Recent Advances in Oncolytic Virotherapy and Immunotherapy for Glioblastoma: A Glimmer of Hope in the Search for an Effective Therapy?
Abstract
1. Introduction
2. Standard Therapy for Adult Glioblastoma: 15 Years of Experience and TTFields
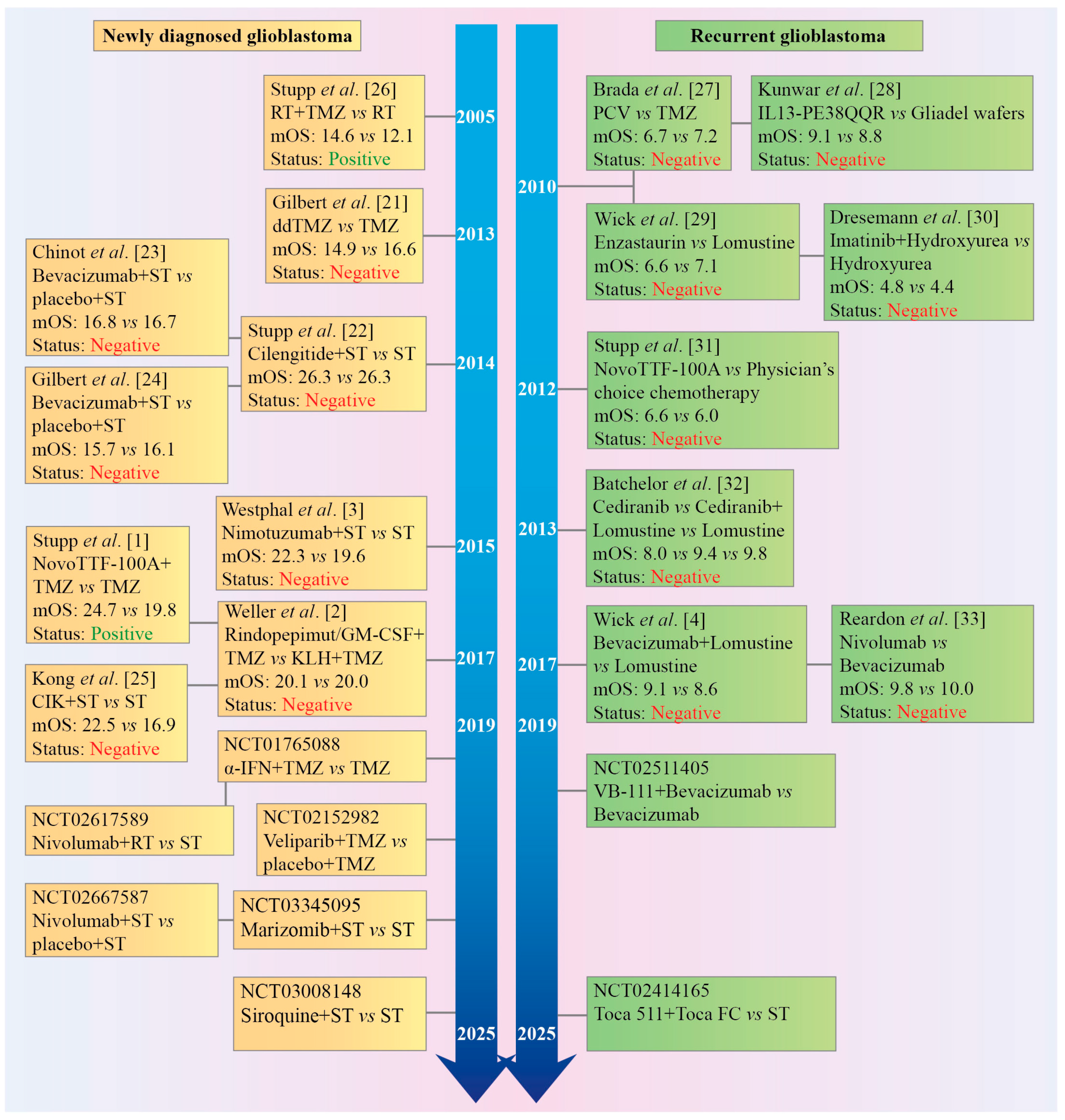
3. Revision of the Landmark Stupp Trial as a Historical Control for Median Overall Survival in Non-Controlled Clinical Trials
4. No Molecularly Targeted Drug(s) for Glioblastoma on the Horizon
5. Dendritic Cell/Peptide Vaccines and CAR T-cells for Glioblastoma Treatment: A Need for Large Controlled Trials to Prove Efficacy
6. Oncolytic Virotherapy for Glioma/Glioblastoma Treatment at Recurrence: Feasibility and Safety in Phase I Trials with Promising Efficacy in Subsets of Patients
