Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy
Abstract
1. Introduction
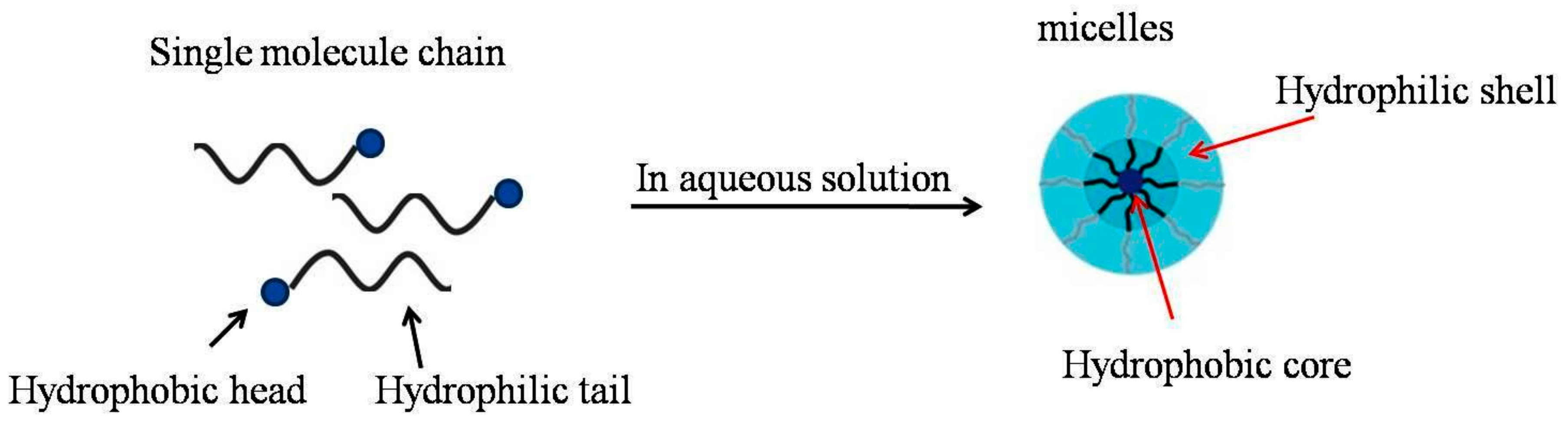
1.1. Identification of Micelles
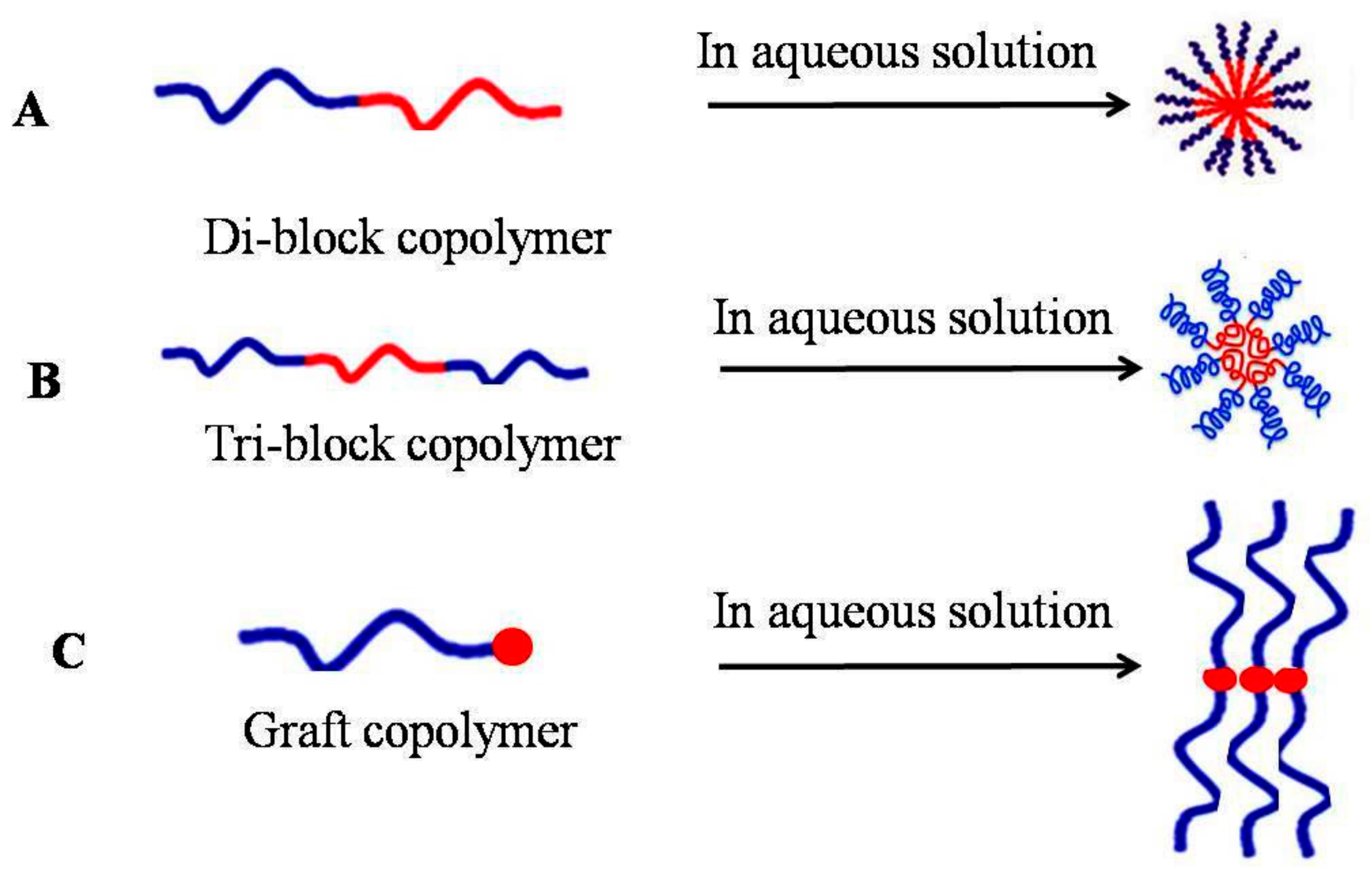
1.2. Micelles Structure
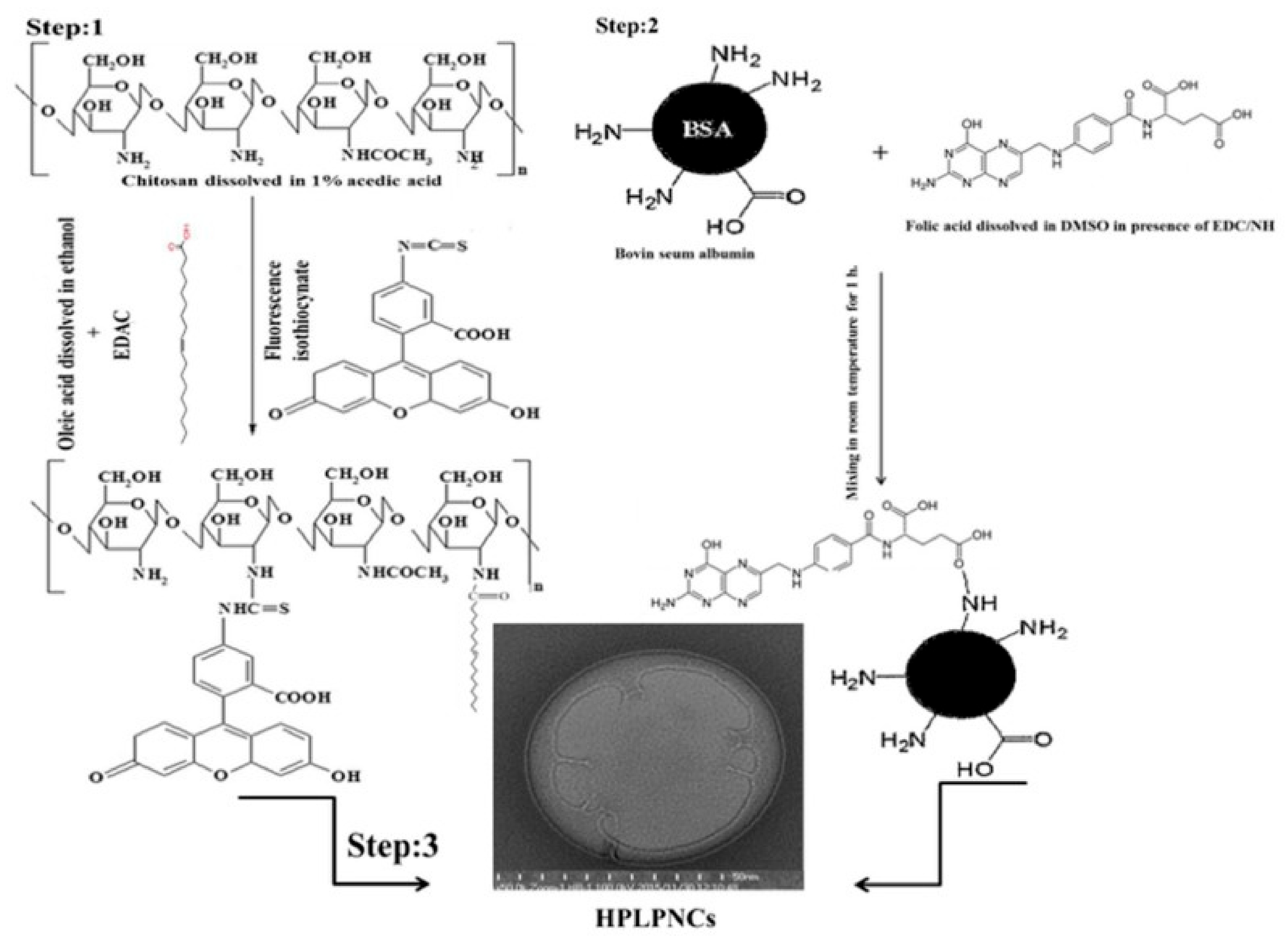
2. Hybrid Polymeric Micelles
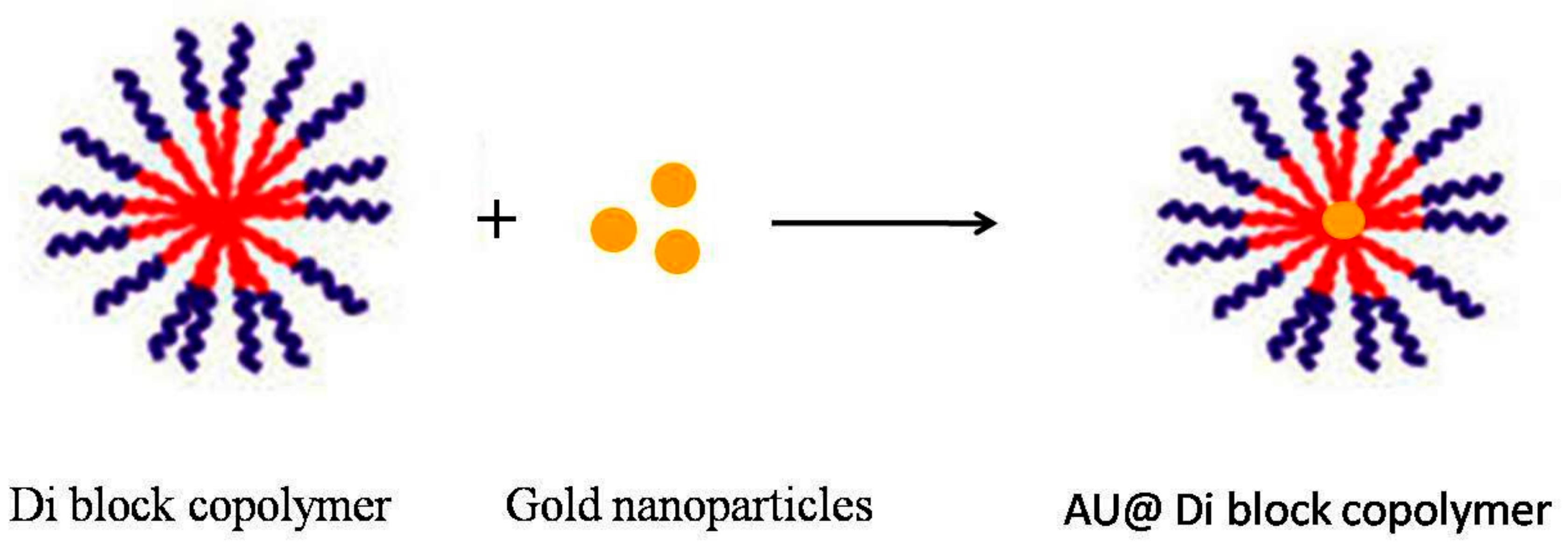
3. Hybrid Nanomicelles with Metal Nanoparticles
4. Micelles Sensitive to Biological Stimuli
5. Drug-Loaded Micelles
6. Advantages and Disadvantages of Micelles
7. Applications of Micelles in Cancer Therapy
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kabanov, A.V.; Batrakova, E.V.; Melik-Nubarov, N.S.; Fedoseev, N.A.; Dorodnich, T.Y.; Alakhov, V.Y.; Chekhonin, P.; Nazarova, I.R.; Kabanov, V.A. A new class of drug carriers: Micelles poly(oxyethylene)–poly(oxypropylene) block copolymers as microcontainers for drug targeting from blood to brain. J. Control. Release 1992, 22, 41–158. [Google Scholar] [CrossRef]
- Torchilin, V.P. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2007, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Schramm, L.L.; Stasiuk, E.N.; Marangoni, D.G. Surfactants and their applications. Annu. Rep. Prog. Chem. Sect. C 2003, 99, 3–48. [Google Scholar] [CrossRef]
- Kellermann, M.; Bauer, W.; Hirsch, A.; Schade, B.; Ludwig, K.; Böttcher, C. The first account of a structurally persistent micelle. Angew. Chem. Int. Ed. 2004, 43, 2959–2962. [Google Scholar] [CrossRef] [PubMed]
- Loppinet, B.; Monteux, C. Dynamics of surfactants and polymers at liquid interfaces. Lect. Notes Phys. 2016, 917, 137–157. [Google Scholar]
- Chen, L.G.; Strasburg, S.H.; Bermudez, H. Micelle co-assembly in surfactant/ionic liquid mixtures. J. Colloid Interface Sci. 2016, 477, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Hu, J.; Dong, J.; Li, X. Light-responsive multillamellar vesicles in coumaric acid/alkyldimethylamine oxide binary systems: Effects of surfactant and hydrotrope structures. J. Colloid Interface Sci. 2016, 477, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.K.; Gawali, I.T.; Usmani, G.A. Synthesis and Properties of Novel Cationic Triazolium Gemini Surfactants. J. Dispers. Sci. Technol. 2016, 37, 1630–1637. [Google Scholar] [CrossRef]
- Gaber, N.N.; Darwis, Y.; Peh, K.K.; Tan, Y.T. Characterization of polymeric micelles for pulmonary delivery of beclomethasone dipropionate. J. Nanosci. Nanotechnol. 2006, 6, 3095–3101. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Kataoka, K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 2006, 112, 630–648. [Google Scholar] [CrossRef] [PubMed]
- Magid, L.J.; Li, Z.; Butler, P.D. Flexibility of elongated sodium dodecyl sulfate micelles in aqueous sodium chloride: A small-angle neutron scattering study. Langmuir 2000, 16, 10028–10036. [Google Scholar] [CrossRef]
- Aoun, B.; Sharma, V.K.; Pellegrini, E.; Mitra, S.; Johnson, M.; Mukhopadhyay, R. Structure and dynamics of ionic micelles: MD simulation and neutron scattering study. J. Phys. Chem. B 2015, 119, 5079–5086. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Nazarova, I.R.; Astafieva, I.V.; Batrakova, E.V.; Alakhov, V.Y.; Yaroslavov, A.A; Kabanov, V.A. Micelle formation and solubilization of fluorescent probes in poly(oxyethylene-b-oxypropylene-b-oxyethylene) solutions. Macromolecules 1995, 28, 2303–2314. [Google Scholar] [CrossRef]
