Racial Disparity and Triple-Negative Breast Cancer in African-American Women: A Multifaceted Affair between Obesity, Biology, and Socioeconomic Determinants
Abstract
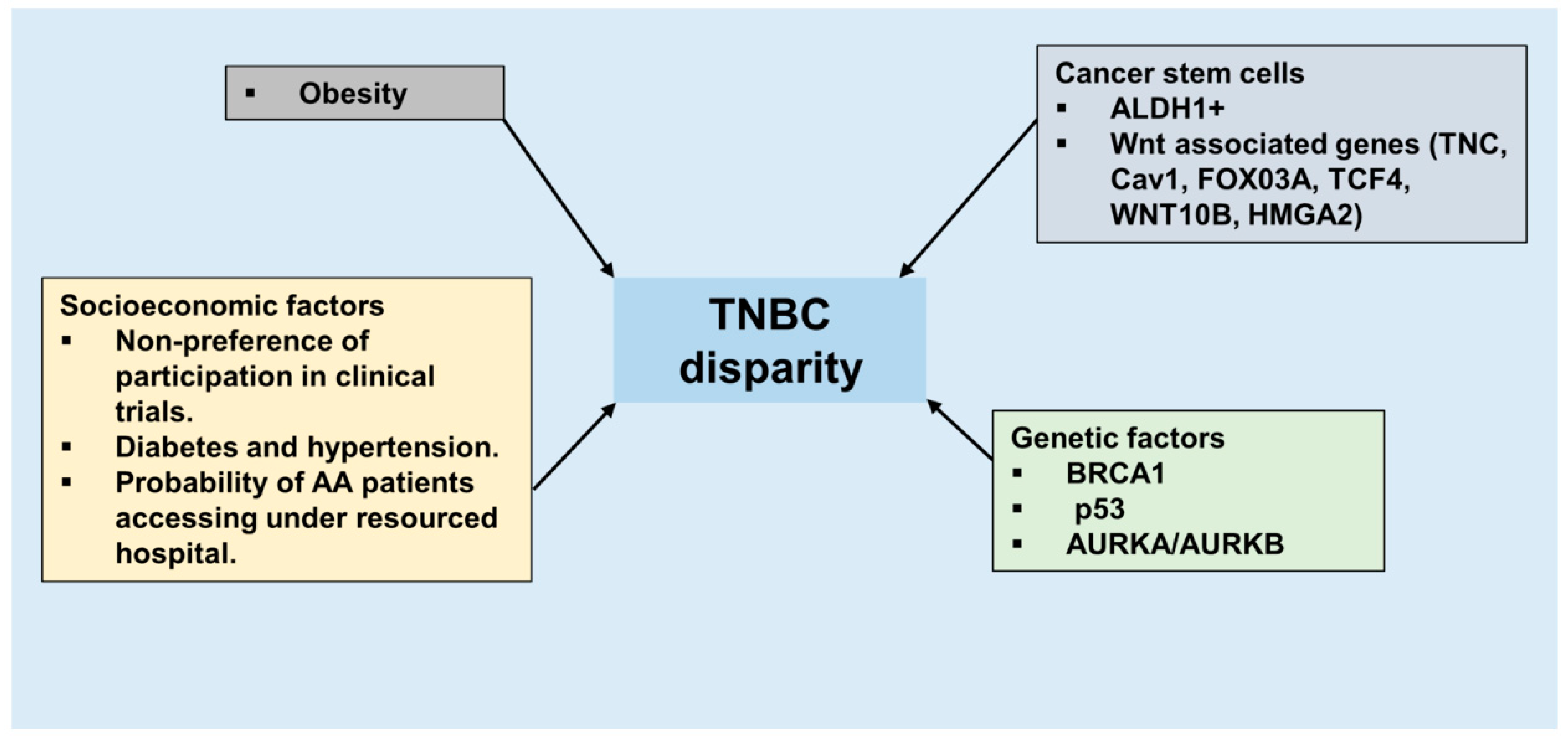
1. Introduction
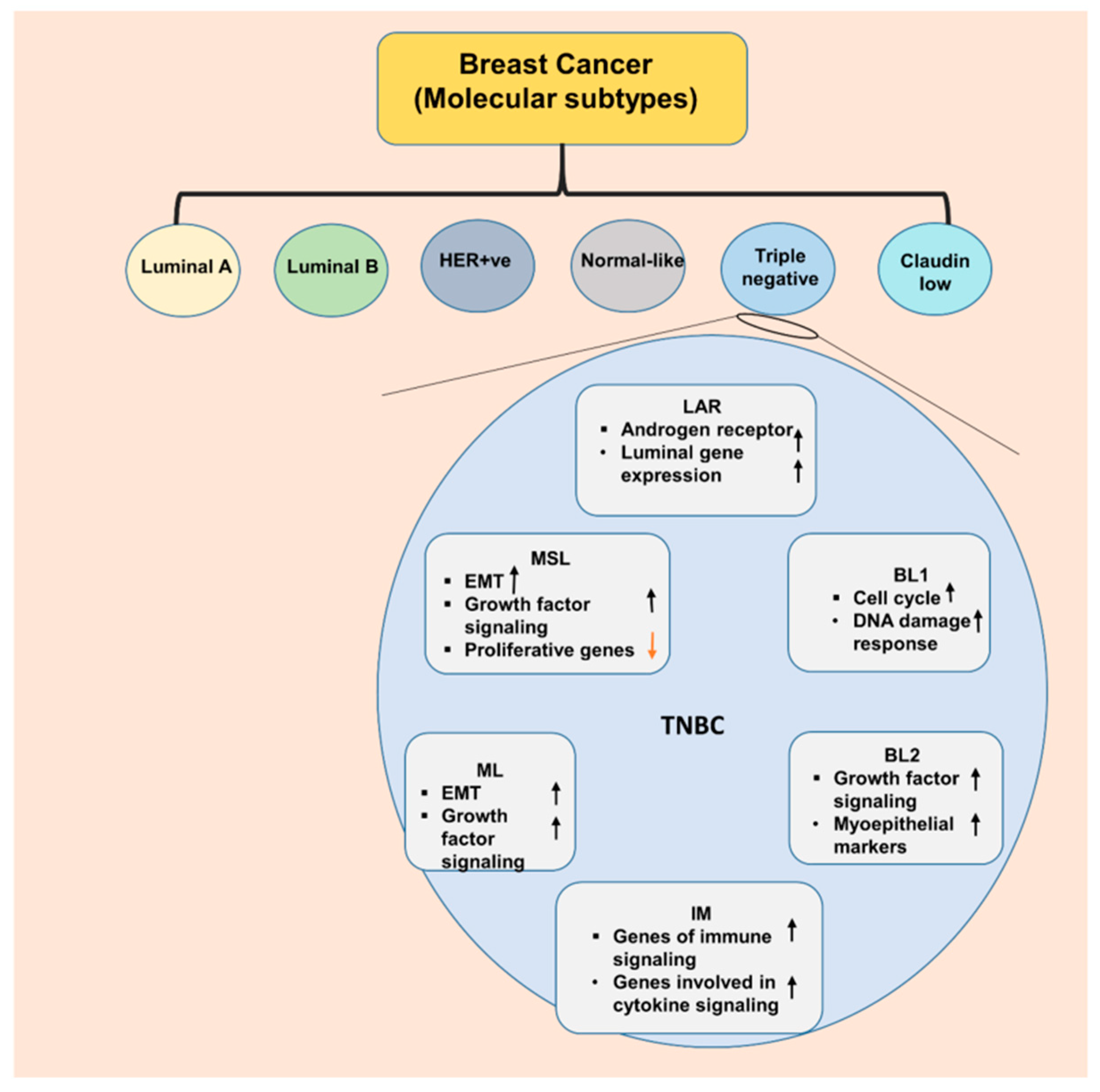
2. Triple Negative Breast Cancer—an Aggressive Subtype
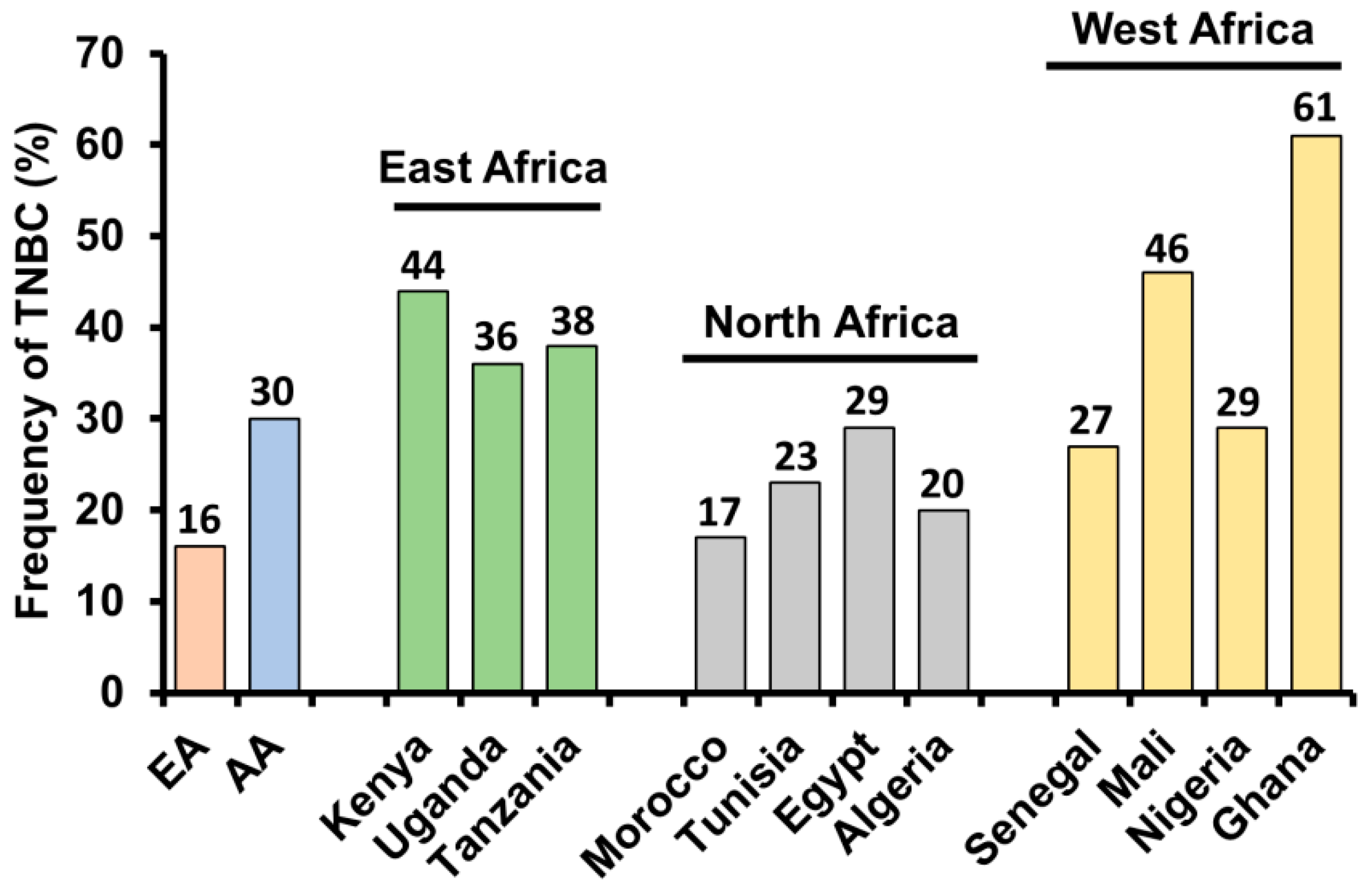
3. Triple Negative Breast Cancer—Higher Prevalence in African American Women
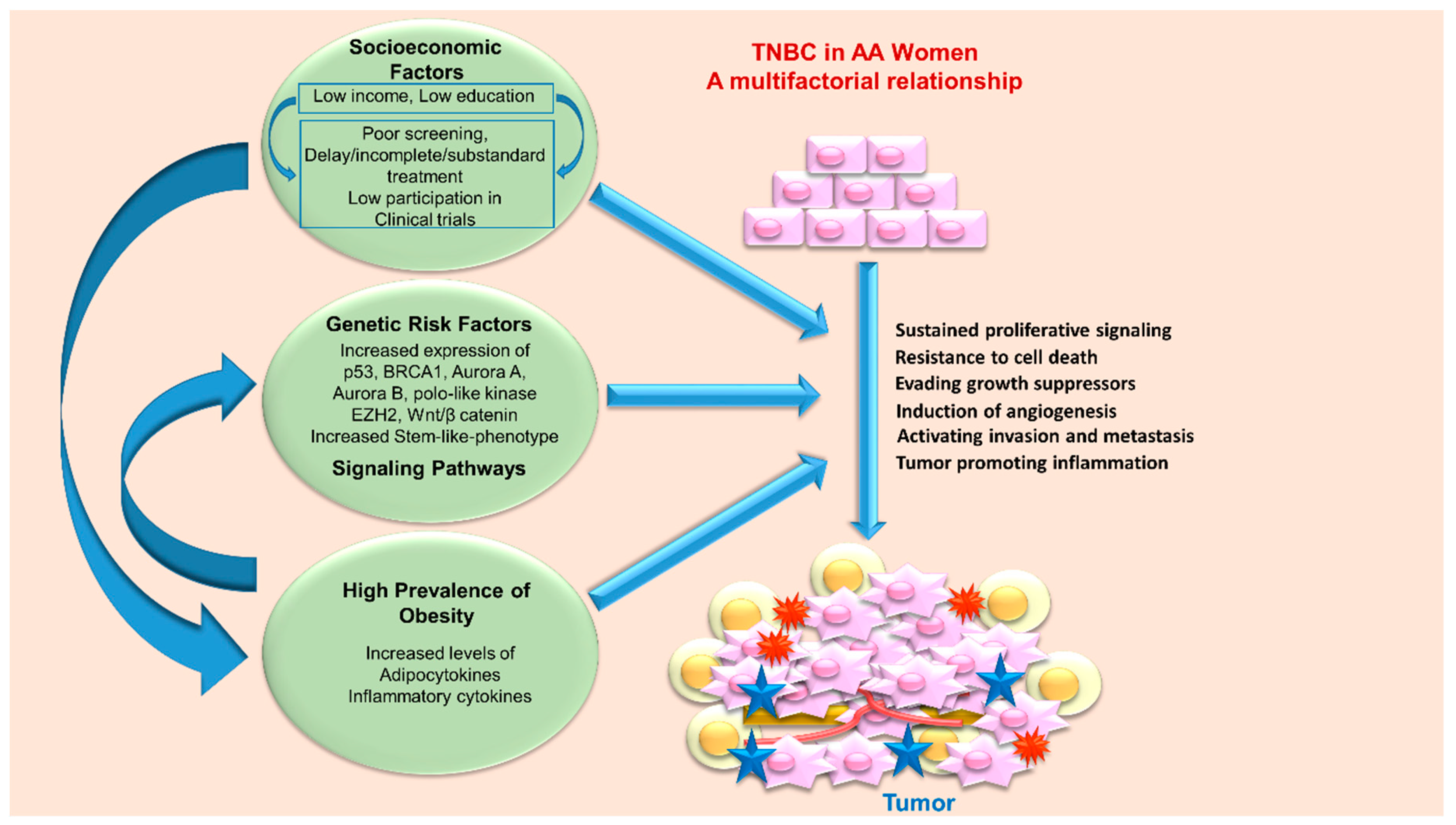
4. Triple Negative Breast Cancer Disparity in African American Women—Biology and Environment
4.1. Genetic Risk Factors
4.2. Socioeconomic Factors
5. Molecular Pathways as Therapeutic Targets in Triple Negative Breast Cancer
6. Cancer Stem Cells, Triple Negative Breast Cancer, and African-American Ancestry
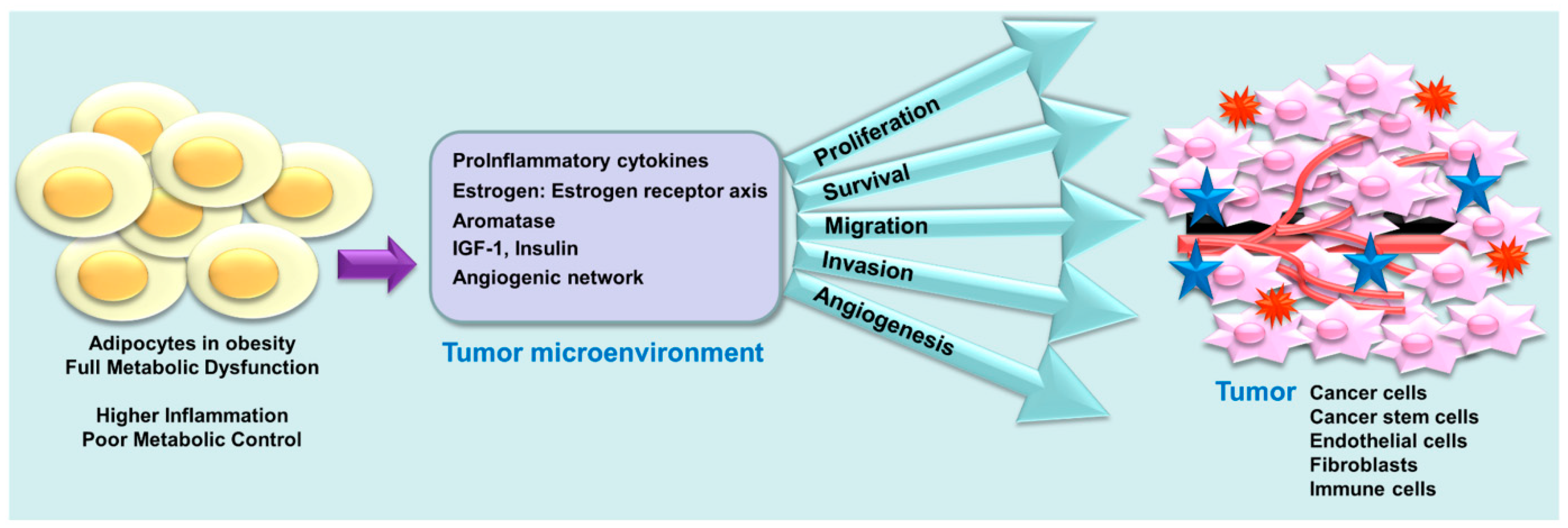
7. Obesity, a Coconspirator in TNBC Disparity
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Lin, C.C.; Mariotto, A.B.; Siegel, R.L.; Stein, K.D.; Kramer, J.L.; Alteri, R.; Robbins, A.S.; Jemal, A. Cancer treatment and survivorship statistics, 2014. CA Cancer J. Clin. 2014, 64, 252–271. [Google Scholar] [CrossRef] [PubMed]
- Engebraaten, O.; Vollan, H.K.M.; Borresen-Dale, A.L. Triple-negative breast cancer and the need for new therapeutic targets. Am. J. Pathol. 2013, 183, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.O.; Hsu, F.D.; Jensen, K.; Cheang, M.; Karaca, G.; Hu, Z.; Hernandez-Boussard, T.; Livasy, C.; Cowan, D.; Dressler, L.; et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin. Cancer Res. 2004, 10, 5367–5374. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Van de Rijn, M.; Perou, C.M.; Tibshirani, R.; Haas, P.; Kallioniemi, O.; Kononen, J.; Torhorst, J.; Sauter, G.; Zuber, M.; Kochli, O.R.; et al. Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am. J. Pathol. 2002, 161, 1991–1996. [Google Scholar] [CrossRef]
- Rakha, E.A.; Putti, T.C.; Abd El-Rehim, D.M.; Paish, C.; Green, A.R.; Powe, D.G.; Lee, A.H.; Robertson, J.F.; Ellis, I.O. Morphological and immunophenotypic analysis of breast carcinomas with basal and myoepithelial differentiation. J. Pathol. 2006, 208, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; El-Sayed, M.E.; Green, A.R.; Lee, A.H.; Robertson, J.F.; Ellis, I.O. Prognostic markers in triple-negative breast cancer. Cancer 2007, 109, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Dietze, E.C.; Sistrunk, C.; Miranda-Carboni, G.; O’Regan, R.; Seewaldt, V.L. Triple-negative breast cancer in African-American women: Disparities versus biology. Nat. Rev. Cancer 2015, 15, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Bonotto, M.; Gerratana, L.; Poletto, E.; Driol, P.; Giangreco, M.; Russo, S.; Minisini, A.M.; Andreetta, C.; Mansutti, M.; Pisa, F.E.; et al. Measures of outcome in metastatic breast cancer: Insights from a real-world scenario. Oncologist 2014, 19, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Reis-Filho, J.S.; Ellis, I.O. Basal-like breast cancer: A critical review. J. Clin. Oncol. 2008, 26, 2568–2581. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Baehner, F.L.; Reis-Filho, J.S. The contribution of gene expression profiling to breast cancer classification, prognostication and prediction: A retrospective of the last decade. J. Pathol. 2010, 220, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, C.; Pusztai, L. Gene-expression signatures in breast cancer. N. Engl. J. Med. 2009, 360, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.R.; Brown, M.; Cress, R.D.; Parise, C.A.; Caggiano, V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California cancer Registry. Cancer 2007, 109, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006, 295, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.; Siegel, R.; Jemal, A. Breast Cancer Facts & Figures 2015–2016. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2015-2016.pdf (accessed on 13 December 2018).