7. Is a Benefit Derived from Immunotherapy/Oncolytic Virotherapy Correlated with a Degree of Immunosuppression?
8. Conclusions
Funding
Conflicts of Interest
References
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients with Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef]
- Westphal, M.; Heese, O.; Steinbach, J.P.; Schnell, O.; Schackert, G.; Mehdorn, M.; Schulz, D.; Simon, M.; Schlegel, U.; Senft, C.; et al. A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur. J. Cancer 2015, 51, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Gorlia, T.; Bendszus, M.; Taphoorn, M.; Sahm, F.; Harting, I.; Brandes, A.A.; Taal, W.; Domont, J.; Idbaih, A.; et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N. Engl. J. Med. 2017, 377, 1954–1963. [Google Scholar] [CrossRef] [PubMed]
- Seystahl, K.; Wick, W.; Weller, M. Therapeutic options in recurrent glioblastoma—An update. Crit. Rev. Oncol. Hematol. 2016, 99, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Omuro, A.; Brandes, A.A.; Rieger, J.; Wick, A.; Sepulveda, J.; Phuphanich, S.; de Souza, P.; Ahluwalia, M.S.; Lim, M.; et al. OS10.3 Randomized Phase 3 Study Evaluating the Efficacy and Safety of Nivolumab vs. Bevacizumab in Patients with Recurrent Glioblastoma: CheckMate 143. Neuro Oncol. 2017, 19, iii21. [Google Scholar] [CrossRef]
- Tipping, M.; Eickhoff, J.; Ian Robins, H. Clinical outcomes in recurrent glioblastoma with bevacizumab therapy: An analysis of the literature. J. Clin. Neurosci. 2017, 44, 101–106. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Batich, K.A.; Gunn, M.D.; Huang, M.-N.; Sanchez-Perez, L.; Nair, S.K.; Congdon, K.L.; Reap, E.A.; Archer, G.E.; Desjardins, A.; et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 2015, 519, 366–369. [Google Scholar] [CrossRef]
- Angelova, A.L.; Geletneky, K.; Nüesch, J.P.F.; Rommelaere, J. Tumor Selectivity of Oncolytic Parvoviruses: From in vitro and Animal Models to Cancer Patients. Front. Bioeng. Biotechnol. 2015, 3, 55. [Google Scholar] [CrossRef]
- Garg, A.D.; Vandenberk, L.; Koks, C.; Verschuere, T.; Boon, L.; Van Gool, S.W.; Agostinis, P. Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma. Sci. Transl. Med. 2016, 8, 328ra27. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Chekhonin, V.P. A compendium of adenovirus genetic modifications for enhanced replication, oncolysis, and tumor immunosurveillance in cancer therapy. Gene 2018, 679, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, A.A.; Chekhonin, V.P. Tropism and transduction of oncolytic adenovirus 5 vectors in cancer therapy: Focus on fiber chimerism and mosaicism, hexon and pIX. Virus Res. 2018, 257, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.; Gromeier, M.; Herndon, J.E.; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Geletneky, K.; Hajda, J.; Angelova, A.L.; Leuchs, B.; Capper, D.; Bartsch, A.J.; Neumann, J.-O.; Schöning, T.; Hüsing, J.; Beelte, B.; et al. Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/IIa Glioblastoma Trial. Mol. Ther. 2017, 25, 2620–2634. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Landolfi, J.; Vogelbaum, M.A.; Ostertag, D.; Elder, J.B.; Bloomfield, S.; Carter, B.; Chen, C.C.; Kalkanis, S.N.; Kesari, S.; et al. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro Oncol. 2018, 20, 1383–1392. [Google Scholar] [CrossRef]
- Bloch, O.; Lim, M.; Sughrue, M.E.; Komotar, R.J.; Abrahams, J.M.; O’Rourke, D.M.; D’Ambrosio, A.; Bruce, J.N.; Parsa, A.T. Autologous Heat Shock Protein Peptide Vaccination for Newly Diagnosed Glioblastoma: Impact of Peripheral PD-L1 Expression on Response to Therapy. Clin. Cancer Res. 2017, 23, 3575–3584. [Google Scholar] [CrossRef]