- Papaioannou, A.I.; Papiris, S.; Papadaki, G.; Manali, E.D.; Roussou, A.; Spathis, A.; Kostikas, K. Surfactant proteins in smoking-related lung disease. Curr. Top. Med. Chem. 2016, 16, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Shahzad, S.; Munir, A.; Nadagouda, M.N.; Khan, G.S.; Shams, D.F.; Rana, U.A. Micelles as soil and water decontamination agents. Chem. Rev. 2016, 116, 6042–6074. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.R.V.; Torres, C.A.V.; Freitas, F.; Sevrin, C.; Grandfils, C.; Reis, M.A.M.; Coelhoso, I.M. Development and characterization of bilayer films of FucoPol and chitosan. Carbohydr. Polym. 2016, 147, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.C.; Leroux, J.C. Polymeric micelles—A new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 1999, 48, 101–111. [Google Scholar] [CrossRef]
- Giorgio, G.; Colafemmina, G.; Mavelli, F.; Murgia, S.; Palazzo, G. The impact of alkanes on the structure of Triton X100 micelles. RSC Adv. 2016, 6, 825–836. [Google Scholar] [CrossRef]
- Pottage, M.J.; Greaves, T.L.; Garvey, C.J.; Tabor, R.F. The effects of alkylammoniumcounterions on the aggregation of fluorinated surfactants and surfactant ionic liquids. J. Colloid Interface Sci. 2016, 475, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.T.; Martin, J.E.; Odinek, J.G.; Newcomer, P.P. Effect of Methanol Concentration on CTAB Micellization and on the Formation of Surfactant-Templated Silica (STS). Chem. Mater. 1998, 10, 1490–1500. [Google Scholar] [CrossRef]
- Kuperkar, K.C.; Mata, J.P.; Bahadur, P. Effect of 1-alkanols/salt on the cationic surfactant micellar aqueous solutions-A dynamic light scattering study. Colloids Surf. A Physicochem. Eng. Asp. 2011, 380, 60–65. [Google Scholar] [CrossRef]
- Techen, A.; Hille, C.; Dosche, C.; Kumke, M.U. Fluorescence study of drug carrier interactions in CTAB/PBS buffer model systems. J. Colloid Interface Sci. 2012, 377, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Gong, Q.T.; Luo, L.; Zhang, L.; Zhao, S.; Yu, J.Y. Aggregation of sodium 2,4,5-(tri-n-Alkyl)-benzene sulfonates in aqueous solution: Micellization and microenvironment characteristics studied by electron paramagnetic resonance and steady-state fluorescence quenching. J. Dispers. Sci. Technol. 2011, 32, 167–173. [Google Scholar] [CrossRef]
- Gibhardt, H.; Haramagatti, C.R.; Islamov, A.K.; Ivankov, O.I.; Kuklin, A.I.; Eckold, G. Universal Behaviour of the structure and dynamics of micelles formed from cationic surfactants. Z. Phys. Chem. 2014, 228, 769–791. [Google Scholar] [CrossRef]
- Helgeson, M.E.; Hodgdon, T.K.; Kaler, E.W.; Wagner, N.J. A systematic study of equilibrium structure, thermodynamics, and rheology of aqueous CTAB/NaNO3 wormlike micelles. J. Colloid Interface Sci. 2010, 349, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Ghamkhari, A.; Massoumi, B.; Jaymand, M. Novel ‘schizophrenic’ di-block copolymer synthesized via RAFT polymerization: Poly (2-succinyloxyethyl methacrylate)-b-poly [(N-4-vinylbenzyl),N,N-diethylamine]. Des. Monomers Polym. 2017, 20, 190–200. [Google Scholar] [CrossRef] [PubMed]
- IUPAC. Glossary of basic terms in polymer science. Pure Appl. Chem. 1996, 68, 2287–2311. [Google Scholar]
