Prediction of Posterior Paraglottic Space and Cricoarytenoid Unit Involvement in Endoscopically T3 Glottic Cancer with Arytenoid Fixation by Magnetic Resonance with Surface Coils
Abstract
1. Introduction
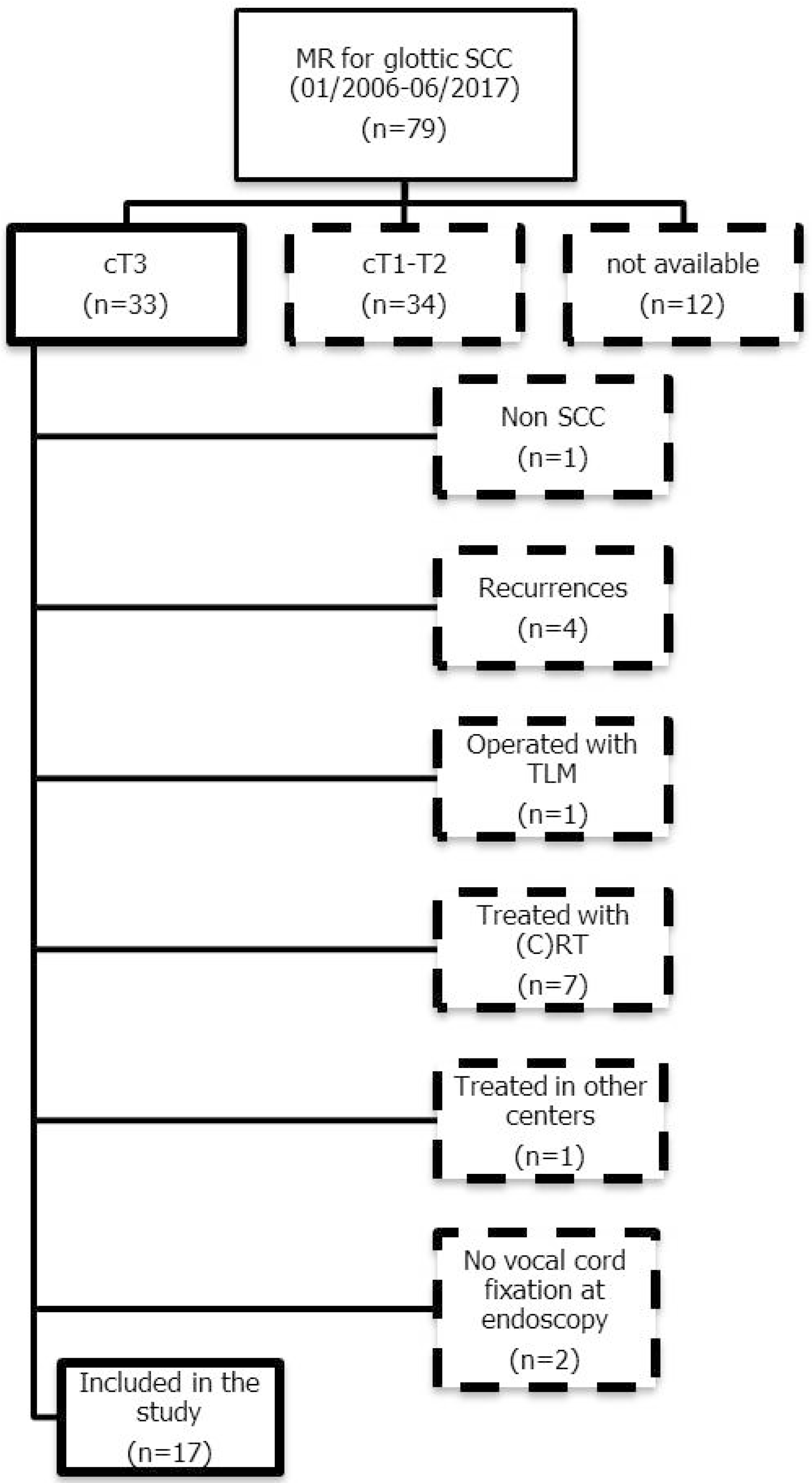
2. Materials and Methods
2.1. Patient Selection
2.2. MR Study Protocol
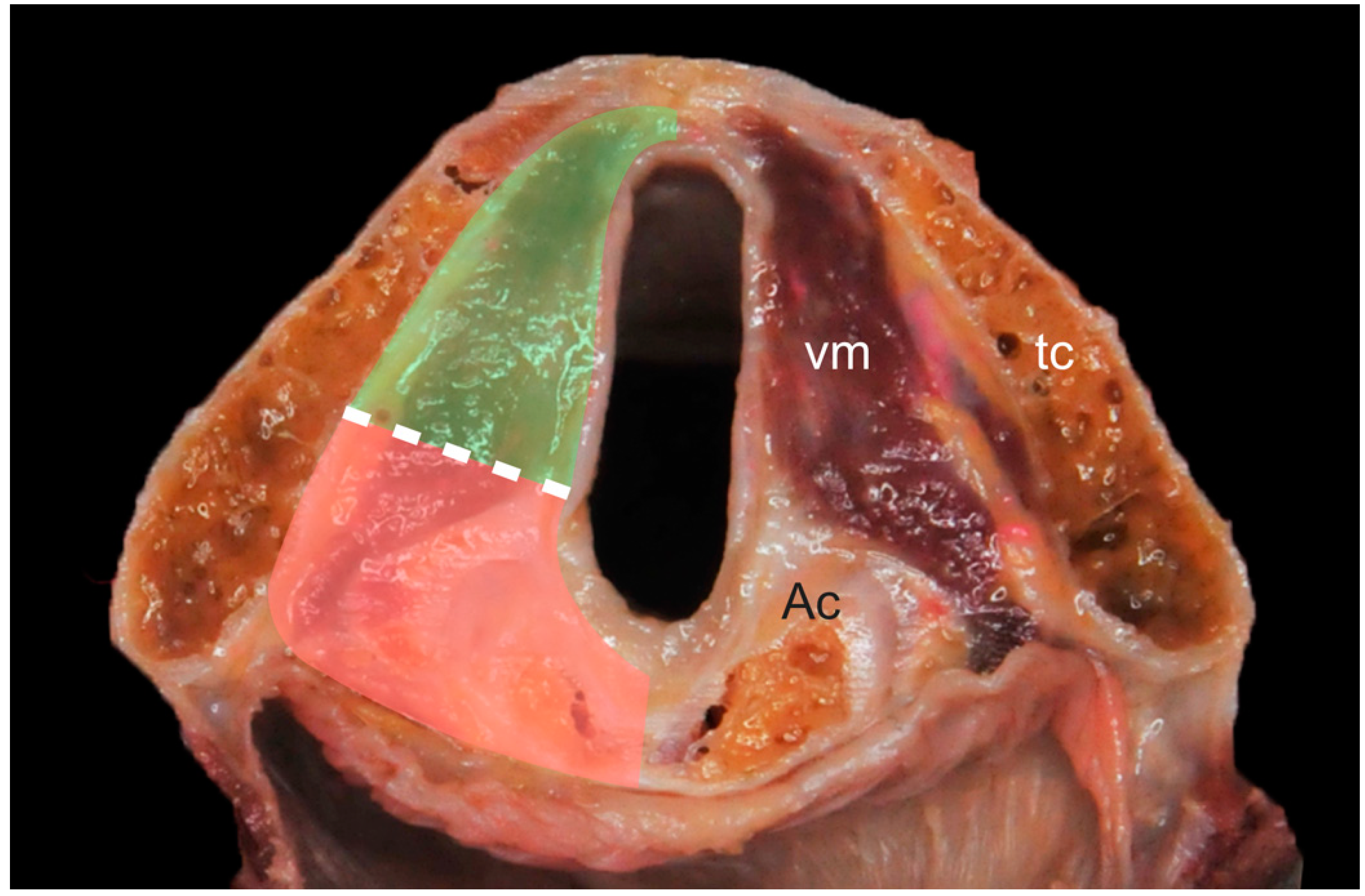
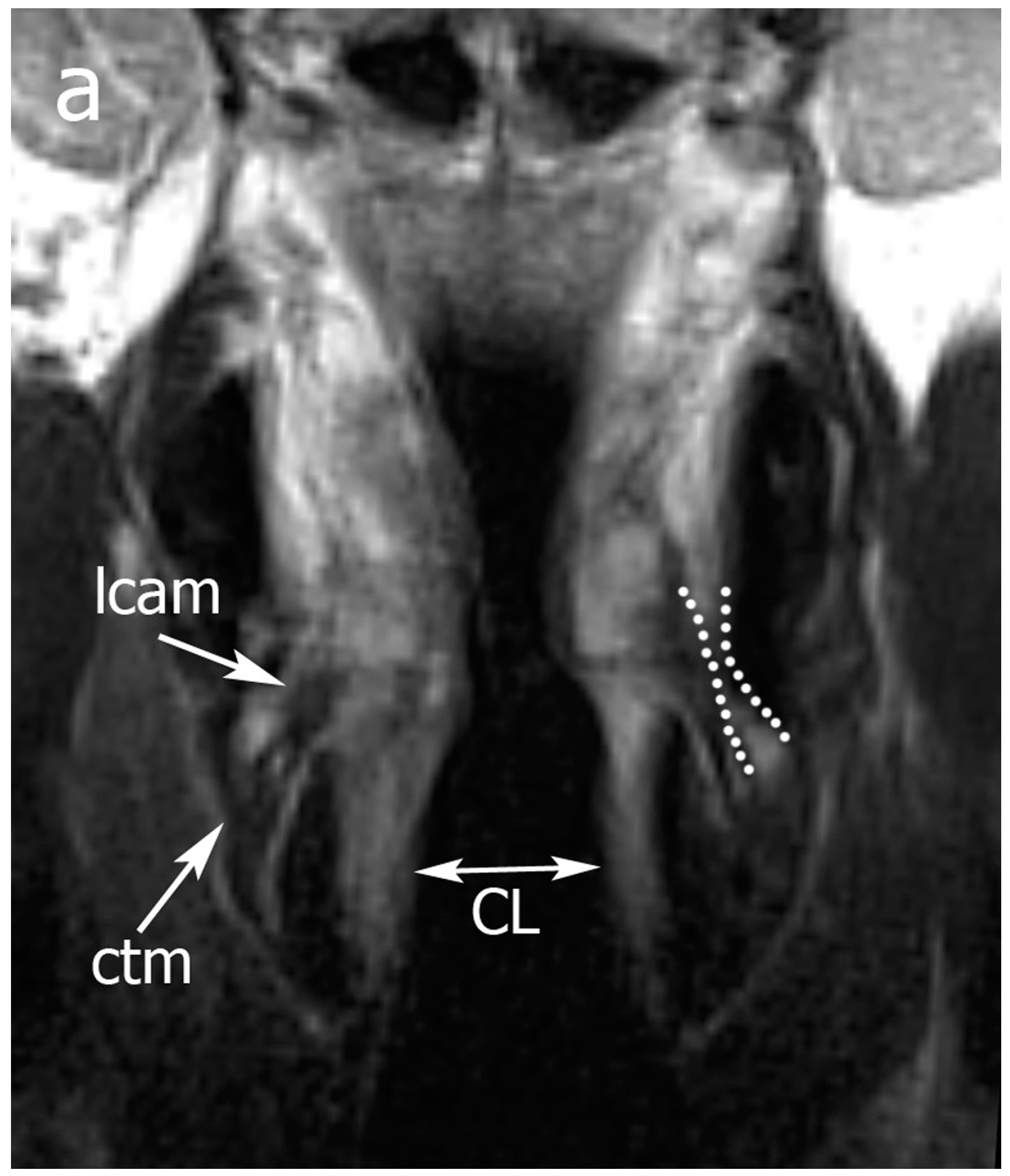
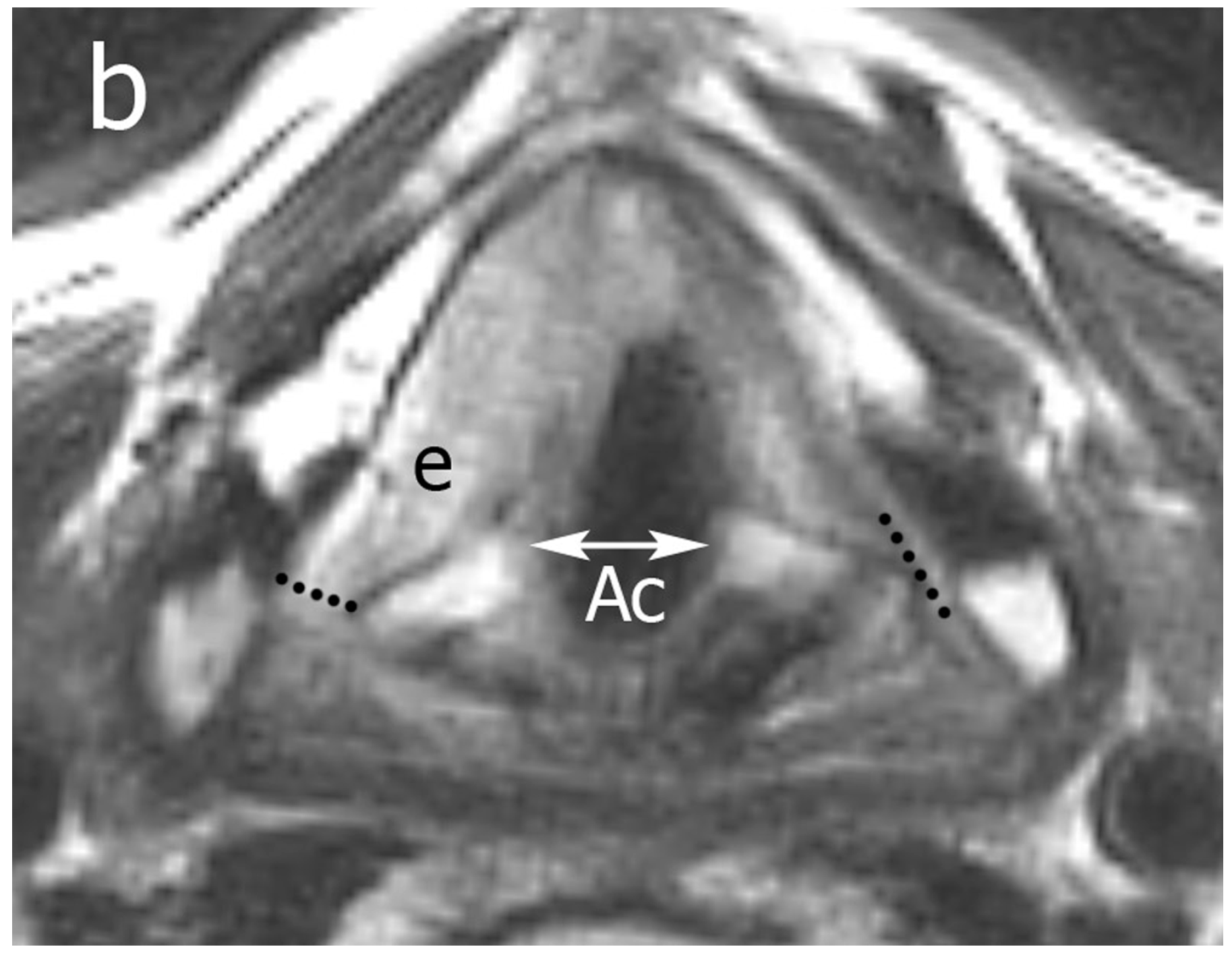
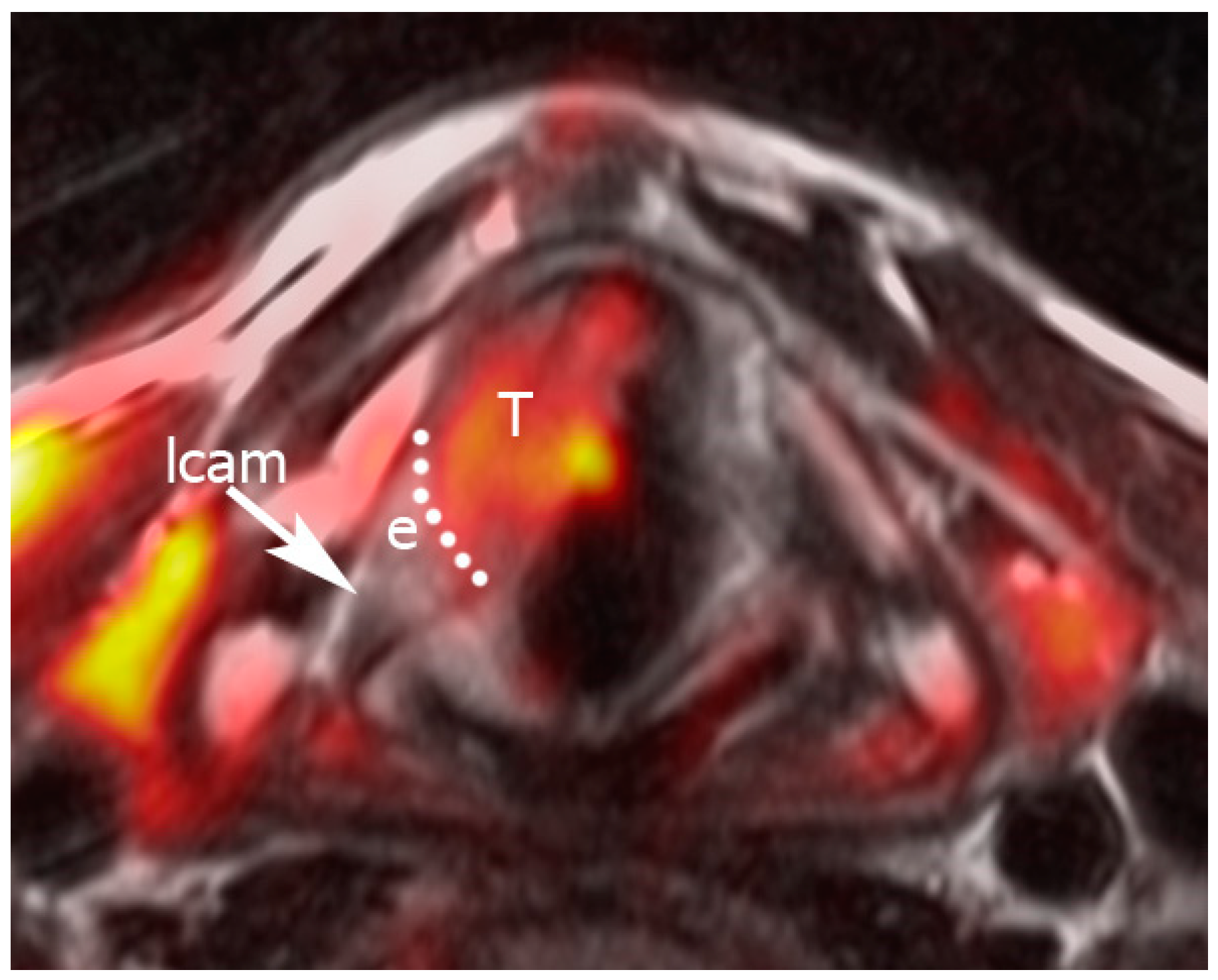
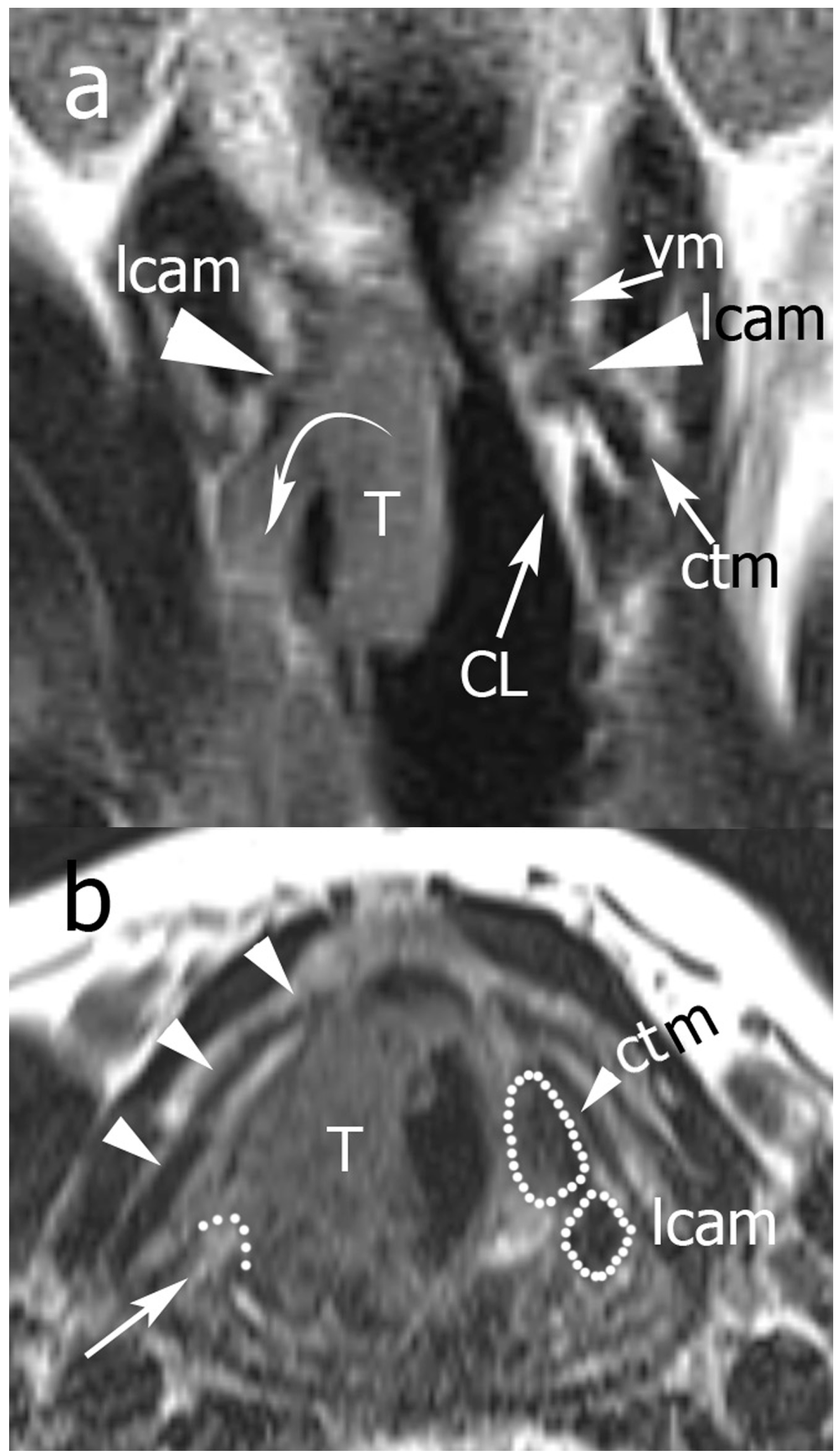
2.3. Image Analysis
Pattern A: normal signal intensity in all sequences;
Pattern B: tissue changes characterized by T2 hyperintensity, no DWI restriction (high apparent diffusion coefficient (ADC)), and variable contrast-enhancement. For ossified cartilages: T2 hyperintensity, T1 hypointensity, and bright contrast enhancement;
Pattern C: tissue abnormalities characterized by T2 intermediate signal, DWI restriction (high DWI signal combined with low ADC value), and variable contrast-enhancement. For ossified cartilages: T2 intermediate signal, T1 hypointensity, and intermediate contrast enhancement.
