; Ylagan, L.R.; Trinkaus, K.M.; Gillanders, W.E.; Naughton, M.J.; Weilbaecher, K.N.; Fleming, T.P.; Aft, R.L. Isolation and molecular profiling of bone marrow micrometastases identifies TWIST1 as a marker of early tumor relapse in breast cancer patients. Clin. Cancer Res. 2007, 13, 5001–5009. [Google Scholar] [CrossRef]
- Fu, M.; Maresh, E.L.; Helguera, G.F.; Kiyohara, M.; Qin, Y.; Ashki, N.; Daniels-Wells, T.R.; Aziz, N.; Gordon, L.K.; Braun, J.; et al. Rationale and preclinical efficacy of a novel anti-EMP2 antibody for the treatment of invasive breast cancer. Mol. Cancer Ther. 2014, 13, 902–915. [Google Scholar] [CrossRef]
- Obermayr, E.; Sanchez-Cabo, F.; Tea, M.K.; Singer, C.F.; Krainer, M.; Fischer, M.B.; Sehouli, J.; Reinthaller, A.; Horvat, R.; Heinze, G.; et al. Assessment of a six gene panel for the molecular detection of circulating tumor cells in the blood of female cancer patients. BMC Cancer 2010, 10, 666. [Google Scholar] [CrossRef]
- Christgen, M.; Geffers, R.; Kreipe, H.; Lehmann, U. IPH-926 lobular breast cancer cells are triple-negative but their microarray profile uncovers a luminal subtype. Cancer Sci. 2013, 104, 1726–1730. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Yao, L.; Burner, D.; Minev, B.; Lu, L.; Wang, M.; Ma, W. Epithelial membrane protein 2: A novel biomarker for circulating tumor cell recovery in breast cancer. Clin. Transl. Oncol. 2019, 21, 433–442. [Google Scholar] [CrossRef]
- Murlidhar, V.; Reddy, R.M.; Fouladdel, S.; Zhao, L.; Ishikawa, M.K.; Grabauskiene, S.; Zhang, Z.; Lin, J.; Chang, A.C.; Carrott, P.; et al. Poor prognosis indicated by venous circulating tumor cell clusters in early-stage lung cancers. Cancer Res. 2017, 77, 5194–5206. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Lim, A.R.; Ghajar, C.M. Circulating and disseminated tumor cells: Harbingers or initiators of metastasis. Mol. Oncol. 2017, 11, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Maresh, E.L.; Soslow, R.A.; Alavi, M.; Mah, V.; Zhou, Q.; Iasonos, A.; Goodglick, L.; Gordon, L.K.; Braun, J.; et al. Epithelial membrane protein-2 is a novel therapeutic target in ovarian cancer. Clin. Cancer Res. 2010, 16, 3954–3963. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Rao, R.; Sudhakar, D.; Hogue, C.P.; Rutta, Z.; Morales, S.; Gordon, L.K.; Braun, J.; Goodglick, L.; Wadehra, M. Epithelial membrane protein-2 promotes endometrial tumor formation through activation of FAK and Src. PLoS ONE 2011, 6, e19945. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.K.; Kiyohara, M.; Fu, M.; Braun, J.; Dhawan, P.; Chan, A.; Goodglick, L.; Wadehra, M. EMP2 regulates angiogenesis in endometrial cancer cells through induction of VEGF. Oncogene 2013, 32, 5369–5376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyohara, M.H.; Dillard, C.; Tsui, J.; Kim, S.R.; Lu, J.; Sachdev, D.; Goodglick, L.; Tong, M.; Torous, V.F.; Aryasomayajula, C.; et al. EMP2 is a novel therapeutic target for endometrial cancer stem cells. Oncogene 2017, 36, 5793–5807. [Google Scholar] [CrossRef] [Green Version]
- Habeeb, O.; Goodglick, L.; Soslow, R.A.; Rao, R.G.; Gordon, L.K.; Schirripa, O.; Horvath, S.; Braun, J.; Seligson, D.B.; Wadehra, M. Epithelial membrane protein-2 expression is an early predictor of endometrial cancer development. Cancer 2010, 116, 4718–4726. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Liu, R.Y.; Zhou, C.; Yuan, M.Z.; Wu, D.M.; Yuan, Z.; Zhang, P.; Lang, J.Y. EMP2 re-expression inhibits growth and enhances radiosensitivity in nasopharyngeal carcinoma. Tumour Biol. 2017, 39, 1010428317695972. [Google Scholar] [CrossRef]