- Iatrou, H.; Avgeropoulos, A.; Hadjichristidis, N. Synthesis of model super H-shaped block copolymers. Macromolecules 1994, 27, 6232–6233. [Google Scholar] [CrossRef]
- Otsu, T.; Yoshida, M.; Kuriyama, A. A model for living radical polymerization. Makromol. Chem. Rapid Commun. 1982, 3, 133–140. [Google Scholar] [CrossRef]
- Otsu, T.; Kuriyama, A. Polymer Design by Iniferter Technique in Radical Polymerization: Synthesis of AB and ABA Block Copolymers Containing Random and Alternating Copolymer Sequences. J. Polym. 1985, 17, 97. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, B.; Dong, T.; Pan, P.; Shuai, X.; Inoue, Y. Interactions between an anticancer drug and polymeric micelles based on biodegradable polyesters. Macromol. Biosci. 2008, 8, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Pierri, E.; Avgoustakis, K. Poly(lactide)-poly(ethylene glycol) micelles as a carrier for grise of ulvin. J. Biomed. Mater. Res. A 2005, 75, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell. Mol. Life Sci. 2004, 61, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Sutton, D.; Nasongkla, N.; Blanco, E.; Gao, J. Functionalized micellar systems for cancer targeted drug delivery. Pharm. Res. 2007, 24, 1029–1046. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.R.; Yokoyama, M.; Okano, T. Inner core segment design for drug delivery control of thermo-responsive polymeric micelles. J. Control. Release 2000, 65, 93–103. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, N.H.; Sant, V.P.; Maysinger, D.; Leroux, J.C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Kessinger, C.W.; Sumer, B.D.; Gao, J. Multifunctional micellar nanomedicine for cancer therapy. Exp. Biol. Med. 2009, 234, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-S.; Matyjaszewski, K. “Living”/controlled radical polymerization. Transition-metal-catalyzed atom transfer radical polymerization in the presence of a conventional radical initiator. Macromolecules 1995, 28, 7572–7573. [Google Scholar] [CrossRef]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Orilall, M.C.; Wiesner, U. Block copolymer based composition and morphology control in nanostructured hybrid materials for energy conversion and storage: Solar cells, batteries, and fuel cells. Chem. Soc. Rev. 2011, 40, 520–535. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Cheng, J. Anticancer Polymeric Nanomedicines. Polym. Rev. 2007, 47, 345–381. [Google Scholar] [CrossRef]
- Cheow, W.S.; Hadinoto, K. Factors affecting drug encapsulation and stability of lipid- polymer hybrid nanoparticles. Colloids Surf. B Biointerfaces 2011, 85, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Trubetskoy, V.S.; Torchilin, V.P. Use of polyoxyethylenelipidconjugates as long-circulating carriers for delivery of therapeutic and diagnostic agents. Adv. Drug Deliv. Rev. 1995, 16, 311–320. [Google Scholar] [CrossRef]
- Torchilin, V.P; Weissig, V. Polymeric micelles for delivery of poorly soluble drugs. Polym. Prepr. 1999, 40, 320–321. [Google Scholar]