2.4. Statistical Analysis
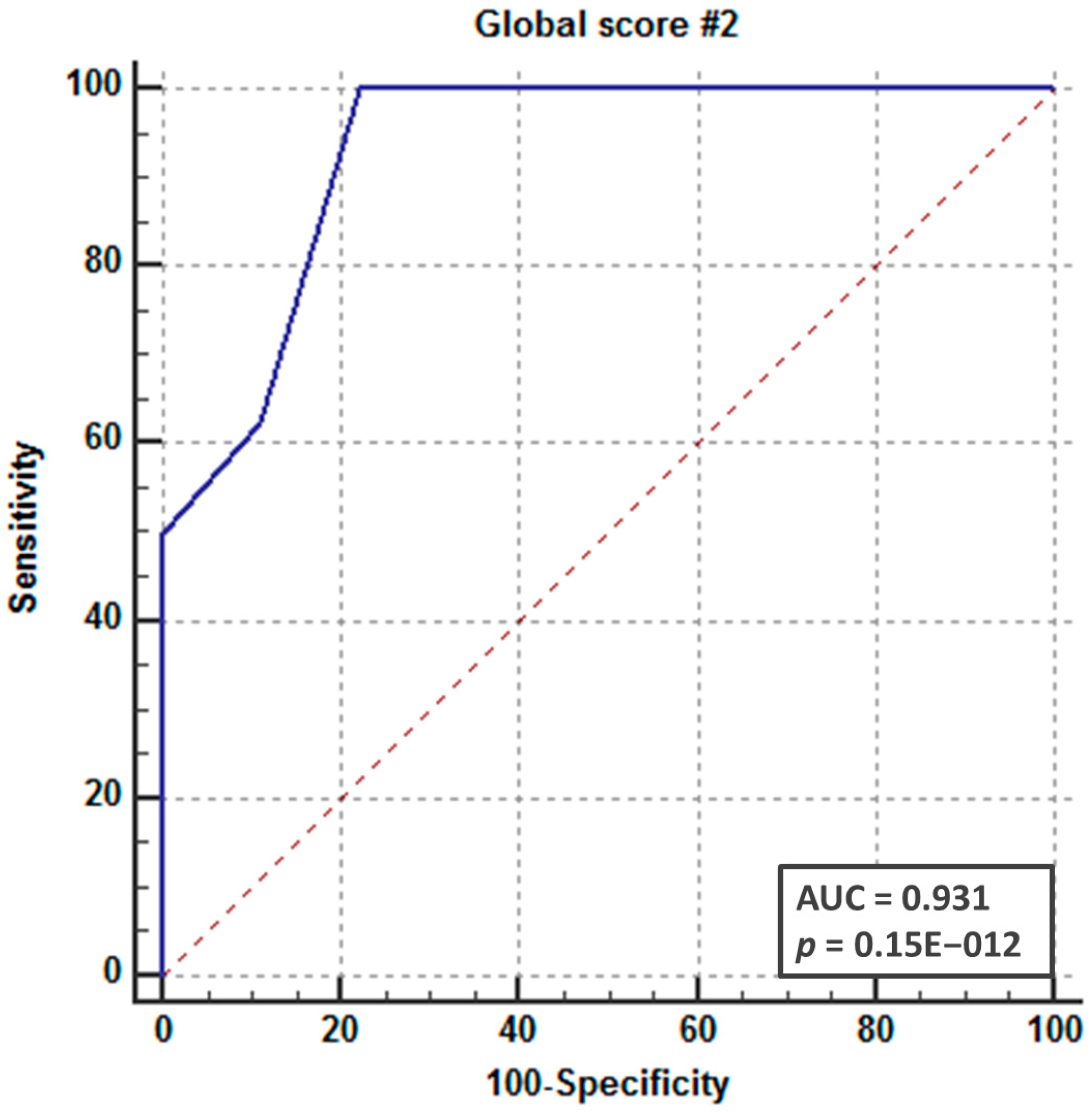
3. Results
Patients and CAU/PLC Infiltration
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sobin, L.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours, 7th ed.; Wiley-Blackwell: Chichester, West Sussex, UK, 2010. [Google Scholar]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Chichester, West Sussex, UK, 2017. [Google Scholar]
- Rosen, C.A.; Mau, T.; Remacle, M.; Hess, M.; Eckel, H.E.; Young, V.N.; Hantzakos, A.; Yung, K.C.; Dikkers, F.G. Nomenclature proposal to describe vocal fold motion impairment. Eur. Arch. Otorhinolaryngol. 2016, 273, 1995–1999. [Google Scholar] [CrossRef] [PubMed]
- Succo, G.; Crosetti, E.; Bertolin, A.; Piazza, C.; Molteni, G.; Cirillo, S.; Petracchini, M.; Tascone, M.; Sprio, A.E.; Berta, G.N.; et al. Treatment for T3 to T4a laryngeal cancer by open partial horizontal laryngectomies: Prognostic impact