- Wang, M.; Li, S.; Zhang, P.; Wang, Y.; Wang, C.; Bai, D.; Jiang, X. EMP2 acts as a suppressor of melanoma and is negatively regulated by mTOR-mediated autophagy. J. Cancer 2019, 10, 3582–3592. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.W.; Li, W.M.; Wu, W.J.; Chai, C.Y.; Chang, T.Y.; Sun, Y.; Cheng, C.J.; Shiue, Y.L.; Su, S.J.; Cheng, H.L.; et al. Epithelial membrane protein 2 is a prognostic indictor for patients with urothelial carcinoma of the upper urinary tract. Am. J. Pathol. 2013, 183, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Scrideli, C.A.; Carlotti, C.G.; Okamoto, O.K.; Andrade, V.S.; Cortez, M.A.; Motta, F.J.; Lucio-Eterovic, A.K.; Neder, L.; Rosemberg, S.; Oba-Shinjo, S.M.; et al. Gene expression profile analysis of primary glioblastomas and non-neoplastic brain tissue: Identification of potential target genes by oligonucleotide microarray and real-time quantitative PCR. J. Neurooncol. 2008, 88, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Wang, Q.; Yan, X.; Wang, J. Whole-genome expression microarray combined with machine learning to identify prognostic biomarkers for high-grade glioma. J. Mol. Neurosci. 2018, 64, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Xu, Q.; Xie, S. High EMP3 expression might independently predict poor overall survival in glioblastoma and its expression is related to DNA methylation. Medicine 2018, 97, e9538. [Google Scholar] [CrossRef]
- Gao, Y.F.; Zhu, T.; Mao, C.X.; Liu, Z.X.; Wang, Z.B.; Mao, X.Y.; Li, L.; Yin, J.Y.; Zhou, H.H.; Liu, Z.Q. PPIC, EMP3 and CHI3L1 are novel prognostic markers for high grade glioma. Int. J. Mol. Sci. 2016, 17, 1808. [Google Scholar] [CrossRef]
- Jun, F.; Hong, J.; Liu, Q.; Guo, Y.; Liao, Y.; Huang, J.; Wen, S.; Shen, L. Epithelial membrane protein 3 regulates TGF-β signaling activation in CD44-high glioblastoma. Oncotarget 2017, 8, 14343–14358. [Google Scholar] [CrossRef]
- Bhat, K.P.L.; Balasubramaniyan, V.; Vaillant, B.; Ezhilarasan, R.; Hummelink, K.; Hollingsworth, F.; Wani, K.; Heathcock, L.; James, J.D.; Goodman, L.D.; et al. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell 2013, 24, 331–346. [Google Scholar] [CrossRef]
- Guo, X.X.; Su, J.; He, X.F. A 4-gene panel predicting the survival of patients with glioblastoma. J. Cell. Biochem. 2019, 120, 16037–16043. [Google Scholar] [CrossRef]
- Ernst, A.; Hofmann, S.; Ahmadi, R.; Becker, N.; Korshunov, A.; Engel, F.; Hartmann, C.; Felsberg, J.; Sabel, M.; Peterziel, H.; et al. Genomic and expression profiling of glioblastoma stem cell-like spheroid cultures identifies novel tumor-relevant genes associated with survival. Clin. Cancer Res. 2009, 15, 6541–6550. [Google Scholar] [CrossRef]
- Zheng, Z.; Luan, X.; Zha, J.; Li, Z.; Wu, L.; Yan, Y.; Wang, H.; Hou, D.; Huang, L.; Huang, F.; et al. TNF-α inhibits the migration of oral squamous cancer cells mediated by miR-765-EMP3-p66Shc axis. Cell. Signal. 2017, 34, 102–109. [Google Scholar] [CrossRef]