- Danforth, D.N., Jr. Disparities in breast cancer outcomes between Caucasian and African American women: A model for describing the relationship of biological and nonbiological factors. Breast Cancer Res. 2013, 15, e208. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.A.; Keegan, T.H.; Yang, J.; Press, D.J.; Kurian, A.W.; Patel, A.H.; Lacey, J.V., Jr. Age-specific incidence of breast cancer subtypes: Understanding the black-white crossover. J. Natl. Cancer Inst. 2012, 104, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Brewster, A.M.; Chavez-MacGregor, M.; Brown, P. Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet. Oncol. 2014, 15, 625–634. [Google Scholar] [CrossRef]
- Kohler, B.A.; Sherman, R.L.; Howlader, N.; Jemal, A.; Ryerson, A.B.; Henry, K.A.; Boscoe, F.P.; Cronin, K.A.; Lake, A.; Noone, A.M.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J. Natl. Cancer Inst. 2015, 107, e048. [Google Scholar] [CrossRef] [PubMed]
- Jiagge, E.; Jibril, A.S.; Chitale, D.; Bensenhaver, J.M.; Awuah, B.; Hoenerhoff, M.; Adjei, E.; Bekele, M.; Abebe, E.; Nathanson, S.D.; et al. Comparative Analysis of Breast Cancer Phenotypes in African American, White American, and West Versus East African patients: Correlation Between African Ancestry and Triple-Negative Breast Cancer. Ann. Surg. Oncol. 2016, 23, 3843–3849. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Ikpatt, F.; Khramtsov, A.; Dangou, J.M.; Nanda, R.; Dignam, J.; Zhang, B.; Grushko, T.; Zhang, C.; Oluwasola, O.; et al. Population differences in breast cancer: Survey in indigenous African women reveals over-representation of triple-negative breast cancer. J. Clin. Oncol. 2009, 27, 4515–4521. [Google Scholar] [CrossRef] [PubMed]
- Ly, M.; Antoine, M.; Dembele, A.K.; Levy, P.; Rodenas, A.; Toure, B.A.; Badiaga, Y.; Dembele, B.K.; Bagayogo, D.C.; Diallo, Y.L.; et al. High incidence of triple-negative tumors in sub-saharan Africa: A prospective study of breast cancer characteristics and risk factors in Malian women seen in a Bamako university hospital. Oncology 2012, 83, 257–263. [Google Scholar] [CrossRef] [PubMed]
- McCormack, V.A.; Joffe, M.; van den Berg, E.; Broeze, N.; Silva Idos, S.; Romieu, I.; Jacobson, J.S.; Neugut, A.I.; Schuz, J.; Cubasch, H. Breast cancer receptor status and stage at diagnosis in over 1200 consecutive public hospital patients in Soweto, South Africa: A case series. Breast Cancer Res. 2013, 15, e84. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.L.; Duffy, S.W.; Ryan, D.A.; Hart, I.R.; Jones, J.L. Early onset of breast cancer in a group of British black women. Br. J. Cancer 2008, 98, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Stead, L.A.; Lash, T.L.; Sobieraj, J.E.; Chi, D.D.; Westrup, J.L.; Charlot, M.; Blanchard, R.A.; Lee, J.C.; King, T.C.; Rosenberg, C.L. Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Res. 2009, 11, e25. [Google Scholar] [CrossRef] [PubMed]
- Amirikia, K.C.; Mills, P.; Bush, J.; Newman, L.A. Higher population-based incidence rates of triple-negative breast cancer among young African-American women: Implications for breast cancer screening recommendations. Cancer 2011, 117, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Lindner, R.; Sullivan, C.; Offor, O.; Lezon-Geyda, K.; Halligan, K.; Fischbach, N.; Shah, M.; Bossuyt, V.; Schulz, V.; Tuck, D.P.; et al. Molecular phenotypes in triple negative breast cancer from African American patients suggest targets for therapy. PLoS ONE 2013, 8, e71915. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.; Moy, B.; Mroz, E.A.; Ross, K.; Niemierko, A.; Rocco, J.W.; Isakoff, S.; Ellisen, L.W.; Bardia, A. Comparison of the Genomic Landscape Between Primary Breast Cancer in African American Versus White Women and the Association of Racial Differences with Tumor Recurrence. J. Clin. Oncol. 2015, 33, 3621–3627. [Google Scholar] [CrossRef] [PubMed]
