Detailed Characterization of Immune Cell Infiltrate and Expression of Immune Checkpoint Molecules PD-L1/CTLA-4 and MMR Proteins in Testicular Germ Cell Tumors Disclose Novel Disease Biomarkers
Abstract
1. Introduction
2. Results
2.1. Immune Cells in Testicular Germ Cell Tumors
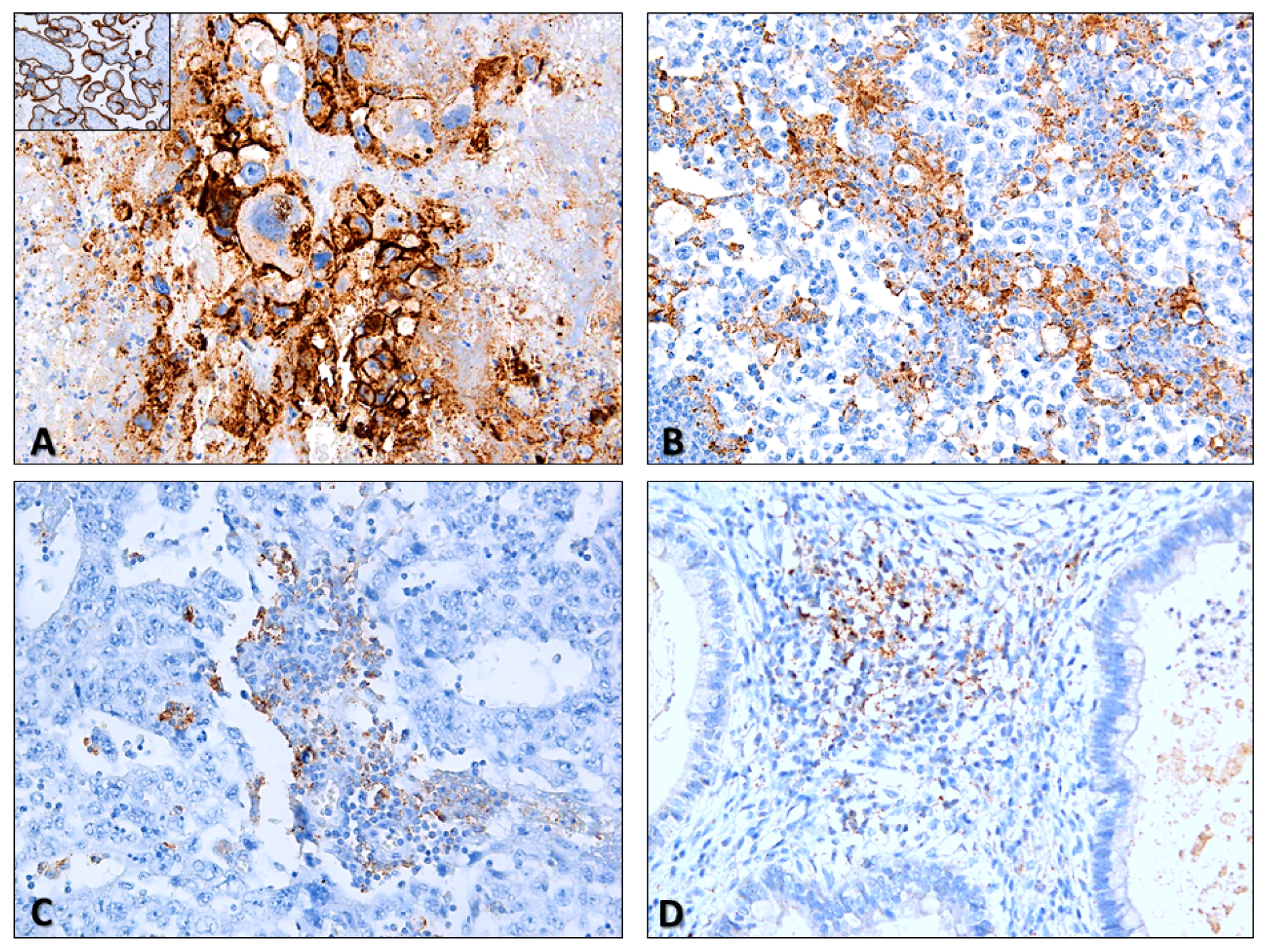
2.1.1. Immune Checkpoints Expression—PD-L1 and CTLA-4
2.1.2. Characterization of the Immune Infiltrate
2.2. Tumor Cells in Testicular Germ Cell Tumors and in Metastases
2.2.1. Immune Checkpoints Expression in Primary Tumors—PD-L1 and CTLA-4
2.2.2. Mismatch Repair Protein Expression in Primary Tumors
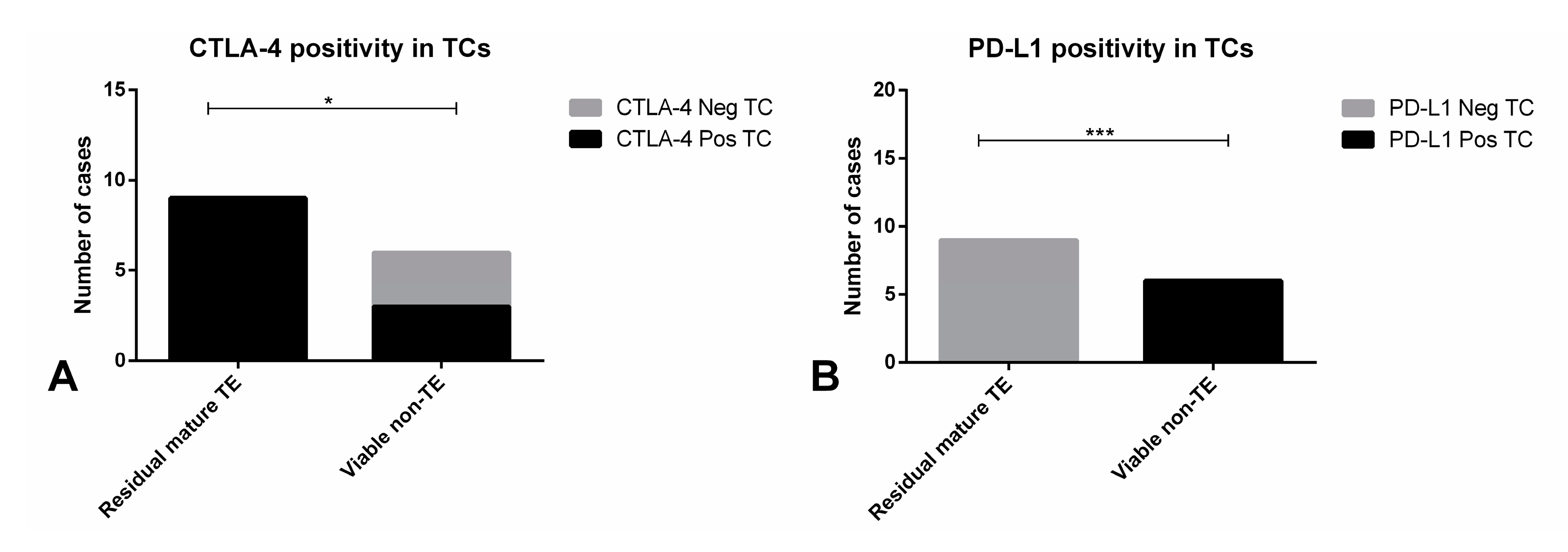
2.2.3. Immunoexpression of the Several Markers in TGCT Metastases
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Immunohistochemistry
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stevenson, S.M.; Lowrance, W.T. Epidemiology and Diagnosis of Testis Cancer. Urol. Clin. N. Am. 2015, 42, 269–275. [Google Scholar] [CrossRef]
- Oing, C.; Skowron, M.A.; Bokemeyer, C.; Nettersheim, D. Epigenetic treatment combinations to effectively target cisplatin-resistant germ cell tumors-past, present, and future considerations. Andrology 2019, 7, 487–497. [Google Scholar] [CrossRef]
- O’Shaughnessy, M.J.; Feldman, D.R.; Carver, B.S.; Sheinfeld, J. Late Relapse of Testicular Germ Cell Tumors. Urol. Clin. N. Am. 2015, 42, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Curreri, S.A.; Fung, C.; Beard, C.J. Secondary malignant neoplasms in testicular cancer survivors. Urol. Oncol. 2015, 33, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, K.A.; Walsh, T.J. Infertility with Testicular Cancer. Urol. Clin. N. Am. 2015, 42, 409–420. [Google Scholar] [CrossRef]
- Chovanec, M.; Albany, C.; Mego, M.; Montironi, R.; Cimadamore, A.; Cheng, L. Emerging Prognostic Biomarkers in Testicular Germ Cell Tumors: Looking Beyond Established Practice. Front. Oncol. 2018, 8, 571. [Google Scholar] [CrossRef]
- Lobo, J.; Gillis, A.J.M.; Jeronimo, C.; Henrique, R.; Looijenga, L.H.J. Human Germ Cell Tumors are Developmental Cancers: Impact of Epigenetics on Pathobiology and Clinic. Int. J. Mol. Sci. 2019, 20, 258. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.L.; Lobo, J.; Jeronimo, C.; Henrique, R. The epigenetics of testicular germ cell tumors: Looking for novel disease biomarkers. Epigenomics 2017, 9, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Ulbright, T.; Humphrey, P.; Reuter, V. WHO Classification of Tumours of the Urinary System and Male Genital Organs, 4th ed.; IARC: Lyon, France, 2016. [Google Scholar]
- Ulbright, T.M. Pitfalls in the interpretation of specimens from patients with testicular tumours, with an emphasis on variant morphologies. Pathology 2018, 50, 88–99. [Google Scholar] [CrossRef]
- Howitt, B.E.; Berney, D.M. Tumors of the Testis: Morphologic Features and Molecular Alterations. Surg. Pathol. Clin. 2015, 8, 687–716. [Google Scholar] [CrossRef]
- Ulbright, T.M. Germ cell tumors of the gonads: A selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Mod. Pathol. 2005, 18 (Suppl. 2), S61–S79. [Google Scholar] [CrossRef] [PubMed]
- Dorantes-Heredia, R.; Motola-Kuba, D.; Murphy-Sanchez, C.; Izquierdo-Tolosa, C.D.; Ruiz-Morales, J.M. Spontaneous regression as a ‘burned-out’ non-seminomatous testicular germ cell tumor: A case report and literature review. J. Surg. Case Rep. 2019, 2019, rjy358. [Google Scholar] [CrossRef] [PubMed]
- Ratta, R.; Zappasodi, R.; Raggi, D.; Grassi, P.; Verzoni, E.; Necchi, A.; Di Nicola, M.; Salvioni, R.; de Braud, F.; Procopio, G. Immunotherapy advances in uro-genital malignancies. Crit. Rev. Oncol. Hematol. 2016, 105, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, C.D.; Curioni-Fontecedro, A.; Allmann, V.; Beyer, J.; Tischler, V.; Sulser, T.; Moch, H.; Bode, P.K. Frequent PD-L1 expression in testicular germ cell tumors. Br. J. Cancer 2015, 113, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Siska, P.J.; Johnpulle, R.A.N.; Zhou, A.; Bordeaux, J.; Kim, J.Y.; Dabbas, B.; Dakappagari, N.; Rathmell, J.C.; Rathmell, W.K.; Morgans, A.K.; et al. Deep exploration of the immune infiltrate and outcome prediction in testicular cancer by quantitative multiplexed immunohistochemistry and gene expression profiling. Oncoimmunology 2017, 6, e1305535. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Ward, J.E.; Bao, R.; Hall, C.R.; Brockstein, B.E.; Luke, J.J. Clinical Response of a Patient to Anti-PD-1 Immunotherapy and the Immune Landscape of Testicular Germ Cell Tumors. Cancer Immunol. Res. 2016, 4, 903–909. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cierna, Z.; Mego, M.; Miskovska, V.; Machalekova, K.; Chovanec, M.; Svetlovska, D.; Hainova, K.; Rejlekova, K.; Macak, D.; Spanik, S.; et al. Prognostic value of programmed-death-1 receptor (PD-1) and its ligand 1 (PD-L1) in testicular germ cell tumors. Ann. Oncol. 2016, 27, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Chovanec, M.; Cierna, Z.; Miskovska, V.; Machalekova, K.; Svetlovska, D.; Kalavska, K.; Rejlekova, K.; Spanik, S.; Kajo, K.; Babal, P.; et al. Prognostic role of programmed-death ligand 1 (PD-L1) expressing tumor infiltrating lymphocytes in testicular germ cell tumors. Oncotarget 2017, 8, 21794–21805. [Google Scholar] [CrossRef] [PubMed]