- Han, M.; Xu, W. EMP3 is induced by TWIST1/2 and regulates epithelial-to-mesenchymal transition of gastric cancer cells. Tumour Biol. 2017, 39, 1010428317718404. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.H.; Hsieh, S.C.; Lee, C.H.; Yang, S.F.; Cheng, C.W.; Tang, M.J.; Lin, C.L.; Lin, C.L.; Chou, R.H. Targeting EMP3 suppresses proliferation and invasion of hepatocellular carcinoma cells through inactivation of PI3K/Akt pathway. Oncotarget 2015, 6, 34859–34874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, X.C.; Fen, Y.J.; Yan, G.C.; Hong, H.; Yan, C.H.; Bing, L.W.; Zhong, Y.H. Epithelial membrane protein 3 functions as an oncogene and is regulated by microRNA-765 in primary breast carcinoma. Mol. Med. Rep. 2015, 12, 6445–6450. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Jiang, Z.; Li, X.; Xu, F.; Liu, Y.; Wen, P.; Kong, L.; Hou, M.; Yu, J. EMP3 overexpression in primary breast carcinomas is not associated with epigenetic aberrations. J. Korean Med. Sci. 2009, 24, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhang, D.; Yang, X.; Song, Y. Estrogen receptor α activates MAPK signaling pathway to promote the development of endometrial cancer. J. Cell. Biochem. 2019, 120, 17593–17601. [Google Scholar] [CrossRef]
- Wang, Y.W.; Li, W.M.; Wu, W.J.; Chai, C.Y.; Liu, H.S.; Lai, M.D.; Chow, N.H. Potential significance of EMP3 in patients with upper urinary tract urothelial carcinoma: Crosstalk with ErbB2-PI3K-Akt pathway. J. Urol. 2014, 192, 242–251. [Google Scholar] [CrossRef]
- Fumoto, S.; Hiyama, K.; Tanimoto, K.; Noguchi, T.; Hihara, J.; Hiyama, E.; Noguchi, T.; Nishiyama, M. EMP3 as a tumor suppressor gene for esophageal squamous cell carcinoma. Cancer Lett. 2009, 274, 25–32. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Liang, H.; Zhang, F.; Liu, F.; Chen, S.; Hu, Y.; Jiang, L.; Hao, Y.; Li, M.; et al. EMP3, which is regulated by miR-663a, suppresses gallbladder cancer progression via interference with the MAPK/ERK pathway. Cancer Lett. 2018, 430, 97–108. [Google Scholar] [CrossRef]
- Xue, Q.; Zhou, Y.; Wan, C.; Lv, L.; Chen, B.; Cao, X.; Ju, G.; Huang, Y.; Ni, R.; Mao, G. Epithelial membrane protein 3 is frequently shown as promoter methylation and functions as a tumor suppressor gene in non-small cell lung cancer. Exp. Mol. Pathol. 2013, 95, 313–318. [Google Scholar] [CrossRef]
- Pareek, S.; Notterpek, L.; Snipes, G.J.; Naef, R.; Sossin, W.; Laliberté, J.; Iacampo, S.; Suter, U.; Shooter, E.M.; Murphy, R.A. Neurons promote the translocation of peripheral myelin protein 22 into myelin. J. Neurosci 1997, 17, 7754–7762. [Google Scholar] [CrossRef]
- Li, J.; Kleeff, J.; Esposito, I.; Kayed, H.; Felix, K.; Giese, T.; Büchler, M.W.; Friess, H. Expression analysis of PMP22/Gas3 in premalignant and malignant pancreatic lesions. J. Histochem. Cytochem. 2005, 53, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Zhu, M.; Tao, Y.; Zhao, Y. Suppression of peripheral myelin protein 22 (PMP22) expression by miR29 inhibits the progression of lung cancer. Neoplasma 2015, 62, 881–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulson, A.; Levy, A.; Gossell-Williams, M. Monoclonal antibodies in cancer therapy: Mechanisms, successes and limitations. West. Indian Med. J. 2014, 63, 650–654. [Google Scholar] [PubMed]
- Salles, G.; Barrett, M.; Foà, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-cell hematologic malignancies: A review of 20 years of clinical experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef]
- Strohl, W.R. Current progress in innovative engineered antibodies. Protein Cell 2018, 9, 86–120. [Google Scholar] [CrossRef]
- Shimazaki, K.; Lepin, E.J.; Wei, B.; Nagy, A.K.; Coulam, C.P.; Mareninov, S.; Fu, M.; Wu, A.M.; Marks, J.D.; Braun, J.; et al. Diabodies targeting epithelial membrane protein 2 reduce tumorigenicity of human endometrial cancer cell lines. Clin. Cancer Res. 2008, 14, 7367–7377. [Google Scholar] [CrossRef]