- Zhang, J.; Chen, X.G.; Huang, L.; Han, J.T.; Zhang, X.F. Self-assembled polymeric nanoparticles based on oleic acid-grafted chitosan oligosaccharide: Biocompatibility, protein adsorption and cellular uptake. J. Mater. Sci. Mater. Med. 2012, 23, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Sabharanjak, S.; Mayor, S. Folate receptor endocytosis and trafficking. Adv. Drug Deliv. Rev. 2004, 56, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Janes, K.A.; Fresneau, M.P.; Marazuela, A.; Fabra, A.; Alonso, M.J. Chitosan nanoparticles as delivery systems for doxorubicin. J. Control. Release 2001, 73, 255–267. [Google Scholar] [CrossRef]
- Esquenet, C.; Terech, P.; Boue, F.; Buhler, E. Structural and rheological properties of hydrophobically modified polysaccharide associative networks. Langmuir 2004, 20, 3583–3592. [Google Scholar] [CrossRef] [PubMed]
- Bedu-Addo, F.K.; Tang, P.; Xu, Y.; Huang, L. Effects of polyethyleneglycol chain length and phospholipid acyl chain composition on the interaction of polyethyleneglycol-phospholipid conjugates with phospholipid: Implications in liposomal drug delivery. Pharm. Res. 1996, 13, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.; Johnsson, M.; Karlsson, G.; Silvander, M. Effect of polyethyleneglycol-phospholipids on aggregate structure in preparations of small unilamellar liposomes. J. Biophys. 1997, 73, 258–266. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Hanafy, N.A.; Dini, L.; Citti, C.; Cannazza, G.; Leporatti, S. Inhibition of Glycolysis by Using a Micro/Nano-Lipid Bromopyruvic Chitosan Carrier as a Promising Tool to Improve Treatment of Hepatocellular Carcinoma. Nanomaterials 2018, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.Y.; Müller, A.H.E. One-dimensional organic-inorganic hybrid nanomaterials. Polymer 2010, 51, 4015–4036. [Google Scholar] [CrossRef]
- Chen, Y.; Cho, J.; Young, A.; Taton, TA. Enhanced stability and bioconjugation of photo-cross-linked polystyrene-shell, Au-core nanoparticles. Langmuir 2007, 23, 7491–7497. [Google Scholar] [CrossRef] [PubMed]
- Dubertret, B.; Skourides, P.; Norris, D.J.; Noireaux, V.; Brivanlou, A.H.; Libchaber, A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 2002, 298, 1759–1762. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Taton, T.A. Core/Shell Gold Nanoparticles by Self-Assembly and Crosslinking of Micellar, Block Copolymer Shells. Angew. Chem. Int. Ed. 2005, 44, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Mantzaridis, C.; Pispas, S. Hybrid Compound Block Copolymer Micelles Encapsulating Gold Nanoparticles. Macromol. Rapid Commun. 2008, 29, 1793–1797. [Google Scholar] [CrossRef]
- Liu, Z.; Torchilin, V.P. Stimulus-responsive nano-preparations for tumor targeting. Integr. Biol. 2013, 5, 96–107. [Google Scholar]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef] [PubMed]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Gao, J.; Zhang, D.; Jia, L.; Liu, Y.; Zheng, D.; Liu, G.; Tian, X.; Wang, F.; Zhang, Q. Galactose-decorated pH-responsive nanogels for hepatoma-targeted delivery of oridonin. Biomacromolecules 2011, 12, 4335–4343. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Bae, Y.H. Recent progress in tumor pH targeting nanotechnology. J. Control. Release 2008, 132, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.N.; Quarta, A.; Ferraro, M.M.; Dini, L.; Nobile, C.; De Giorgi, M.L.; Carallo, S.; Citti, C.; Gaballo, A.; Cannazza, G.; et al. Polymeric Nano-Micelles as Novel Cargo-Carriers for LY2157299 Liver Cancer Cells Delivery. Int. J. Mol Sci. 2018, 19, 748. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Z.; Hsieh, J.-H.; Fan, X.-C.; Yang, J.-D.; Chung, T.-W. Synthesis, characterization and drug delivery behaviors of new PCP polymeric micelles. Carbohydr. Polym. 2007, 8, 544–554. [Google Scholar] [CrossRef]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine 2010, 6, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Khemtong, C.; Yang, X.; Chang, X.; Gao, J. Nanonization strategies for poorly water-soluble drugs. Drug Discov. Today 2011, 16, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Sant, V.P.; Smith, D.; Leroux, J.-C. Enhancement of oral bioavailability of poorly water-soluble drugs by poly(ethylene glycol)-block-poly(alkyl acrylate-co-methacrylic acid) self-assemblies. J. Control. Release 2005, 104, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, C.H. Self-assembled biodegradable nanoparticles developed by direct dialysis for the delivery of paclitaxel. Pharm. Res. 2005, 22, 2079–2090. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.T.; Ma, G.H.; Qiu, W.; Su, Z.G. W/O/W double emulsion technique using ethyl acetate as organic solvent: Effects of its diffusion rate on the characteristics of microparticles. J. Control. Release 2003, 91, 407–416. [Google Scholar] [CrossRef]
- Jette, K.; Law, D.; Schmitt, E.; Kwon, G. Preparation and drug loading of poly (ethylene glycol)-block-poly(ε-caprolactone) micelles through the evaporation of a cosolvent azeotrope. Pharm. Res. 2004, 21, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Fournier, E.; Dufresne, M.H.; Smith, D.C.; Ranger, M.; Leroux, J.C. A novel one-step drug- Drug Loading of Polymeric Micelles. Pharm. Res. 2013, 30, 584–595. [Google Scholar]
- Kabanov, A.V.; Alakhov, V.Y. Micelles of amphilphilic block copolymers as vehicles for drug delivery. In Amphiphilic Block Copolymers: Self-Assembly and Applications; Alexandridis, P., Lindman, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 347–376. [Google Scholar]
- Yokoyama, M. Clinical Applications of Polymeric Micelle Carrier Systems in Chemotherapy and Image Diagnosis of Solid Tumors. J. Exp. Clin. Med. 2011, 3, 151–158. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Zhang, R.; Li, V.; Liu, X.; Sun, R.W.; Che, C.M.; Wong, K.K. Enhancement of anticancer efficacy using modified lipophilic nanoparticle drug encapsulation. Int. J. Nanomed. 2012, 7, 731–737. [Google Scholar]
- Savić, R.; Eisenberg, A.; Maysinger, D. Block copolymer micelles as delivery vehicles of hydrophobic drugs: Micelle–cell interactions. J. Drug Target 2006, 14, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Soga, O.; van Nostrum, C.F.; Fens, M.; Rijcken, C.J.; Schiffelers, R.M.; Storm, G.; Hennink, W.E. Thermosensitive and biodegradable polymeric micelles for paclitaxel delivery. J. Control. Release 2005, 103, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lammers, T.; Storm, G.; Hennink, W.E. Physico-Chemical Strategies to Enhance Stability and Drug Retention of Polymeric Micelles for Tumor-Targeted Drug Delivery. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.C.; Chan, D.P.Y.; Shoichet, M.S. Polymeric micelle stability. Nanotoday 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release 2014, 190, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Nagasaki, Y.; Okada, T.; Kato, M.; Kataoka, K. Core-Polymerized Reactive Micelles from Heterotelechelic Amphiphilic Block Copolymers. Macromolecules 1999, 32, 1140–1146. [Google Scholar] [CrossRef]