- Hinsch, A.; Blessin, N.C.; Simon, R.; Kluth, M.; Fischer, K.; Hube-Magg, C.; Li, W.; Makrypidi-Fraune, G.; Wellge, B.; Mandelkow, T.; et al. Expression of the immune checkpoint receptor TIGIT in seminoma. Oncol. Lett. 2019, 18, 1497–1502. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Q.; Gao, Z.; Lao, X.M.; Lin, W.M.; Chen, D.P.; Mu, M.; Huang, C.X.; Liu, Z.Y.; Li, B.; et al. The local immune landscape determines tumor PD-L1 heterogeneity and sensitivity to therapy. J. Clin. Investig. 2019, 129, 3347–3360. [Google Scholar] [CrossRef]
- Chi, E.A.; Schweizer, M.T. Durable Response to Immune Checkpoint Blockade in a Platinum-Refractory Patient With Nonseminomatous Germ Cell Tumor. Clin. Genitourin. Cancer 2017, 15, e855–e857. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.A.; Ring, K.L.; Willis, B.C.; Modesitt, S.C.; Mills, A.M. PD-L1 Expression in Mismatch Repair-deficient Endometrial Carcinomas, Including Lynch Syndrome-associated and MLH1 Promoter Hypermethylated Tumors. Am. J. Surg. Pathol. 2017, 41, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.W.; Bledsoe, J.R.; Morales-Oyarvide, V.; Huynh, T.G.; Mino-Kenudson, M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod. Pathol. 2016, 29, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Honecker, F.; Wermann, H.; Mayer, F.; Gillis, A.J.; Stoop, H.; van Gurp, R.J.; Oechsle, K.; Steyerberg, E.; Hartmann, J.T.; Dinjens, W.N.; et al. Microsatellite instability, mismatch repair deficiency, and BRAF mutation in treatment-resistant germ cell tumors. J. Clin. Oncol. 2009, 27, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, C.; Melau, C.; Nielsen, J.E.; Vile Jensen, K.; Liu, D.; Pena-Diaz, J.; Rajpert-De Meyts, E.; Rasmussen, L.J.; Jorgensen, A. Involvement of the DNA mismatch repair system in cisplatin sensitivity of testicular germ cell tumours. Cell. Oncol. (Dordr.) 2017, 40, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.; Costa, A.L.; Vilela-Salgueiro, B.; Rodrigues, A.; Guimaraes, R.; Cantante, M.; Lopes, P.; Antunes, L.; Jeronimo, C.; Henrique, R. Testicular germ cell tumors: Revisiting a series in light of the new WHO classification and AJCC staging systems, focusing on challenges for pathologists. Hum. Pathol. 2018, 82, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Gwynn, M.E.; DeRemer, D.L. The Emerging Role of PD-1/PD-L1-Targeting Immunotherapy in the Treatment of Metastatic Urothelial Carcinoma. Ann. Pharmacother. 2018, 52, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Nazzani, S.; Bazinet, A.; Karakiewicz, P.I. Role of immunotherapy in kidney cancer. Curr. Opin. Support. Palliat. Care 2018, 12, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Mullane, S.A.; Werner, L.; Fay, A.P.; Callea, M.; Leow, J.J.; Taplin, M.E.; Choueiri, T.K.; Hodi, F.S.; Freeman, G.J.; et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann. Oncol. 2015, 26, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.M.; Hirsch, F.R. Programmed Death Ligand-1 Immunohistochemistry: Friend or Foe? Arch. Pathol. Lab. Med. 2016, 140, 326–331. [Google Scholar] [CrossRef]
- Salama, A.K.; Hodi, F.S. Cytotoxic T-lymphocyte-associated antigen-4. Clin. Cancer Res. 2011, 17, 4622–4628. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.S.; Sansom, D.M. Confusing signals: Recent progress in CTLA-4 biology. Trends Immunol. 2015, 36, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Nakanoma, T.; Nakamura, K.; Deguchi, N.; Fujimoto, J.; Tazaki, H.; Hata, J. Immunohistological analysis of tumour infiltrating lymphocytes in seminoma using monoclonal antibodies. Virchows Arch. A 1992, 421, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Casanova, J.F.; Nistal, M.; Regadera, J. Quantification of immunocompetent cells in testicular germ cell tumours. Histopathology 1997, 30, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Grobholz, R.; Verbeke, C.S.; Schleger, C.; Kohrmann, K.U.; Hein, B.; Wolf, G.; Bleyl, U.; Spagnoli, G.C.; Coplan, K.; Kolb, D.; et al. Expression of MAGE antigens and analysis of the inflammatory T-cell infiltrate in human seminoma. Urol. Res. 2000, 28, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Yakirevich, E.; Lefel, O.; Sova, Y.; Stein, A.; Cohen, O.; Izhak, O.B.; Resnick, M.B. Activated status of tumour-infiltrating lymphocytes and apoptosis in testicular seminoma. J. Pathol. 2002, 196, 67–75. [Google Scholar] [CrossRef]