- Filippakopoulos, P.; Qi, J.; Picaud, S.; Shen, Y.; Smith, W.B.; Fedorov, O.; Morse, E.M.; Keates, T.; Hickman, T.T.; Felletar, I.; et al. Selective inhibition of BET bromodomains. Nature 2010, 468, 1067–1073. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.J.; Lee, E.F.; Checco, J.W.; Evangelista, M.; Gellman, S.H.; Fairlie, W.D. Structure-guided rational design of α/β-peptide foldamers with high affinity for BCL-2 family prosurvival proteins. ChemBioChem 2013, 14, 1564–1572. [Google Scholar] [CrossRef]
- Zhao, Y.; Aguilar, A.; Bernard, D.; Wang, S. Small-molecule inhibitors of the MDM2−p53 protein−protein interaction (MDM2 inhibitors) in clinical trials for cancer treatment. J. Med. Chem. 2015, 58, 1038–1052. [Google Scholar] [CrossRef]
- Haase, H.S.; Peterson-Kaufman, K.J.; Lan Levengood, S.K.; Checco, J.W.; Murphy, W.L.; Gellman, S.H. Extending foldamer design beyond α-helix mimicry: α/β-peptide inhibitors of vascular endothelial growth factor signaling. J. Am. Chem. Soc. 2012, 134, 7652–7655. [Google Scholar] [CrossRef]
- Yoshida, A.; Shimizu, A.; Asano, H.; Kadonosono, T.; Kondoh, S.K.; Geretti, E.; Mammoto, A.; Klagsbrun, M.; Seo, M.K. VEGF-A/NRP1 stimulates GIPC1 and Syx complex formation to promote RhoA activation and proliferation in skin cancer cells. Biol. Open 2015, 4, 1063–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Type of Cancer | In Vitro Model | In Vivo Model | Patient Samples | Remarks | |||
|---|---|---|---|---|---|---|---|
| mRNA | Protein | mRNA | Protein | mRNA | Protein | ||
| Pro-metastatic property | |||||||
| GBM | [59,60] | [59,60] | [59] | [59,60] | [59,60] | Promotion of cancer cell proliferation and invasion; Correlation with poor clinical outcome | |
| Uveal melanoma | [65] | [65] | [65] | [63] | Possible relationship with a high risk for metastatic death | ||
| NSCLC | [66] | [66,67] | [67] | [68] | [67] | Increase in cancer cell proliferation; Impairment of drug sensitivity | |
| ALL | [69] | [69] | [69] | Decrease in apoptosis; Increase in cancer cell migration, adhesion and proliferation; Impairment of drug sensitivity | |||
| Anti-metastatic property | |||||||
| Nasopharyngeal cancer | [70] | [70] | [70] | Inhibition of cancer cell migration and invasion; Increase in apoptosis; Improvement of the patients’ survival rates | |||
| Esophageal cancer | [71] | [71] | Decrease in cancer cell proliferation | ||||
| Gastric cancer | [72] | [72] | [72] | Correlation with reduced cancer invasion and metastasis and with elongation of the patients’ survival | |||
| Colorectal cancer | [73] | [73] | [73] | Decrease in cancer cell proliferation; Increase in apoptosis; Improvement of the patients’ survival rates | |||
| Ovarian cancer | [74] | Possible association with reduction of the severity of the cancer | |||||
| Paradoxical effect on metastasis | |||||||
| Breast cancer | [76] | [76] | [75,76] | Possible correlation with promotion of cancer invasion; Biomarker to distinguish the histological types of the cancer Inhibition of cancer cell migration, proliferation and invasion; Improvement of the patients’ survival rates | |||
| rostate cancer | [22,77] | [22,77] | [22] | [22] | [22,77] | Promotion of cancer cell migration, invasion and metastasis Inhibition of cancer cell migration and invasion | |
| Type of Cancer | In Vitro Model | In Vivo Model | Patient Samples | Remarks | |||