of different pathologic tumor subcategories. Head Neck 2018, 40, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Maroldi, R.; Ravanelli, M.; Farina, D. Magnetic resonance for laryngeal cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Zbaren, P.; Casselman, J.W.; Kohler, R.; Dulguerov, P.; Becker, C.D. Neoplastic invasion of laryngeal cartilage: Reassessment of criteria for diagnosis at MR imaging. Radiology 2008, 249, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, G.; Unsal, O.; Celebi, I.; Ayhan, B.; Guliyev, U.; Akova, P.; Basak, T.; Coskun, B.U. Does CT help in predicting preepiglottic space invasion in laryngeal carcinoma? Auris Nasus Larynx 2018, 45, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Agnello, F.; Cupido, F.; Sparacia, G.; Midiri, F.; Miroddi, M.; Grassedonio, E.; Galia, M. Computerised tomography and magnetic resonance imaging of laryngeal squamous cell carcinoma: A practical approach. Neuroradiol. J. 2017, 30, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Dankbaar, J.W.; Oosterbroek, J.; Jager, E.A.; de Jong, H.W.; Raaijmakers, C.P.; Willems, S.M.; Terhaard, C.H.; Philippens, M.E.; Pameijer, F.A. Detection of cartilage invasion in laryngeal carcinoma with dynamic contrast-enhanced CT. Laryngoscope Investig. Otolaryngol. 2017, 2, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Kuno, H.; Sakamaki, K.; Fujii, S.; Sekiya, K.; Otani, K.; Hayashi, R.; Yamanaka, T.; Sakai, O.; Kusumoto, M. Comparison of MR Imaging and Dual-Energy CT for the Evaluation of Cartilage Invasion by Laryngeal and Hypopharyngeal Squamous Cell Carcinoma. AJNR Am. J. Neuroradiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kuno, H.; Onaya, H.; Fujii, S.; Ojiri, H.; Otani, K.; Satake, M. Primary staging of laryngeal and hypopharyngeal cancer: CT, MR imaging and dual-energy CT. Eur. J. Radiol. 2014, 83, e23–e35. [Google Scholar] [CrossRef] [PubMed]