- Hvarness, T.; Nielsen, J.E.; Almstrup, K.; Skakkebaek, N.E.; Rajpert-De Meyts, E.; Claesson, M.H. Phenotypic characterisation of immune cell infiltrates in testicular germ cell neoplasia. J. Reprod. Immunol. 2013, 100, 135–145. [Google Scholar] [CrossRef]
- Klein, B.; Haggeney, T.; Fietz, D.; Indumathy, S.; Loveland, K.L.; Hedger, M.; Kliesch, S.; Weidner, W.; Bergmann, M.; Schuppe, H.C. Specific immune cell and cytokine characteristics of human testicular germ cell neoplasia. Hum. Reprod. 2016, 31, 2192–2202. [Google Scholar] [CrossRef]
- Loveland, K.L.; Klein, B.; Pueschl, D.; Indumathy, S.; Bergmann, M.; Loveland, B.E.; Hedger, M.P.; Schuppe, H.C. Cytokines in Male Fertility and Reproductive Pathologies: Immunoregulation and Beyond. Front. Endocrinol. (Lausanne) 2017, 8, 307. [Google Scholar] [CrossRef]
- Hadrup, S.R.; Braendstrup, O.; Jacobsen, G.K.; Mortensen, S.; Pedersen, L.O.; Seremet, T.; Andersen, M.H.; Becker, J.C.; Straten, P.T. Tumor infiltrating lymphocytes in seminoma lesions comprise clonally expanded cytotoxic T cells. Int. J. Cancer 2006, 119, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chung, J.H. PD-L1 Testing in Non-small Cell Lung Cancer: Past, Present, and Future. J. Pathol. Transl. Med. 2019, 53, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Guleria, I.; Khosroshahi, A.; Ansari, M.J.; Habicht, A.; Azuma, M.; Yagita, H.; Noelle, R.J.; Coyle, A.; Mellor, A.L.; Khoury, S.J.; et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J. Exp. Med. 2005, 202, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, E.E.; Kilvaer, T.K.; Rakaee, M.; Richardsen, E.; Hald, S.M.; Andersen, S.; Busund, L.T.; Bremnes, R.M.; Donnem, T. CTLA-4 expression in the non-small cell lung cancer patient tumor microenvironment: Diverging prognostic impact in primary tumors and lymph node metastases. Cancer Immunol. Immunother. 2017, 66, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, K.A.; Bowen, J.A.; Tsai, A.F.; Bluestone, J.A.; Hunt, J.S.; Ober, C. The CTLA-4 gene is expressed in placental fibroblasts. Mol. Hum. Reprod. 1999, 5, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Adra, N.; Einhorn, L.H.; Althouse, S.K.; Ammakkanavar, N.R.; Musapatika, D.; Albany, C.; Vaughn, D.; Hanna, N.H. Phase II trial of pembrolizumab in patients with platinum refractory germ-cell tumors: A Hoosier Cancer Research Network Study GU14-206. Ann. Oncol. 2018, 29, 209–214. [Google Scholar] [CrossRef]
- Necchi, A.; Giannatempo, P.; Raggi, D.; Mariani, L.; Colecchia, M.; Fare, E.; Monopoli, F.; Calareso, G.; Ali, S.M.; Ross, J.S.; et al. An Open-label Randomized Phase 2 study of Durvalumab Alone or in Combination with Tremelimumab in Patients with Advanced Germ Cell Tumors (APACHE): Results from the First Planned Interim Analysis. Eur. Urol. 2019, 75, 201–203. [Google Scholar] [CrossRef]
- Mego, M.; Svetlovska, D.; Chovanec, M.; Reckova, M.; Rejlekova, K.; Obertova, J.; Palacka, P.; Sycova-Mila, Z.; De Giorgi, U.; Mardiak, J. Phase II study of avelumab in multiple relapsed/refractory germ cell cancer. Investig. New Drugs 2019, 37, 748–754. [Google Scholar] [CrossRef]
- Olasz, J.; Mandoky, L.; Geczi, L.; Bodrogi, I.; Csuka, O.; Bak, M. Influence of hMLH1 methylation, mismatch repair deficiency and microsatellite instability on chemoresistance of testicular germ-cell tumors. Anticancer. Res. 2005, 25, 4319–4324. [Google Scholar]
- Lobo, J.; Pinto, C.; Pinheiro, M.; Lobo, F.; Sousa, N.; Lopes, P.; Looijenga, L.H.J.; Jeronimo, C.; Teixeira, M.R.; Henrique, R. Widening the spectrum of Lynch syndrome: First report of testicular seminoma attributable to MSH2 loss. Histopathology 2019. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Shih, J.; Hollern, D.P.; Wang, L.; Bowlby, R.; Tickoo, S.K.; Thorsson, V.; Mungall, A.J.; Newton, Y.; Hegde, A.M.; et al. Integrated Molecular Characterization of Testicular Germ Cell Tumors. Cell Rep. 2018, 23, 3392–3406. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.; Riquelme, E.; Schultz, M.; Wistuba, I.I.; Villarroel, L.; Pizarro, J.; Berlin, A.; Ittmann, M.; Koh, M.S.; Leach, F.S. Mismatch repair gene expression and genetic instability in testicular germ cell tumor. Cancer Biol. Ther. 2004, 3, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Rieke, D.T.; Ochsenreither, S.; Klinghammer, K.; Seiwert, T.Y.; Klauschen, F.; Tinhofer, I.; Keilholz, U. Methylation of RAD51B, XRCC3 and other homologous recombination genes is associated with expression of immune checkpoints and an inflammatory signature in squamous cell carcinoma of the head and neck, lung and cervix. Oncotarget 2016, 7, 75379–75393. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: Berlin, Germany, 2017. [Google Scholar]
- International Germ Cell Consensus Classification: A prognostic factor-based staging system for metastatic germ cell cancers. Available online: https://ascopubs.org/doi/abs/10.1200/JCO.1997.15.2.594 (accessed on 1 July 2019).