- Xu, L. Sacrificial PSS doped CaCO3 template to prepare chitosan capsules and their deformation under bulk pressure. Polym. Bull. 2013, 70, 455–465. [Google Scholar] [CrossRef]
- Kim, S.; Shi, Y.; Kim, J.Y.; Park, K.; Cheng, J.X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin. Drug Deliv. 2010, 7, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; van der Meel, R.; Theek, B.; Oude Blenke, E.; Pieters, E.H.; Fens, M.H.; Ehling, J.; Schiffelers, R.M. Storm, G.; van Nostrum, C.F.; et al. Complete Regression of Xenograft Tumors upon Targeted Delivery of Paclitaxel via Π-Π Stacking Stabilized Polymeric Micelles. ACS Nano 2015, 9, 3740–3752. [Google Scholar] [CrossRef] [PubMed]
- Shuai, X.; Merda, T.; Schaper, A.K.; Xi, F.; Kissel, T. Core-cross-linked polymeric micelles as paclitaxel carriers. Bioconjug. Chem. 2004, 15, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Kim, D.W.; Chung, J.Y.; Shin, S.G.; Kim, S.C.; Heo, D.S.; Kim, N.K.; Bang, Y.J. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin. Cancer Res. 2004, 10, 3708–3716. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Chung, H.C.; Im, S.A.; Park, Y.H.; Kim, C.S.; Kim, S.B.; Rha, S.Y.; Lee, M.Y.; Ro, J. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2008, 108, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kim, S.Y.; Kim, H.K.; Kim, S.W.; Shin, S.W.; Kim, J.S.; Park, K.; Lee, M.Y.; Heo, D.S. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann. Oncol. 2007, 18, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Danson, S.; Ferry, D.; Alakhov, V.; Margison, J.; Kerr, D.; Jowle, D.; Brampton, M.; Halbert, G.; Ranson, M. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br. J. Cancer. 2004, 90, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Hamaguchi, T.; Ura, T.; Muro, K.; Yamada, Y.; Shimada, Y.; Shirao, K.; Okusaka, T.; Ueno, H.; Ikeda, M.; et al. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br. J. Cancer 2004, 91, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Lukyanov, A.N.; Singhal, A.; Torchilin, V.P. Diacyl lipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Lett. 2002, 2, 979–982. [Google Scholar] [CrossRef]
- Mu, L.; Chrastina, A.; Levchenko, T.; Torchilin, V.P. Micelles from poly(ethylene glycol) phosphatidyl ethanolamine conjugates (PEG-PE) as pharmaceutical nanocarriers for poorly soluble drug camptothecin. J. Biomed. Nanotechnol. 2005, 1, 190–195. [Google Scholar] [CrossRef]
- Wang, J.; Mongayt, D.A.; Lukyanov, A.N.; Levchenko, T.S.; Torchilin, V.P. Preparation and in vitro synergistic anticancer effect of vitamin K3 and 1,8-diazabicyclo[5,4,0]undec-7-ene in poly(ethylene glycol)-diacyl lipid micelles. Int. J. Pharm. 2004, 272, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Okano, T.; Sakurai, Y.; Ekimoto, H.; Shibazaki, C.; Kataoka, K. Toxicity and antitumor activity against solid tumors of micelle-forming polymeric anticancer drug and its extremely long circulation in blood. Cancer Res. 1991, 51, 3229–3236. [Google Scholar] [PubMed]