- Marchi, F.; Piazza, C.; Ravanelli, M.; Gaggero, G.; Parrinello, G.; Paderno, A.; Perotti, P.; Filauro, M.; Maroldi, R.; Peretti, G. Role of imaging in the follow-up of T2-T3 glottic cancer treated by transoral laser microsurgery. Eur. Arch. Otorhinolaryngol. 2017, 274, 3679–3686. [Google Scholar] [CrossRef] [PubMed]
- Peretti, G.; Piazza, C.; Del Bon, F.; Mora, R.; Grazioli, P.; Barbieri, D.; Mangili, S.; Nicolai, P. Function preservation using transoral laser surgery for T2-T3 glottic cancer: Oncologic, vocal, and swallowing outcomes. Eur. Arch. Otorhinolaryngol. 2013, 270, 2275–2281. [Google Scholar] [CrossRef] [PubMed]
- Peretti, G.; Piazza, C.; Mora, F.; Garofolo, S.; Guastini, L. Reasonable limits for transoral laser microsurgery in laryngeal cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Canis, M.; Ihler, F.; Martin, A.; Wolff, H.A.; Matthias, C.; Steiner, W. Results of 226 patients with T3 laryngeal carcinoma after treatment with transoral laser microsurgery. Head Neck 2014, 36, 652–659. [Google Scholar] [CrossRef]
- Vilaseca, I.; Bernal-Sprekelsen, M.; Luis Blanch, J. Transoral laser microsurgery for T3 laryngeal tumors: Prognostic factors. Head Neck 2010, 32, 929–938. [Google Scholar] [CrossRef] [PubMed]