- Fankhauser, C.D.; Gerke, T.A.; Roth, L.; Sander, S.; Grossmann, N.C.; Kranzbuhler, B.; Eberli, D.; Sulser, T.; Beyer, J.; Hermanns, T. Pre-orchiectomy tumor marker levels should not be used for International Germ Cell Consensus Classification (IGCCCG) risk group assignment. J. Cancer Res. Clin. Oncol. 2019, 145, 781–785. [Google Scholar] [CrossRef]
- Lobo, J.; Costa, A.L.; Cantante, M.; Guimaraes, R.; Lopes, P.; Antunes, L.; Braga, I.; Oliveira, J.; Pelizzola, M.; Henrique, R.; et al. m(6)A RNA modification and its writer/reader VIRMA/YTHDF3 in testicular germ cell tumors: A role in seminoma phenotype maintenance. J. Transl. Med. 2019, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.L.; Moreira-Barbosa, C.; Lobo, J.; Vilela-Salgueiro, B.; Cantante, M.; Guimaraes, R.; Lopes, P.; Braga, I.; Oliveira, J.; Antunes, L.; et al. DNA methylation profiling as a tool for testicular germ cell tumors subtyping. Epigenomics 2018, 10, 1511–1523. [Google Scholar] [CrossRef]
- Vilela-Salgueiro, B.; Barros-Silva, D.; Lobo, J.; Costa, A.L.; Guimaraes, R.; Cantante, M.; Lopes, P.; Braga, I.; Oliveira, J.; Henrique, R.; et al. Germ cell tumour subtypes display differential expression of microRNA371a-3p. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170388. [Google Scholar] [CrossRef]
- Teixido, C.; Vilarino, N.; Reyes, R.; Reguart, N. PD-L1 expression testing in non-small cell lung cancer. Ther. Adv. Med. Oncol. 2018, 10, 1758835918763493. [Google Scholar] [CrossRef]
- Hodgson, A.; Slodkowska, E.; Jungbluth, A.; Liu, S.K.; Vesprini, D.; Enepekides, D.; Higgins, K.; Katabi, N.; Xu, B.; Downes, M.R. PD-L1 Immunohistochemistry Assay Concordance in Urothelial Carcinoma of the Bladder and Hypopharyngeal Squamous Cell Carcinoma. Am. J. Surg. Pathol. 2018, 42, 1059–1066. [Google Scholar] [CrossRef]
- Lobo, J.; Henrique, R.; Jeronimo, C. The Role of DNA/Histone Modifying Enzymes and Chromatin Remodeling Complexes in Testicular Germ Cell Tumors. Cancers 2018, 11, 6. [Google Scholar] [CrossRef]
- Jostes, S.; Nettersheim, D.; Schorle, H. Epigenetic drugs and their molecular targets in testicular germ cell tumours. Nat. Rev. Urol. 2019, 16, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Chovanec, M.; De Giorgi, U.; Mego, M. Immune-Related Concepts in Biology and Treatment of Germ-Cell Tumors. Adv. Urol. 2018, 2018, 3718165. [Google Scholar] [CrossRef] [PubMed]
- Semaan, A.; Haddad, F.G.; Eid, R.; Kourie, H.R.; Nemr, E. Immunotherapy: Last bullet in platinum refractory germ cell testicular cancer. Future Oncol. 2019, 15, 533–541. [Google Scholar] [CrossRef] [PubMed]