- Ademuyiwa, F.O.; Tao, Y.; Luo, J.; Weilbaecher, K.; Ma, C.X. Differences in the mutational landscape of triple-negative breast cancer in African Americans and Caucasians. Breast Cancer Res. Treat. 2017, 161, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.A.; Luks, J.; Roycik, M.D.; Sang, Q.X.; Zhang, J. Differentially expressed transcripts and dysregulated signaling pathways and networks in African American breast cancer. PLoS ONE 2013, 8, e82460. [Google Scholar] [CrossRef] [PubMed]
- Mavaddat, N.; Barrowdale, D.; Andrulis, I.L.; Domchek, S.M.; Eccles, D.; Nevanlinna, H.; Ramus, S.J.; Spurdle, A.; Robson, M.; Sherman, M.; et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: Results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol. Biomark. Prev. 2012, 21, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.; Schumm, L.P.; Cummings, S.; Fackenthal, J.D.; Sveen, L.; Ademuyiwa, F.; Cobleigh, M.; Esserman, L.; Lindor, N.M.; Neuhausen, S.L.; et al. Genetic testing in an ethnically diverse cohort of high-risk women: A comparative analysis of BRCA1 and BRCA2 mutations in American families of European and African ancestry. JAMA 2005, 294, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.I.; King, M.C. Population genetics of BRCA1 and BRCA2. Am. J. Hum. Genet. 1997, 60, 1013–1020. [Google Scholar]
- Gonzalez, M.E.; DuPrie, M.L.; Krueger, H.; Merajver, S.D.; Ventura, A.C.; Toy, K.A.; Kleer, C.G. Histone methyltransferase EZH2 induces Akt-dependent genomic instability and BRCA1 inhibition in breast cancer. Cancer Res. 2011, 71, 2360–2370. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Toy, K.A.; Griffith, K.A.; Awuah, B.; Quayson, S.; Newman, L.A.; Kleer, C.G. Invasive breast carcinomas in Ghana: High frequency of high grade, basal-like histology and high EZH2 expression. Breast Cancer Res. Treat. 2012, 135, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Pietersen, A.M.; Horlings, H.M.; Hauptmann, M.; Langerod, A.; Ajouaou, A.; Cornelissen-Steijger, P.; Wessels, L.F.; Jonkers, J.; van de Vijver, M.J.; van Lohuizen, M. EZH2 and BMI1 inversely correlate with prognosis and TP53 mutation in breast cancer. Breast Cancer Res. 2008, 10, e19. [Google Scholar] [CrossRef] [PubMed]
- Shavers, V.L.; Brown, M.L. Racial and ethnic disparities in the receipt of cancer treatment. J. Natl. Cancer Inst. 2002, 94, 334–357. [Google Scholar] [CrossRef]
- Owens, O.L.; Jackson, D.D.; Thomas, T.L.; Friedman, D.B.; Hebert, J.R. African American men’s and women’s perceptions of clinical trials research: Focusing on prostate cancer among a high-risk population in the South. J. Health Care Poor Underserved 2013, 24, 1784–1800. [Google Scholar] [CrossRef] [PubMed]
- Haynes-Maslow, L.; Godley, P.; Dimartino, L.; White, B.; Odom, J.; Richmond, A.; Carpenter, W. African American women’s perceptions of cancer clinical trials. Cancer Med. 2014, 3, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.A.; Griffith, K.A.; Jatoi, I.; Simon, M.S.; Crowe, J.P.; Colditz, G.A. Meta-analysis of survival in African American and white American patients with breast cancer: Ethnicity compared with socioeconomic status. J. Clin. Oncol. 2006, 24, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Tammemagi, C.M.; Nerenz, D.; Neslund-Dudas, C.; Feldkamp, C.; Nathanson, D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA 2005, 294, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.; Weinberg, M.; Rosner, Z.; Alexis, K.; Tiersten, A.; Grann, V.R.; Troxel, A.; Neugut, A.I. Ethnic neutropenia and treatment delay in African American women undergoing chemotherapy for early-stage breast cancer. J. Natl. Cancer Inst. 2003, 95, 1545–1548. [Google Scholar] [CrossRef]