|---|---|---|---|---|---|---|---|
| mRNA | Protein | mRNA | Protein | mRNA | Protein | ||
| Pro-metastatic property | |||||||
| GBM | [84] | [83,84] | [83,84] | [82] | [81,83] | Promotion of cancer cell migration, invasion and angiogenesis | |
| Breast cancer | [92] | [90,93] | [92] | [86,87,88,89,91] | [90,93] | Promotion of cancer cell invasion and metastasis | |
| Ovarian cancer | [97] | [97] | [97] | Decrease in cancer cell death; Association with the malignant type of the cancer | |||
| Endometrial cancer | [99,100] | [98,99,100] | [98,99,100] | [101] | Promotion of angiogenesis; Correlation with cancer progression | ||
| Anti-metastatic property | |||||||
| Nasopharyngeal cancer | [102] | [102] | [102] | Decrease in cancer cell growth; Enhancement of the sensitivity of radiotherapy; Improvement of the clinical outcome | |||
| Cutaneous melanoma | [103] | [103] | [103] | Increase in apoptosis | |||
| Urothelial cancer | [104] | [104] | [104] | [104] | Decrease in cancer cell proliferation | ||
| Type of Cancer | In Vitro Model | In Vivo Model | Patient Samples | Remarks | |||
|---|---|---|---|---|---|---|---|
| mRNA | Protein | mRNA | Protein | mRNA | Protein | ||
| Pro-metastatic property | |||||||
| GBM | [109] | [109] | [109] | [105,106,107,108,109,111,112] | [112] | Increase in cancer cell proliferation; Decrease in apoptosis; Correlation with poor clinical outcome | |
| Oral squamous cancer | [113] | [113] | [113] | Increase in cancer cell migration | |||
| Gastric cancer | [114] | [114] | [114] | Induction of EMT; Correlation with poor clinical outcome | |||
| Hepatocellular cancer | [115] | [115] | [115] | Promotion of cancer cell proliferation, migration and invasion; Negative correlation with tumor differentiation | |||
| Breast cancer | [116] | [116] | [116,117] | [116] | Promotion of cancer cell proliferation, invasion and metastasis | ||
| Endometrial cancer | [118] | Possible correlation with development of the cancer | |||||
| Urothelial cancer | [119] | [119] | [119] | [119] | Increase in cancer cell proliferation and migration | ||
| Anti-metastatic property | |||||||
| Esophageal cancer | [120] | [120] | [120] | [120] | Increase in cancer cell death; Improvement of the survival rate | ||
| Gallbladder cancer | [121] | [121] | [121] | [121] | [121] | Inhibition of cancer cell proliferation, migration and invasion; Improvement of the patients’ survival rates | |
| NSCLC | [122] | [122] | Decrease in cancer cell proliferation | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmat Amin, M.K.B.; Shimizu, A.; Ogita, H. The Pivotal Roles of the Epithelial Membrane Protein Family in Cancer Invasiveness and Metastasis. Cancers 2019, 11, 1620. https://doi.org/10.3390/cancers11111620
Ahmat Amin MKB, Shimizu A, Ogita H. The Pivotal Roles of the Epithelial Membrane Protein Family in Cancer Invasiveness and Metastasis. Cancers. 2019; 11(11):1620. https://doi.org/10.3390/cancers11111620
Chicago/Turabian StyleAhmat Amin, Mohammad Khusni B., Akio Shimizu, and Hisakazu Ogita. 2019. "The Pivotal Roles of the Epithelial Membrane Protein Family in Cancer Invasiveness and Metastasis" Cancers 11, no. 11: 1620. https://doi.org/10.3390/cancers11111620
APA StyleAhmat Amin, M. K. B., Shimizu, A., & Ogita, H. (2019). The Pivotal Roles of the Epithelial Membrane Protein Family in Cancer Invasiveness and Metastasis. Cancers, 11(11), 1620. https://doi.org/10.3390/cancers11111620