| Variables | Patient Cohort (n = 162 #) Tumor Samples (n = 271) |
|---|---|
| Age [years (median, IQR)] | 30 (25–36) |
| Laterality (n, %) | |
| Right | 88/160 (55.0) |
| Left | 71/160 (44.4%) |
| Bilateral synchronous | 1/160 (0.6%) |
| Pre-operative AFP (n, %) | |
| Within normal range | 102/157 (65.0) |
| Elevated | 55/157 (35.0) |
| Pre-operative HCG (n, %) | |
| Within normal range | 77/158 (48.7) |
| Elevated | 81/158 (51.3) |
| Pre-operative LDH (n, %) | |
| Within normal range | 75/131 (57.3) |
| Elevated | 56/131 (42.7) |
| Histologic subtypes (n, %) | |
| Pure SE | 86/164 (52.4) |
| Pure EC | 12/164 (7.3) |
| Pure TE | 4/164 (2.5) |
| Mixed tumor | 62/164 (37.8) |
| Tumor components (n, %) | |
| SE | 109/271 (40.2) |
| EC | 61/271 (22.5) |
| YST | 41/271 (15.1) |
| CH | 12/271 (4.5) |
| TE | 48/271 (17.7) |
| Largest tumor size [cm (median, IQR)] | 4.2 (2.4-6.5) |
| Rete testis invasion (n, %) | |
| Absent | 76/160 (47.5) |
| Present, stromal | 84/160 (52.5) |
| Vascular invasion (n, %) | |
| Absent | 84/164 (51.2) |
| Present | 80/164 (48.8) |
| Stage (n, %) | |
| I | 102/163 (62.5) |
| II | 34/163 (20.9) |
| III | 27/163 (16.6) |
| IGCCCG group* (n, %) | |
| Good | 45/61 (73.8) |
| Intermediate | 9/61 (14.7) |
| Poor | 7/61 (11.5) |
| Histology of metastases (n, %) | |
| SE | 1/17 (5.9) |
| EC | 5/17 (29.4) |
| YST | 1/17 (5.9) |
| CH | 1/17 (5.9) |
| Residual mature TE | 9/17 (52.9) |
| Relapse (n, %) | |
| No | 151/162 (93.2) |
| Yes | 11/162 (6.8) |
| Treatments performed (n) | |
| CT | 102 |
| RT | 47 |
| Vital status at last follow-up (n, %) | |
| A-NED | 155/162 (95.7) |
| AWD | 3/162 (1.9) |
| D-NED | 1/162 (0.5) |
| DFD | 3/162 (1.9) |
| Variables | CTLA-4 in ICs: Negative | CTLA-4 in ICs: Positive | p-Value |
|---|---|---|---|
| Histology (n, % within Histology) | 1.0 | ||
| SE (n = 86) | 3 (3.5) | 83 (96.5) | |
| NS (n = 78) | 3 (3.8) | 75 (96.2) | |
| LVI (n, % within LVI) | 0.6822 | ||
| Yes (n = 80) | 2 (2.5) | 78 (97.5) | |
| No (n = 84) | 4 (4.8) | 80 (95.2) | |
| pT Stage (n, % within pT Stage) | 1.0 | ||
| pT1 (n = 71) | 2 (2.8) | 69 (97.2) | |
| pT2-3 (n = 92) | 3 (3.3) | 89 (96.7) | |
| N Stage (n, % within N Stage) | 0.6599 | ||
| N0 (n = 106) | 4 (3.8) | 102 (96.2) | |
| N+ (n = 56) | 1 (1.8) | 55 (98.2) | |
| Stage, TNM (n, % within Stage TNM) | 0.6514 | ||
| I (n = 102) | 4 (3.9) | 98 (96.1) | |
| II–III (n = 61) | 1 (1.6) | 60 (98.4) | |
| IGCCCG grouping (n, % within IGCCCG) | 1.0 | ||
| Good (n = 45) | 1 (2.2) | 44 (97.8) | |
| Intermediate/Poor (n = 16) | 0 (0) | 16 (100) | |