- Deeb, D.; Jiang, H.; Gao, X.; Al-Holou, S.; Danyluk, A.L.; Dulchavsky, S.A.; Gautam, S.C. Curcumin [1,7-Bis(4-hydroxy-3-methoxyphenyl)-1–6-heptadine-3,5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-κB via inhibition of the pro survival Akt signaling pathway. J. Pharmacol. Exp. Ther. 2007, 321, 616–625. [Google Scholar] [PubMed]
- Jiang, M.C.; Yang-Yen, H.F.; Yen, J.J.; Lin, J.K. Curcumin induces apoptosis in immortalized NIH 3T3 and malignant cancer cell lines. Nutr. Cancer 1996, 26, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Holy, J. Curcumin inhibits cell motility and alters microfilament organization and function in prostate cancer cells. Cell. Motil. Cytoskelet. 2004, 58, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.H.; Kim, Y.H.; Choi, Y.J.; Kim, D.G.; Lee, K.S.; Bae, J.H.; Min, D.S.; Chang, J.S.; Jeong, Y.J.; Lee, Y.H.; et al. Molecular mechanisms of curcumin-induced cytotoxicity: Induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome C and inhibition of Akt. Carcinogenesis 2003, 24, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Yoysungnoen, P.; Wirachwong, P.; Bhattarakosol, P.; Niimi, H.; Patumraj, S. Effects of curcumin on tumor angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular carcinoma cell-implanted nude mice. Clin. Hemorheol. Microcirc. 2006, 34, 109–115. [Google Scholar] [PubMed]
- Anuchapreeda, S.; Limtrakul, P.; Thanarattanakorn, P.; Sittipreechacharn, S.; Chanarat, P. Inhibitory effect of curcumin on WT1 gene expression in patient leukemic cells. Arch. Pharm. Res. 2006, 29, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Odot, J.; Albert, P.; Carlier, A.; Tarpin, M.; Devy, J.; Madoulet, C. In vitro and in vivo anti-tumoral effect of curcumin against melanoma cells. Int. J. Cancer 2004, 111, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Murphy, C.J.; Zhang, B.; Shen, Y.; Sui, M.; Van Kirk, E.A.; Feng, X.; Murdoch, W.J. Amphiphilic curcumin conjugate-forming nanoparticles as anticancer prodrug and drug carriers: In vitro and in vivo effects. Nanomedicine 2010, 5, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, J.; Gao, X.; Li, J.; Zhao, W.; Sun, M.; Stolz, D.B.; Venkataramanan, R.; Rohan, L.C.; Li, S. PEG-derivatizedembelin as a dual functional carrier for the delivery of paclitaxel. Bioconjug. Chem. 2012, 23, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Varela-Moreira, A.; Shi, Y.; Fens, M.H.A.M.; Lammers, T.; Hennink, W.E.; Schiffelers, R.M. Clinical application of polymeric micelles for the treatment of cancer. Mater. Chem. Front. 2017, 1, 1485–1501. [Google Scholar] [CrossRef]
- Roby, A.; Erdogan, S.; Torchilin, V.P. Enhanced in vivo antitumor efficacy of poorly soluble PDT agent, meso-tetraphenylporphine, in PEG-PEbased tumortargeted immune-micelles. Cancer Biol. Ther. 2007, 6, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, E.S.; Oh, K.T.; Gao, Z.G.; Bae, Y.H. Doxorubicin-loaded polymeric micelle overcomes multidrug resistance of cancer by double-targeting folate receptor and early endosomal pH. Small 2008, 4, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Armstrong, A.; Newman, C.; Alakhov, V.; Pietrzynski, G.; Brewer, J.; Campbell, S.; Corrie, P.; Rowinsky, E.K.; Ranson, M. A phase 2 study of SP1049C, doxorubicin in P-glycoprotein-targeting pluronics, in patients with advanced adenocarcinoma of the esophagus and gastro-esophageal junction. Investig. New Drugs 2011, 29, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Cable, H.C.; Lloyd, J.B.; Rejmanova, P.J. Kopeček, Polymers containing enzymatically degradable bonds. 7. Design of oligopeptide side-chains in poly[n-(2-hydroxypropyl)methacrylamide] copolymers to promote efficient degradation by lysosomal-enzymes, Macromol. Chem. Macromol. Chem. Phys. 1983, 184, 1997–2008. [Google Scholar]