- Albain, K.S.; Unger, J.M.; Crowley, J.J.; Coltman, C.A., Jr.; Hershman, D.L. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J. Natl. Cancer Inst. 2009, 101, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Akinyemiju, T.F.; Pisu, M.; Waterbor, J.W.; Altekruse, S.F. Socioeconomic status and incidence of breast cancer by hormone receptor subtype. Springerplus 2015, 4, 015–1282. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Dieras, V.; Glaspy, J.; Brufsky, A.; Miller, K.; Miles, D.; Koralewski, P.; Phan, S.; Bhattacharya, S. Comparison of Subgroup Analyses of PFS from Three Phase III Studies of Bevacizumab in Combination with Chemotherapy in Patients with HER2-Negative Metastatic Breast Cancer (MBC). Cancer Res. 2009, 69, 207. [Google Scholar] [CrossRef]
- Turner, N.; Tutt, A.; Ashworth, A. Hallmarks of “BRCAness” in sporadic cancers. Nat. Rev. Cancer. 2004, 4, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.C.; Boss, D.S.; Yap, T.A.; Tutt, A.; Wu, P.; Mergui-Roelvink, M.; Mortimer, P.; Swaisland, H.; Lau, A.; O’Connor, M.J.; et al. Inhibition of poly (ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009, 361, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.; Robson, M.; Garber, J.E.; Domchek, S.M.; Audeh, M.W.; Weitzel, J.N.; Friedlander, M.; Arun, B.; Loman, N.; Schmutzler, R.K.; et al. Oral poly (ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet 2010, 376, 235–244. [Google Scholar] [CrossRef]
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmana, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 2015, 33, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Gelmon, K.A.; Tischkowitz, M.; Mackay, H.; Swenerton, K.; Robidoux, A.; Tonkin, K.; Hirte, H.; Huntsman, D.; Clemons, M.; Gilks, B.; et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: A phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011, 12, 852–861. [Google Scholar] [CrossRef]
- Brown, J.S.; O’Carrigan, B.; Jackson, S.P.; Yap, T.A. Targeting DNA Repair in Cancer: Beyond PARP Inhibitors. Cancer Discov. 2017, 7, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.A.; Lindeman, G.J.; Clemons, M.; Wildiers, H.; Chan, A.; McCarthy, N.J.; Singer, C.F.; Lowe, E.S.; Watkins, C.L.; Carmichael, J. Phase I trial of the oral PARP inhibitor olaparib in combination with paclitaxel for first- or second-line treatment of patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2013, 15, e88. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Olopade, O.I.; DeMichele, A.; Yau, C.; van’t Veer, L.J.; Buxton, M.B.; Hogarth, M.; Hylton, N.M.; Paoloni, M.; Perlmutter, J.; et al. Adaptive Randomization of Veliparib-Carboplatin Treatment in Breast Cancer. N. Engl. J. Med. 2016, 375, 23–34. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Osborne, C.; Pippen, J.E.; Yoffe, M.; Patt, D.; Rocha, C.; Koo, I.C.; Sherman, B.M.; Bradley, C. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N. Engl. J. Med. 2011, 364, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Niemeier, L.A.; Dabbs, D.J.; Beriwal, S.; Striebel, J.M.; Bhargava, R. Androgen receptor in breast cancer: Expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod. Pathol. 2010, 23, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Barton, V.N.; D’Amato, N.C.; Gordon, M.A.; Christenson, J.L.; Elias, A.; Richer, J.K. Androgen Receptor Biology in Triple Negative Breast Cancer: A Case for Classification as AR+ or Quadruple Negative Disease. Horm. Cancer 2015, 6, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Proverbs-Singh, T.; Feldman, J.L.; Morris, M.J.; Autio, K.A.; Traina, T.A. Targeting the androgen receptor in prostate and breast cancer: Several new agents in development. Endocr. Relat. Cancer 2015, 22, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Farmer, P.; Bonnefoi, H.; Becette, V.; Tubiana-Hulin, M.; Fumoleau, P.; Larsimont, D.; Macgrogan, G.; Bergh, J.; Cameron, D.; Goldstein, D.; et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene 2005, 24, 4660–4671. [Google Scholar] [CrossRef] [PubMed]