| PD-L1 in ICs: Negative | PD-L1 in ICs: Positive | p-Value | |
| Histology (n, % within Histology) | 0.2099 | ||
| SE (n = 86) | 11 (12.8) | 75 (87.2) | |
| NS (n = 78) | 16 (20.5) | 62 (79.5) | |
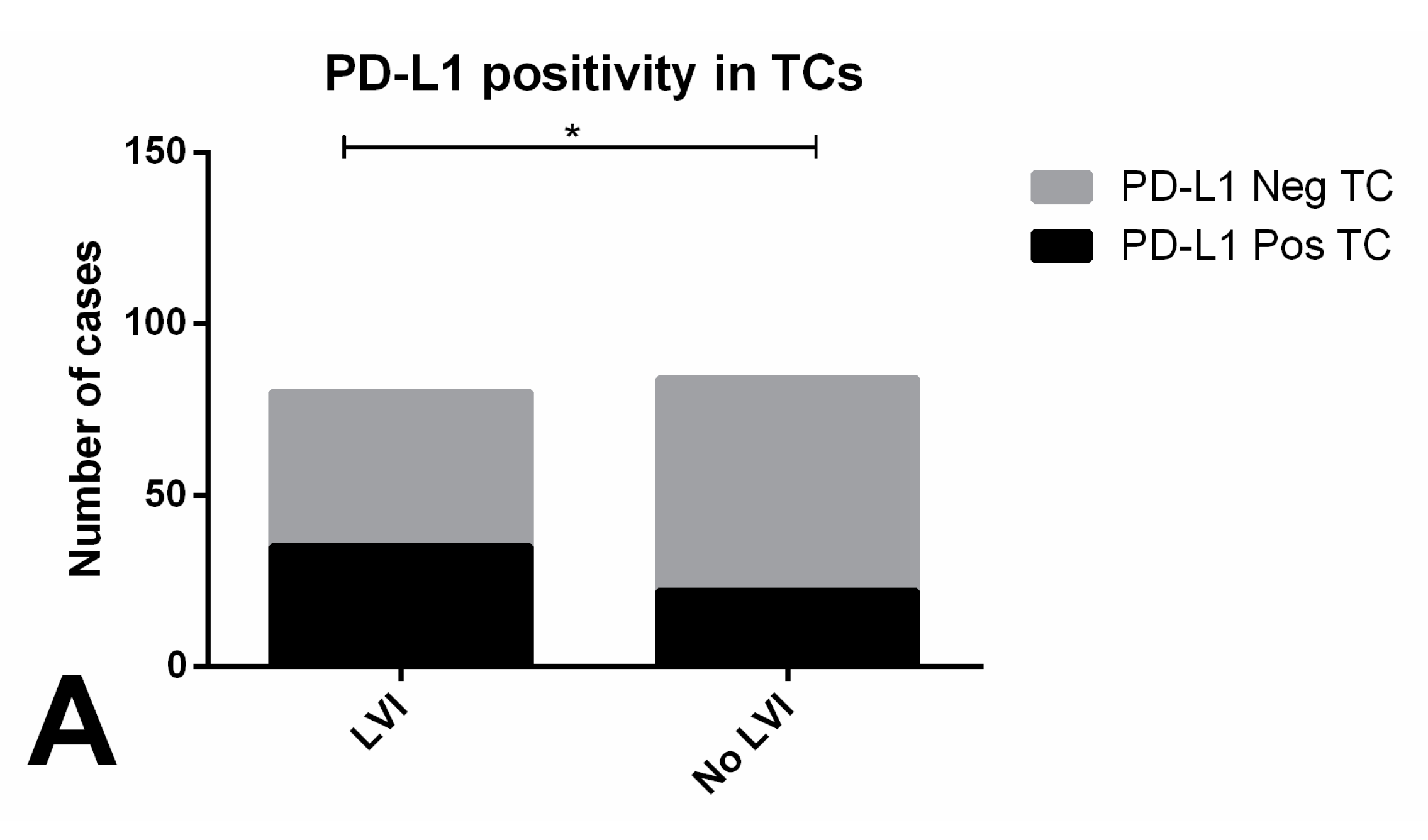
| LVI (n, % within LVI) | 0.0939 | ||
| Yes (n = 80) | 9 (11.3) | 71 (88.7) | |
| No (n = 84) | 18 (21.4) | 66 (78.6) | |
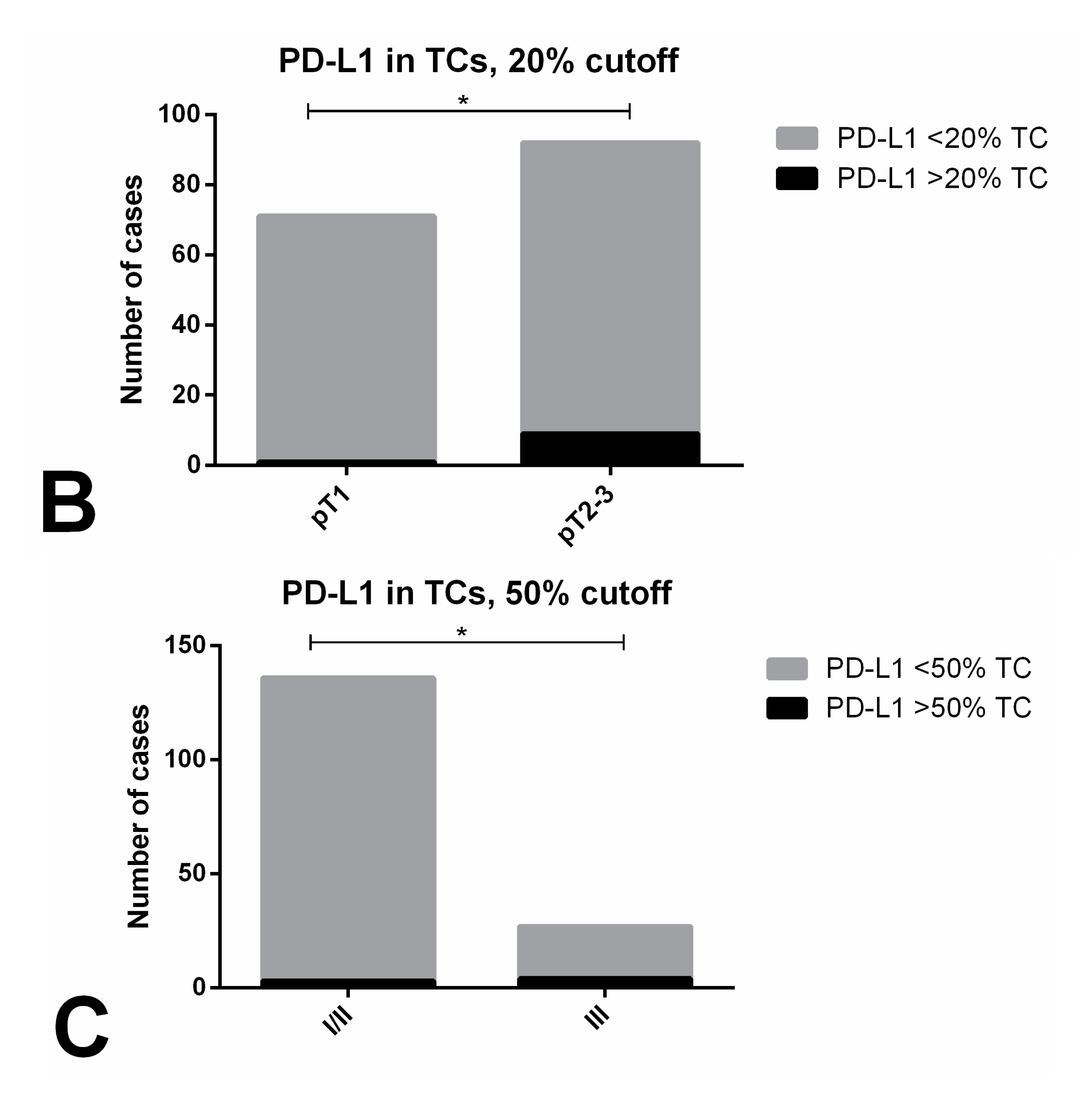
| pT Stage (n, % within pT Stage) | 0.1334 | ||
| pT1 (n = 71) | 15 (21.1) | 56 (78.9) | |
| pT2-3 (n = 92) | 11 (12.0) | 81 (88.0) | |
| N Stage (n, % within N Stage) | 0.5020 | ||
| N0 (n = 106) | 18 (17.0) | 88 (83.0) | |
| N+ (n = 56) | 7 (12.5) | 49 (87.5) | |
| Stage, TNM (n, % within Stage TNM) | 1.0 | ||
| I (n = 102) | 16 (15.7) | 86 (84.3) | |
| II–III (n = 61) | 10 (16.4) | 51 (83.6) | |
| IGCCCG grouping (n, % within IGCCCG) | 0.1097 | ||
| Good (n = 45) | 5 (11.1) | 40 (88.9) | |
| Intermediate/Poor (n = 16) | 5 (31.3) | 11 (68.7) |
| Relapse-Free Survival | |
|---|---|
| Variables # | Univariable |
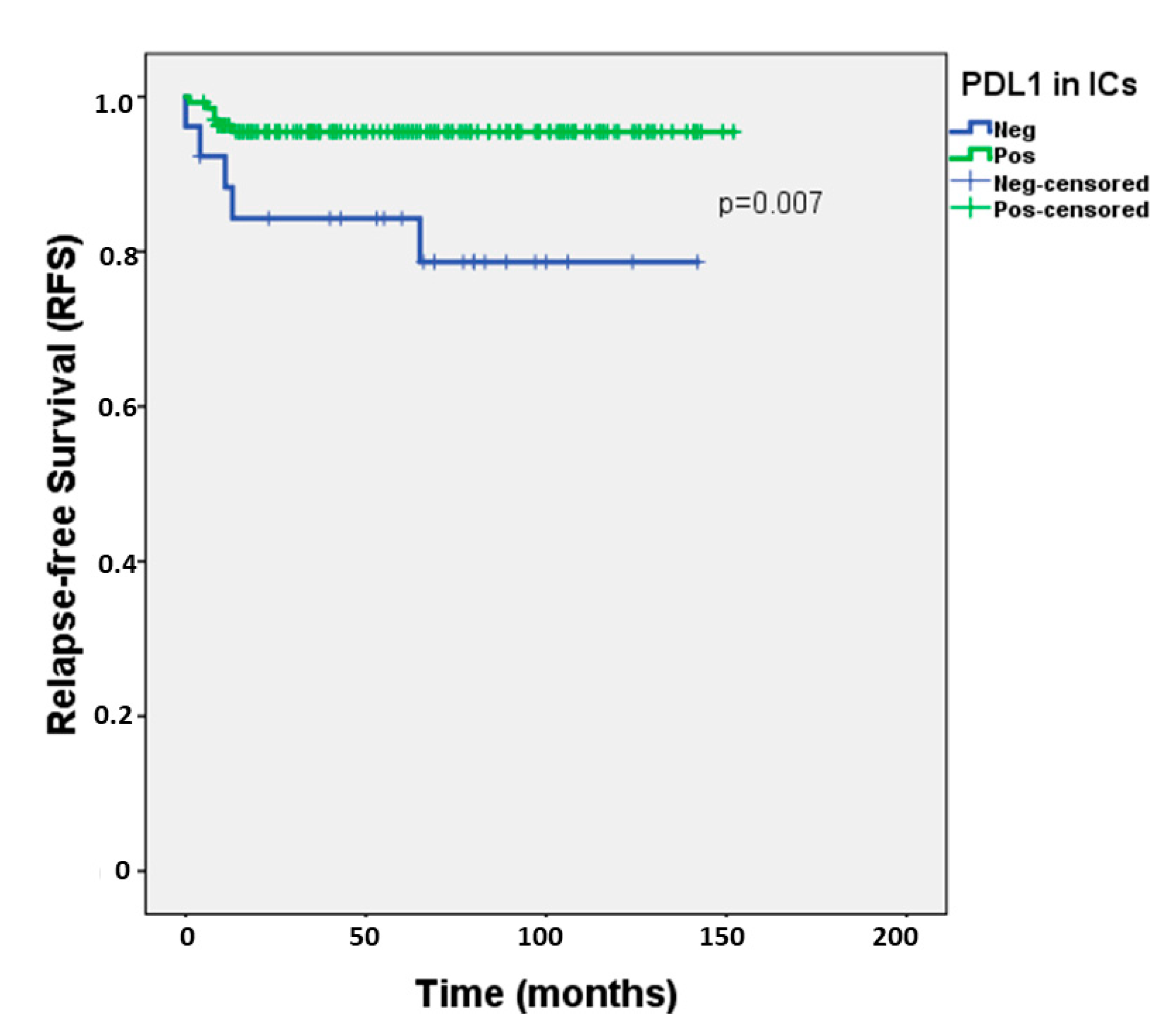
| PD-L1 expression in ICs | HR = 4.481, 95%CI 1.366–14.697, p = 0.013 * |
| Multivariable (PD-L1 expression in ICs, adjusted for…) | |
| Histology (SE vs NS) | HR = 4.198, 95%CI 1.268–13.899, p = 0.019 * |
| LVI | HR = 4.647, 95%CI 1.380–15.651, p = 0.013 * |
| pT Stage | HR = 4.683, 95%CI 1.397–15.700, p = 0.012 * |
| N Stage | HR = 3.938, 95%CI 1.108–13.996, p = 0.034 * |
| Stage (TNM) | HR = 4.209, 95%CI 1.275–13.896, p = 0.018 * |
| IGCCCG grouping | HR = 5.398, 95%CI 0.902–32.302, p = 0.065 |
| Variables | CD20 in ICs: Low | CD20 in ICs: High | p-value |
|---|---|---|---|
| LVI (n, % within LVI) | 0.6537 | ||
| Yes (n = 31) | 17 (54.8) | 14 (45.2) | |
| No (n = 55) | 26 (47.3) | 29 (52.7) | |
| Rete testis invasion (n, % within rete testis invasion) | 0.0079* | ||
| Yes (n = 46) | 29 (63.0) | 17 (37.0) | |
| No (n = 37) | 12 (32.4) | 25 (67.6) | |
| pT Stage (n, % within pT Stage) | 0.1949 | ||
| pT1 (n = 45) | 19 (42.2) | 26 (57.8) | |
| pT2-3 (n = 41) | 24 (58.5) | 17 (41.5) | |
| N Stage (n, % within N Stage) | 0.3155 | ||
| N0 (n = 65) | 30 (46.2) | 35 (53.8) | |
| N+ (n = 20) | 12 (60.0) | 8 (40.0) | |
| Stage, TNM (n, % within Stage, TNM) | 0.0216* | ||
| I (n = 65) | 30 (46.2) | 35 (53.8) | |
| II (n = 14) | 6 (42.9) | 8 (57.1) | |
| III (n = 7) | 7 (100) | 0 (0) | |
| IGCCCG grouping (n, % within IGCCCG) | 0.5048 | ||
| Good (n = 19) | 11 (57.9) | 8 (42.1) | |
| Intermediate/Poor (n = 2) | 2 (100) | 0 (0) | |
| CD3 in ICs: Low | CD3 in ICs: High | p-Value | |
| LVI (n, % within LVI) | 0.3353 | ||