- Wilson, R.H.; Plummer, R.; Adam, J.; Eatock, M.M.; Boddy, A.V.; Griffin, M.; Miller, R.; Matsumura, Y.; Shimizu, T.; Calvert, H. Phase I and pharmacokinetic study of NC-6004, a new platinum entity of cisplatin conjugated polymer forming micelles. J. Clin. Oncol. 2008, 26, 2573. [Google Scholar] [CrossRef]
- Singer, J.W. Paclitaxel poliglumex (XYOTAX, CT-2103): A macromolecular taxane. J. Control. Release 2005, 109, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, F.; Kitagawa, M.; Negishi, T.; Onda, S.; Matsumoto, S.; Hamaguchi, T.; Matsumura, Y. Novel SN-38-incorporating polymeric micelles, NK012, eradicate vascular endothelial growth factor-secreting bulky tumors. Cancer Res. 2006, 66, 10048–10056. [Google Scholar] [CrossRef] [PubMed]
| Micelles | Formulation |
|---|---|
| Lipid micelle | Phospholipid or cholesterol |
| Polymeric micelles | Polymers having hydrophobic and hydrophilic properties |
| Hybrid polymeric lipids micelles | Polymers integrated into lipids |
| Hybrid micelles with metal nanoparticles | Micelles assembled with gold, silver or iron oxide nanoparticles |
| Micelles coated by layer by layer technique | Micelles incorporated into calcium carbonate and coated by polymers |
| Stimuli-responsive micelles | Micelles doped with stimuli such as pH-sensitive components |
| Name | Drug | Block Copolymer | Drug Loading (%w Drug/w Polymer) | Size (nm) | Company | Indication |
|---|---|---|---|---|---|---|
| NK105 | Paclitaxel | PEG-b-poly(α,β-aspartic acid) | 23 | 85 | Nippon Kayaku, Co. | Gastric cancer/Breast cancer |
| NK012 | SN-38 | PEG-b-poly(l-glutamic acid) | 20 | 20 | Nippon Kayaku, Co. | Triple negative breast cancer |
| NK911 | Doxorubicin | PEG-b-poly(α,β-aspartic acid) | 17 | 40 | Nippon Kayaku, Co. | Various solid tumors |
| NC-6004 | Cisplatin | PEG-b-poly(l-glutamic acid) | 30 | 20 | Nanocarrier, Co. | Pancreatic cancer |
| NC-4016 | Oxaliplatin | PEG-b-poly(l-glutamic acid) | 30 | 30 | Nanocarrier, Co. | Various solid tumors |
| NC-6300 | Epirubicin | PEG-b-poly(aspartate-hydrazone) | 20 | 60 | Nanocarrier, Co. | Various solid tumors |
| siRNA micelles | siRNA | PEG-b-polycations | Various | 40–60 | Nanocarrier, Co. | – |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy. Cancers 2018, 10, 238. https://doi.org/10.3390/cancers10070238
Hanafy NAN, El-Kemary M, Leporatti S. Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy. Cancers. 2018; 10(7):238. https://doi.org/10.3390/cancers10070238
Chicago/Turabian StyleHanafy, Nemany A. N., Maged El-Kemary, and Stefano Leporatti. 2018. "Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy" Cancers 10, no. 7: 238. https://doi.org/10.3390/cancers10070238
APA StyleHanafy, N. A. N., El-Kemary, M., & Leporatti, S. (2018). Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy. Cancers, 10(7), 238. https://doi.org/10.3390/cancers10070238