- Doane, A.S.; Danso, M.; Lal, P.; Donaton, M.; Zhang, L.; Hudis, C.; Gerald, W.L. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 2006, 25, 3994–4008. [Google Scholar] [CrossRef] [PubMed]
- Gucalp, A.; Tolaney, S.; Isakoff, S.J.; Ingle, J.N.; Liu, M.C.; Carey, L.A.; Blackwell, K.; Rugo, H.; Nabell, L.; Forero, A.; et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin. Cancer Res. 2013, 19, 5505–5512. [Google Scholar] [CrossRef] [PubMed]
- A Traina, T.; O’Shaughnessy, J.; Nanda, R.; Schwartzberg, L.; Abramson, V.; Cortes, J.; Peterson, A.; Tudor, I.; Blaney, M.; L Steinberg, J.; et al. Abstract P5-19-09: Preliminary results from a phase 2 single-arm study of enzalutamide, an androgen receptor (AR) inhibitor, in advanced AR+ triple-negative breast cancer (TNBC). Cancer Res. 2015, 75, 5–19. [Google Scholar] [CrossRef]
- Traina, T.A.; Miller, K.; Yardley, D.A.; O’Shaughnessy, J.; Cortes, J.; Awada, A.; Kelly, C.M.; Trudeau, M.E.; Schmid, P.; Gianni, L.; et al. Results from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR+ triple-negative breast cancer (TNBC). J. Clin. Oncol. 2015, 33, 1003. [Google Scholar]
- Arce-Salinas, C.; Riesco-Martinez, M.C.; Hanna, W.; Bedard, P.; Warner, E. Complete Response of Metastatic Androgen Receptor-Positive Breast Cancer to Bicalutamide: Case Report and Review of the Literature. J. Clin. Oncol. 2016, 34, e2. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.A.; Rugo, H.S.; Marcom, P.K.; Mayer, E.L.; Esteva, F.J.; Ma, C.X.; Liu, M.C.; Storniolo, A.M.; Rimawi, M.F.; Forero-Torres, A.; et al. TBCRC 001: Randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J. Clin. Oncol. 2012, 30, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Gomez, P.; Greil, R.; Braga, S.; Climent, M.A.; Wardley, A.M.; Kaufman, B.; Stemmer, S.M.; Pego, A.; Chan, A.; et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J. Clin. Oncol. 2013, 31, 2586–2592. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Dering, J.; Ginther, C.; Wilson, C.A.; Glaspy, P.; Tchekmedyian, N.; Slamon, D.J. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/"triple-negative" breast cancer cell lines growing in vitro. Breast Cancer Res. Treat. 2007, 105, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Tryfonopoulos, D.; Walsh, S.; Collins, D.M.; Flanagan, L.; Quinn, C.; Corkery, B.; McDermott, E.W.; Evoy, D.; Pierce, A.; O’Donovan, N.; et al. Src: A potential target for the treatment of triple-negative breast cancer. Ann. Oncol. 2011, 22, 2234–2240. [Google Scholar] [CrossRef] [PubMed]
- Fornier, M.N.; Morris, P.G.; Abbruzzi, A.; D’Andrea, G.; Gilewski, T.; Bromberg, J.; Dang, C.; Dickler, M.; Modi, S.; Seidman, A.D.; et al. A phase I study of dasatinib and weekly paclitaxel for metastatic breast cancer. Ann. Oncol. 2011, 22, 2575–2581. [Google Scholar] [CrossRef] [PubMed]
- Saal, L.H.; Holm, K.; Maurer, M.; Memeo, L.; Su, T.; Wang, X.; Yu, J.S.; Malmstrom, P.O.; Mansukhani, M.; Enoksson, J.; et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005, 65, 2554–2559. [Google Scholar] [CrossRef] [PubMed]
- Ellard, S.L.; Clemons, M.; Gelmon, K.A.; Norris, B.; Kennecke, H.; Chia, S.; Pritchard, K.; Eisen, A.; Vandenberg, T.; Taylor, M.; et al. Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND.163. J. Clin. Oncol. 2009, 27, 4536–4541. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, D.; Kusdra, L.; Huskey, N.E.; Chandriani, S.; Lenburg, M.E.; Gonzalez-Angulo, A.M.; Creasman, K.J.; Bazarov, A.V.; Smyth, J.W.; Davis, S.E.; et al. MYC pathway activation in triple-negative breast cancer is synthetic lethal with CDK inhibition. J. Exp. Med. 2012, 209, 679–696. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Brufsky, A.; Kim, S.B.; Velu, T.; Garcia-Saenz, J.A.; Tan-Chiu, E.; Sohn, J.H.; Dirix, L.; Borms, M.V.; Liu, M.C.; Moezi, M.M.; et al. Cobimetinib (C) combined with paclitaxel (P) as a first-line treatment in patients (pts) with advanced triple-negative breast cancer (COLET study): Updated clinical and biomarker results. Cancer Res. 2017, 77, 4–22. [Google Scholar] [CrossRef]