| Yes (n = 31) | 12 (38.7) | 19 (61.3) | |
| No (n = 55) | 15 (27.3) | 40 (72.7) | |
| pT Stage (n, % within pT Stage) | 0.0211* | ||
| pT1 (n = 45) | 9 (20.0) | 36 (80.0) | |
| pT2-3 (n = 41) | 18 (43.9) | 23 (56.1) | |
| N Stage (n, % within N Stage) | 0.4054 | ||
| N0 (n = 65) | 18 (27.7) | 47 (72.3) | |
| N+ (n = 20) | 8 (40.0) | 12 (60.0) | |
| Stage, TNM (n, % within Stage TNM) | 0.0291* | ||
| I–II (n = 79) | 22 (27.8) | 57 (72.2) | |
| III (n = 7) | 5 (71.4) | 2 (28.6) | |
| IGCCCG grouping (n, % IGCCCG) | 0.1714 | ||
| Good (n = 19) | 7 (36.8) | 12 (63.2) | |
| Intermediate/Poor (n = 2) | 2 (100) | 0 (0) |
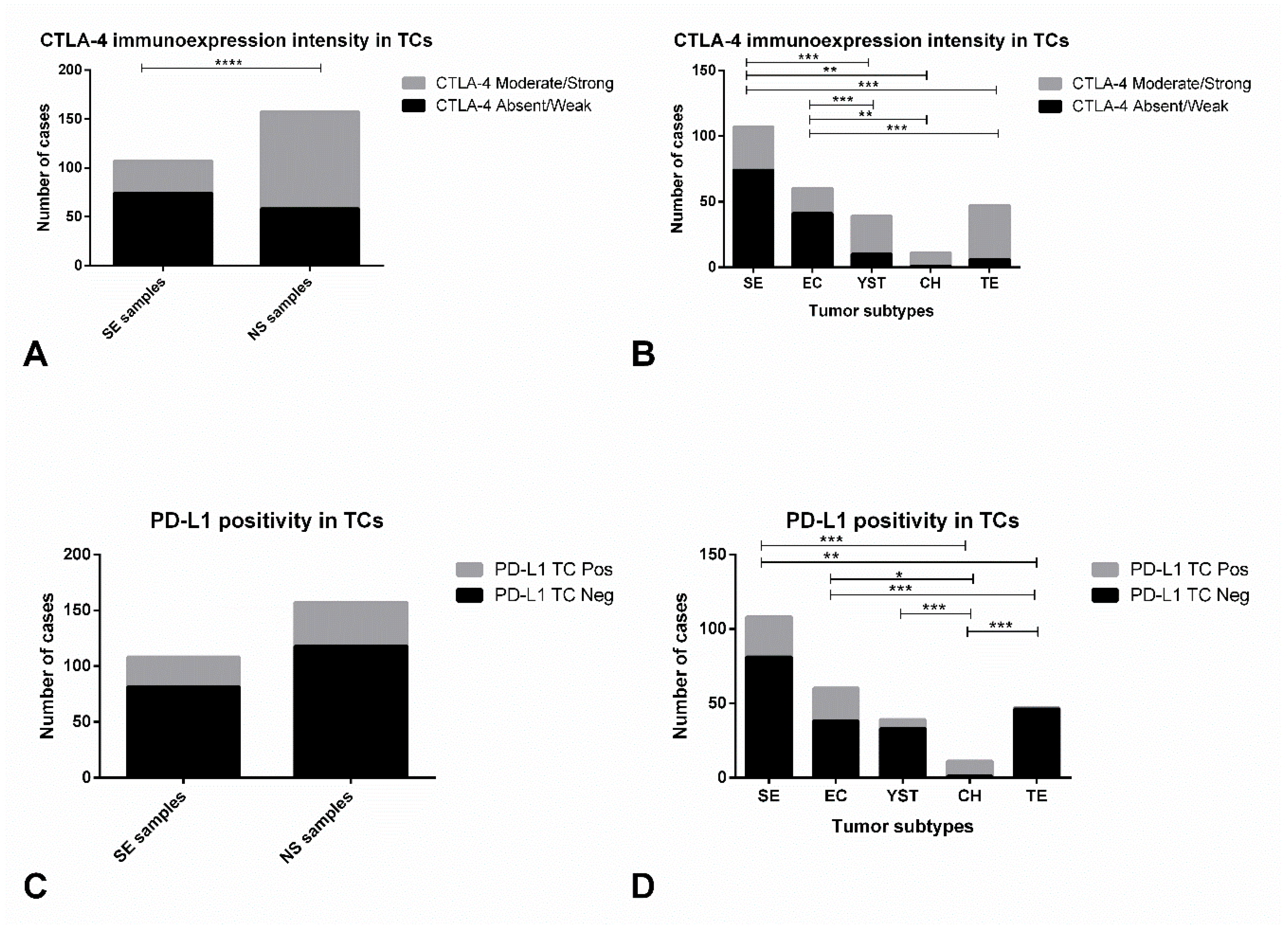
| Variables | CTLA-4 in TCs: Absent/Weak | CTLA-4 in TCs: Moderate/Strong | p-Value |
|---|---|---|---|
| Primary tumor histology (n, % within Histology) | <0.0001 * | ||
| SE (n = 107) | 74 (69.2) | 33 (30.8) | |
| NS (n = 157) | 58 (36.9) | 99 (63.1) | |
| Primary tumor subtype (n, % within subtype) | |||
| SE (n = 107) | 74 (69.2) | 33 (30.8) | <0.0001 * |
| EC (n = 60) | 41 (68.3) | 19 (31.7) | |
| YST (n = 39) | 10 (25.6) | 29 (74.4) | |
| CH (n = 11) | 1 (9.1) | 10 (90.9) | |
| TE (n = 47) | 6 (12.8) | 41 (87.2) | |
| PD-L1 in TCs: Negative | PD-L1 in TCs: Positive | p-Value | |
| Histology (n, % within Histology) | 1.0 | ||
| SE (n = 108) | 81 (75.0) | 27 (25.0) | |
| NS (n = 157) | 118 (75.2) | 39 (24.8) | |
| Primary tumor subtype (n, % within subtype) | |||
| SE (n = 108) | 81 (75.0) | 27 (25.0) | <0.0001* |
| EC (n = 60) | 38 (63.3) | 22 (36.7) | |
| YST (n = 39) | 33 (84.6) | 6 (15.4) | |
| CH (n = 11) | 1 (9.1) | 10 (90.9) | |
| TE (n = 47) | 46 (97.9) | 1 (2.1) | |
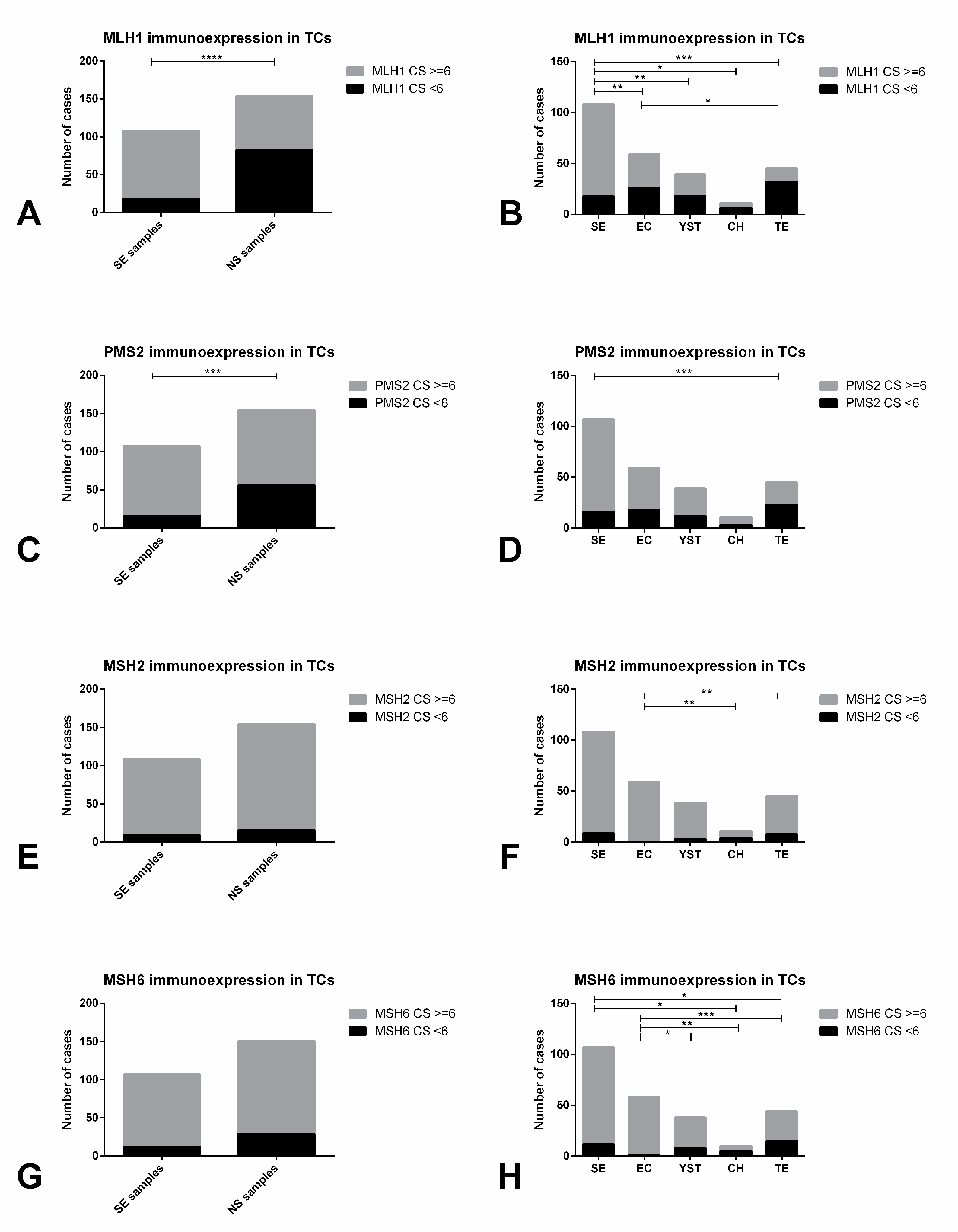
| MLH1 in TCs: CS<6 | MLH1 in TCs: CS≥6 | p-Value | |
| Histology (n, % within Histology) | <0.0001* | ||
| SE (n = 108) | 18 (16.7) | 90 (83.3) | |
| NS (n = 154) | 82 (53.2) | 72 (46.8) | |
| Primary tumor subtype (n, % within subtype) | |||
| SE (n = 108) | 18 (16.7) | 90 (83.3) | <0.0001* |
| EC (n = 59) | 26 (44.1) | 33 (55.9) | |
| YST (n = 39) | 18 (46.2) | 21 (53.8) | |
| CH (n = 11) | 6 (54.5) | 5 (45.5) | |
| TE (n = 45) | 32 (71.1) | 13 (28.9) | |
| PMS2 in TCs: CS<6 | PMS2 in TCs: CS≥6 | p-Value | |
| Histology (n, % within Histology) | 0.0001* | ||
| SE (n = 107) | 16 (15.0) | 91 (85.0) | |
| NS (n = 154) | 56 (36.4) | 98 (63.6) | |
| Primary tumor subtype (n, % within subtype) | |||
| SE (n = 107) | 16 (15.0) | 91 (85.0) | 0.0003* |
| EC (n = 59) | 18 (30.5) | 41 (69.5) | |
| YST (n = 39) | 12 (30.8) | 27 (69.2) | |
| CH (n = 11) | 3 (27.3) | 8 (72.7) | |