| Mean Age (range) | 64.9 years (45–82) years |
| Gender | 13 M, 4 F |
| cT | 17 cT3 |
| cN | 11 cN0 6 cN+ |
| pT | 13 pT3 4 pT4a |
| pN | 8 pN0 2 pN2a 5 pN2b 2 pN2c |
| Surgery | 10 OPHL 7 TL |
| ND | 10 bilatera l7 unilateral |
| ID | POST VM | POST PGS | INF PGS | ARY | CRIC LAM | LCAM | CTM | Overall Score | Partial Score of Pattern B | Partial Score of Pattern C | Histological CAU/PLC Infiltration |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| 2 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 4 | 4 | 0 | 0 |
| 3 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 4 | 0 |
| 4 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 3 | 3 | 0 | 0 |
| 5 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 3 | 3 | 0 | 0 |
| 6 | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 10 | 0 | 10 | 1 |
| 7 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 3 | 3 | 0 | 0 |
| 8 | 2 | 1 | 1 | 0 | 0 | 2 | 0 | 6 | 2 | 4 | 1 |
| 9 | 2 | 2 | 2 | 1 | 0 | 2 | 0 | 9 | 1 | 8 | 1 |
| 10 | 2 | 2 | 2 | 1 | 0 | 2 | 1 | 10 | 2 | 8 | 1 |
| 11 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 7 | 5 | 2 | 1 |
| 12 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 2 | 0 |
| 13 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 1 | 2 | 1 |
| 16 | 2 | 2 | 1 | 2 | 0 | 1 | 0 | 8 | 2 | 6 | 1 |
| 17 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 2 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravanelli, M.; Paderno, A.; Del Bon, F.; Montalto, N.; Pessina, C.; Battocchio, S.; Farina, D.; Nicolai, P.; Maroldi, R.; Piazza, C. Prediction of Posterior Paraglottic Space and Cricoarytenoid Unit Involvement in Endoscopically T3 Glottic Cancer with Arytenoid Fixation by Magnetic Resonance with Surface Coils. Cancers 2019, 11, 67. https://doi.org/10.3390/cancers11010067
Ravanelli M, Paderno A, Del Bon F, Montalto N, Pessina C, Battocchio S, Farina D, Nicolai P, Maroldi R, Piazza C. Prediction of Posterior Paraglottic Space and Cricoarytenoid Unit Involvement in Endoscopically T3 Glottic Cancer with Arytenoid Fixation by Magnetic Resonance with Surface Coils. Cancers. 2019; 11(1):67. https://doi.org/10.3390/cancers11010067
Chicago/Turabian StyleRavanelli, Marco, Alberto Paderno, Francesca Del Bon, Nausica Montalto, Carlotta Pessina, Simonetta Battocchio, Davide Farina, Piero Nicolai, Roberto Maroldi, and Cesare Piazza. 2019. "Prediction of Posterior Paraglottic Space and Cricoarytenoid Unit Involvement in Endoscopically T3 Glottic Cancer with Arytenoid Fixation by Magnetic Resonance with Surface Coils" Cancers 11, no. 1: 67. https://doi.org/10.3390/cancers11010067
APA StyleRavanelli, M., Paderno, A., Del Bon, F., Montalto, N., Pessina, C., Battocchio, S., Farina, D., Nicolai, P., Maroldi, R., & Piazza, C. (2019). Prediction of Posterior Paraglottic Space and Cricoarytenoid Unit Involvement in Endoscopically T3 Glottic Cancer with Arytenoid Fixation by Magnetic Resonance with Surface Coils. Cancers, 11(1), 67. https://doi.org/10.3390/cancers11010067