- Gerratana, L.; Fanotto, V.; Pelizzari, G.; Agostinetto, E.; Puglisi, F. Do platinum salts fit all triple negative breast cancers? Cancer Treat Rev. 2016, 48, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Poggio, F.; Bruzzone, M.; Ceppi, M.; Ponde, N.F.; La Valle, G.; Del Mastro, L.; de Azambuja, E.; Lambertini, M. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis. Ann. Oncol. 2018, 29, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, Y.; Kong, X.; Zhai, J.; Li, Y.; Song, Y.; Wang, J.; Feng, X.; Fang, Y. Recent Advances in the Research of Immunotherapy for Triple-Negative Breast Cancer. Cancer Lett. 2018, 442, 409–428. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Neaves, W.B. Normal stem cells and cancer stem cells: The niche matters. Cancer Res. 2006, 66, 4553–4557. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Nalwoga, H.; Arnes, J.B.; Wabinga, H.; Akslen, L.A. Expression of aldehyde dehydrogenase 1 (ALDH1) is associated with basal-like markers and features of aggressive tumours in African breast cancer. Br. J. Cancer 2010, 102, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Clevers, H. Mining the Wnt pathway for cancer therapeutics. Nat. Rev. Drug. Discov. 2006, 5, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Bayet-Robert, M.; Kwiatkowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, X.; Wang, Y.; Zhang, K.; Wu, J.; Yuan, Y.C.; Deng, X.; Chen, L.; Kim, C.C.; Lau, S.; et al. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene 2011, 30, 4437–4446. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Hassan, H.; Vilardo, L.; Kumar, S.K.; Kumar, A.V.; Kelsch, R.; Schneider, C.; Kiesel, L.; Eich, H.T.; Zucchi, I.; et al. Syndecan-1 (CD138) modulates triple-negative breast cancer stem cell properties via regulation of LRP-6 and IL-6-mediated STAT3 signaling. PLoS ONE 2013, 8, e85737. [Google Scholar] [CrossRef] [PubMed]
- Getz, J.E.; Teoh, D.B.; Nasser, S.; Waibhav, T.; Christophe, L.R.; Yellapantula, V.; Ahearn, M.E.; Gomez, C.R.; Jorda, M.; Pegram, M.D.; et al. Abstract A74: Differential Wnt signaling in African American and Caucasian women with triple-negative breast cancer. Cancer Epidemiol. Biomark. Prev. 2015, 24, e74. [Google Scholar] [CrossRef]
- Wend, P.; Runke, S.; Wend, K.; Anchondo, B.; Yesayan, M.; Jardon, M.; Hardie, N.; Loddenkemper, C.; Ulasov, I.; Lesniak, M.S.; et al. WNT10B/beta-catenin signalling induces HMGA2 and proliferation in metastatic triple-negative breast cancer. EMBO 2013, 5, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.M.; Pohlig, R.T.; Sims-Mourtada, J. Co-activation of Hedgehog and Wnt signaling pathways is associated with poor outcomes in triple negative breast cancer. Oncol. Lett. 2017, 14, 5285–5292. [Google Scholar] [CrossRef] [PubMed]
- Polkinghorn, W.R.; Tarbell, N.J. Medulloblastoma: Tumorigenesis, current clinical paradigm, and efforts to improve risk stratification. Nat. Clin. Pract. Oncol. 2007, 4, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Nakshatri, H.; Anjanappa, M.; Bhat-Nakshatri, P. Ethnicity-Dependent and -Independent Heterogeneity in Healthy Normal Breast Hierarchy Impacts Tumor Characterization. Sci. Rep. 2015, 5, e13526. [Google Scholar] [CrossRef] [PubMed]
- CDC. Available online: https://www.cdc.gov/obesity/adult/defining.html (accessed on 25 September 2018).
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. Available online: http://apps.who.int/iris/bitstream/handle/10665/44583/?sequence=1 (accessed on 14 December 2018).
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016, 315, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Seidell, J.C.; Halberstadt, J. Obesity: The obesity epidemic in the USA-no end in sight? Nat. Rev. Endocrinol. 2016, 12, 499–500. [Google Scholar] [CrossRef] [PubMed]