| TE (n = 45) | 23 (51.1) | 22 (48.9) | |
| MSH2 in TCs: CS<6 | MSH2 in TCs: CS≥6 | p-Value | |
| Histology (n, % within Histology) | 0.8287 | ||
| SE (n = 108) | 9 (8.3) | 99 (91.7) | |
| NS (n = 154) | 15 (9.7) | 139 (90.3) | |
| Primary tumor subtype (n, % within subtype) | |||
| SE (n = 108) | 9 (8.3) | 99 (91.7) | 0.0005* |
| EC (n = 59) | 0 (0) | 59 (100) | |
| YST (n = 39) | 3 (7.7) | 36 (92.3) | |
| CH (n = 11) | 4 (36.4) | 7 (63.6) | |
| TE (n = 45) | 8 (17.8) | 37 (82.2) | |
| MSH6 in TCs: CS<6 | MSH6 in TCs: CS≥6 | p-Value | |
| Histology (n, % within Histology) | 0.0865 | ||
| SE (n = 107) | 12 (11.2) | 95 (88.8) | |
| NS (n = 150) | 29 (19.3) | 121 (80.7) | |
| Primary tumor subtype (n, % within subtype) | |||
| SE (n = 107) | 12 (11.2) | 95 (88.8) | <0.0001 * |
| EC (n = 58) | 1 (1.7) | 57 (98.3) | |
| YST (n = 38) | 8 (21.1) | 30 (78.9) | |
| CH (n = 10) | 5 (50.0) | 5 (50.0) | |
| TE (n = 44) | 15 (34.1) | 29 (65.9) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobo, J.; Rodrigues, Â.; Guimarães, R.; Cantante, M.; Lopes, P.; Maurício, J.; Oliveira, J.; Jerónimo, C.; Henrique, R. Detailed Characterization of Immune Cell Infiltrate and Expression of Immune Checkpoint Molecules PD-L1/CTLA-4 and MMR Proteins in Testicular Germ Cell Tumors Disclose Novel Disease Biomarkers. Cancers 2019, 11, 1535. https://doi.org/10.3390/cancers11101535
Lobo J, Rodrigues Â, Guimarães R, Cantante M, Lopes P, Maurício J, Oliveira J, Jerónimo C, Henrique R. Detailed Characterization of Immune Cell Infiltrate and Expression of Immune Checkpoint Molecules PD-L1/CTLA-4 and MMR Proteins in Testicular Germ Cell Tumors Disclose Novel Disease Biomarkers. Cancers. 2019; 11(10):1535. https://doi.org/10.3390/cancers11101535
Chicago/Turabian StyleLobo, João, Ângelo Rodrigues, Rita Guimarães, Mariana Cantante, Paula Lopes, Joaquina Maurício, Jorge Oliveira, Carmen Jerónimo, and Rui Henrique. 2019. "Detailed Characterization of Immune Cell Infiltrate and Expression of Immune Checkpoint Molecules PD-L1/CTLA-4 and MMR Proteins in Testicular Germ Cell Tumors Disclose Novel Disease Biomarkers" Cancers 11, no. 10: 1535. https://doi.org/10.3390/cancers11101535
APA StyleLobo, J., Rodrigues, Â., Guimarães, R., Cantante, M., Lopes, P., Maurício, J., Oliveira, J., Jerónimo, C., & Henrique, R. (2019). Detailed Characterization of Immune Cell Infiltrate and Expression of Immune Checkpoint Molecules PD-L1/CTLA-4 and MMR Proteins in Testicular Germ Cell Tumors Disclose Novel Disease Biomarkers. Cancers, 11(10), 1535. https://doi.org/10.3390/cancers11101535