- Vona-Davis, L.; Rose, D.P.; Hazard, H.; Howard-McNatt, M.; Adkins, F.; Partin, J.; Hobbs, G. Triple-negative breast cancer and obesity in a rural Appalachian population. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3319–3324. [Google Scholar] [CrossRef] [PubMed]
- Millikan, R.C.; Newman, B.; Tse, C.K.; Moorman, P.G.; Conway, K.; Dressler, L.G.; Smith, L.V.; Labbok, M.H.; Geradts, J.; Bensen, J.T.; et al. Epidemiology of basal-like breast cancer. Breast Cancer Res. Treat. 2008, 109, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Berstad, P.; Coates, R.J.; Bernstein, L.; Folger, S.G.; Malone, K.E.; Marchbanks, P.A.; Weiss, L.K.; Liff, J.M.; McDonald, J.A.; Strom, B.L.; et al. A case-control study of body mass index and breast cancer risk in white and African-American women. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.A.; Durham, D.D.; Tse, C.K.; Millikan, R.C. Determinants of breast cancer treatment delay differ for African American and White women. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Ambrosone, C.B.; Ciupak, G.L.; Bandera, E.V.; Jandorf, L.; Bovbjerg, D.H.; Zirpoli, G.; Pawlish, K.; Godbold, J.; Furberg, H.; Fatone, A.; et al. Conducting Molecular Epidemiological Research in the Age of HIPAA: A Multi-Institutional Case-Control Study of Breast Cancer in African-American and European-American Women. J. Oncol. 2009, 871250, e25. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, L.; Adams-Campbell, L.; Palmer, J.R. The Black Women’s Health Study: A follow-up study for causes and preventions of illness. J. Am. Med. Womens Assoc. 1972, 50, 56–58. [Google Scholar]
- Kolonel, L.N.; Henderson, B.E.; Hankin, J.H.; Nomura, A.M.; Wilkens, L.R.; Pike, M.C.; Stram, D.O.; Monroe, K.R.; Earle, M.E.; Nagamine, F.S. A multiethnic cohort in Hawaii and Los Angeles: Baseline characteristics. Am. J. Epidemiol. 2000, 151, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Bandera, E.V.; Chandran, U.; Hong, C.C.; Troester, M.A.; Bethea, T.N.; Adams-Campbell, L.L.; Haiman, C.A.; Park, S.Y.; Olshan, A.F.; Ambrosone, C.B.; et al. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res. Treat. 2015, 150, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.R.; Ambrosone, C.B.; Olshan, A.F. A collaborative study of the etiology of breast cancer subtypes in African American women: The AMBER consortium. Cancer Causes Control 2014, 25, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Capers, P.L.; Kinsey, A.W.; Miskell, E.L.; Affuso, O. Visual Representation of Body Shape in African-American and European American Women: Clinical Considerations. Clin. Med. Insights Womens Health 2016, 9, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Niswender, K.D.; Fazio, S.; Gower, B.A.; Silver, H.J. Balanced high fat diet reduces cardiovascular risk in obese women although changes in adipose tissue, lipoproteins, and insulin resistance differ by race. Metabolism 2018, 82, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Davidson, N.E. Obesity and breast cancer: A multipartite connection. J. Mammary Gland Biol. Neoplasia 2013, 18, 253–255. [Google Scholar] [CrossRef] [PubMed]
| Phase | Clinical Trial | Treatment | ClinicalTrials.gov Identifier |
|---|---|---|---|
| Phase 1 | A pilot study of Olaparib and Durvalumab in patients with metastatic triple negative breast cancer (TNBC) | Olaparib Durvalumab | NCT03544125 |
| Phase 1 | A phase I of Olaparib with Radiation Therapy in patients with inflammatory, loco-regional advanced or metastatic TNBC | Olaparib Radiation Therapy | NCT03109080 |
| Phase 1 | An open, non-randomized, multi-center Phase I study to access the safety and efficacy of Fluzoparib given in combination with Apatinib | Fluzoparib Apatinib | NCT03075462 |
| Phase 1 | A phase 1 study of PARP inhibitor Olaparib and HSP90 inhibitor AT13387 | Olaparib Onalespib | NCT02898207 |
| Phase 2 | A phase II open-label, randomized study of PARP inhibition either alone or in combination with anti-PD-L1 Therapy | Atezolizumab Olaparib | NCT02849496 |
| Phase 1 Phase 2 | Phase 1/2 clinical study of Niraparib in combination with Pembrilizumab (MK-3475) | Niraparib Pembrolizumab | NCT02657889 |
| Phase 2 | A phase II clinical trial of the PARP inhibitor Talazoparib | Talazoparib Tosylate | NCT02401347 |
| Phase | Clinical Trial | Treatment | ClinicalTrials.gov Identifier |
|---|---|---|---|
| Phase 1/2 | CYP17 Lyase and Androgen Receptor Inhibitor treatment with Seviteronel | Seviteronel | NCT02580448 |
| Phase 1 Phase 2 | A phase I/II, single arm, non-randomized study of Ribociclib (LEE011), a CDK 4/6 inhibitor, in combination with Bicalutamide, an androgen receptor (AR) inhibitor | Ribociclib Bicalutamide | NCT03090165 |
| Phase 1 Phase 2 | A phase Ib/II trial of Taselisib (GDC-0032), a PI3K inhibitor, in combination with Enzalutamide in patient with AR+veTNBC | Enzalutamide Taselisib | NCT02457910 |
| Phase 2 | A phase 2 open-label study to evaluate the efficacy and safety of VT-464, previous treatment with Enzalutamide | VT-464 | NCT02130700 |
| Phase 1 | Clinical Trial | Treatment | ClinicalTrials.gov Identifier |
|---|---|---|---|
| Phase 2 | Neoadjuvant chemotherapy Docetaxel with or without Selumetinib in patients with TNBC | Selumetinib Doxorubicin Cyclophosphamide Docetaxel | NCT02685657 |
| Early phase 1 | Defining the TNBC kinome response to GSK1120212, MEK inhibitor | GSK1120212 | NCT01467310 |
| Phase 2 | A single arm, phase II study of single agent Trametinib followed by Trametinib in combination with GSK21411795 | Tramedtinib GSK21411795 | NCT01964924 |
| Phase 1 | Safety, pharmacokinetics (PK) of AKT and MEK combination | GSK1120212 GSK21411795 | NCT01138085 |
| Phase 1 | A study to investigate safety, pharmacokinetics and pharmacodynamics of BKM120 plus GSK1120212 | BKM120 GSK1121212 | NCT01155453 |
| Phase 1 | Safety, pharmacokinetics and pharmacodynamics of BKM120 plus MEK162 | BEZ235 MEK162 | NCT01337765 |
| Phase 1 | A phase Ib, Open-label, Multi-center, Dose-escalation, and Expansion Study of an Orally Administered Combination of BKM120 Plus MEK162 | BKM120 MEK162 | NCT01363232 |
| Phase | Clinical Trial | Treatment | ClinicalTrials.gov Identifier |
|---|---|---|---|
| Phase 2 | Study of CB-839 in combination w/Pacliatxel in patients of African Ancestry (AA) and Non-African (Non AA) Ancestry with Advanced TNBC | Glutaminase Inhibitor CB-839 in combination with Paclitaxel Cohort 1: AA, 3rd line+ Cohort 2: AA, 1st line Cohort 3: non-AA, 1st line Cohort 4: non-AA, 1st line | NCT03057600 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddharth, S.; Sharma, D. Racial Disparity and Triple-Negative Breast Cancer in African-American Women: A Multifaceted Affair between Obesity, Biology, and Socioeconomic Determinants. Cancers 2018, 10, 514. https://doi.org/10.3390/cancers10120514
Siddharth S, Sharma D. Racial Disparity and Triple-Negative Breast Cancer in African-American Women: A Multifaceted Affair between Obesity, Biology, and Socioeconomic Determinants. Cancers. 2018; 10(12):514. https://doi.org/10.3390/cancers10120514
Chicago/Turabian StyleSiddharth, Sumit, and Dipali Sharma. 2018. "Racial Disparity and Triple-Negative Breast Cancer in African-American Women: A Multifaceted Affair between Obesity, Biology, and Socioeconomic Determinants" Cancers 10, no. 12: 514. https://doi.org/10.3390/cancers10120514
APA StyleSiddharth, S., & Sharma, D. (2018). Racial Disparity and Triple-Negative Breast Cancer in African-American Women: A Multifaceted Affair between Obesity, Biology, and Socioeconomic Determinants. Cancers, 10(12), 514. https://doi.org/10.3390/cancers10120514