- Ji, N.; Zhang, Y.; Liu, Y.; Xie, J.; Wang, Y.; Hao, S.; Gao, Z. Heat shock protein peptide complex-96 vaccination for newly diagnosed glioblastoma: A phase I, single-arm trial. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Batich, K.A.; Reap, E.A.; Archer, G.E.; Sanchez-Perez, L.; Nair, S.K.; Schmittling, R.J.; Norberg, P.; Xie, W.; Herndon, J.E.; Healy, P.; et al. Long-term Survival in Glioblastoma with Cytomegalovirus pp65-Targeted Vaccination. Clin. Cancer Res. 2017, 23, 1898–1909. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D’Andre, S.D.; et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 2018, 16, 142. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Wang, M.; Aldape, K.D.; Stupp, R.; Hegi, M.E.; Jaeckle, K.A.; Armstrong, T.S.; Wefel, J.S.; Won, M.; Blumenthal, D.T.; et al. Dose-Dense Temozolomide for Newly Diagnosed Glioblastoma: A Randomized Phase III Clinical Trial. J. Clin. Oncol. 2013, 31, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Gorlia, T.; Erridge, S.C.; Perry, J.; Hong, Y.-K.; Aldape, K.D.; Lhermitte, B.; Pietsch, T.; Grujicic, D.; et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1100–1108. [Google Scholar] [CrossRef]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus Radiotherapy–Temozolomide for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.-S.; Nam, D.-H.; Kang, S.-H.; Lee, J.W.; Chang, J.-H.; Kim, J.-H.; Lim, Y.-J.; Koh, Y.-C.; Chung, Y.-G.; Kim, J.-M.; et al. Phase III randomized trial of autologous cytokine-induced killer cell immunotherapy for newly diagnosed glioblastoma in Korea. Oncotarget 2017, 8, 7003–7013. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Brada, M.; Stenning, S.; Gabe, R.; Thompson, L.C.; Levy, D.; Rampling, R.; Erridge, S.; Saran, F.; Gattamaneni, R.; Hopkins, K.; et al. Temozolomide Versus Procarbazine, Lomustine, and Vincristine in Recurrent High-Grade Glioma. J. Clin. Oncol. 2010, 28, 4601–4608. [Google Scholar] [CrossRef]
- Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z.; Piepmeier, J.; Prados, M.; et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro. Oncol. 2010, 12, 871–881. [Google Scholar] [CrossRef]
- Wick, W.; Puduvalli, V.K.; Chamberlain, M.C.; van den Bent, M.J.; Carpentier, A.F.; Cher, L.M.; Mason, W.; Weller, M.; Hong, S.; Musib, L.; et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J. Clin. Oncol. 2010, 28, 1168–1174. [Google Scholar] [CrossRef]
- Dresemann, G.; Weller, M.; Rosenthal, M.A.; Wedding, U.; Wagner, W.; Engel, E.; Heinrich, B.; Mayer-Steinacker, R.; Karup-Hansen, A.; Fluge, Ø.; et al. Imatinib in combination with hydroxyurea versus hydroxyurea alone as oral therapy in patients with progressive pretreated glioblastoma resistant to standard dose temozolomide. J. Neurooncol. 2010, 96, 393–402. [Google Scholar] [CrossRef]
- Stupp, R.; Wong, E.T.; Kanner, A.A.; Steinberg, D.; Engelhard, H.; Heidecke, V.; Kirson, E.D.; Taillibert, S.; Liebermann, F.; Dbalý, V.; et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur. J. Cancer 2012, 48, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.T.; Mulholland, P.; Neyns, B.; Nabors, L.B.; Campone, M.; Wick, A.; Mason, W.; Mikkelsen, T.; Phuphanich, S.; Ashby, L.S.; et al. Phase III Randomized Trial Comparing the Efficacy of Cediranib As Monotherapy, and in Combination With Lomustine, Versus Lomustine Alone in Patients With Recurrent Glioblastoma. J. Clin. Oncol. 2013, 31, 3212–3218. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Omuro, A.; Brandes, A.A.; Rieger, J.; Wick, A.; Sepulveda, J.; Phuphanich, S.; de Souza, P.; Ahluwalia, M.S.; Lim, M.; et al. OS10.3 Randomized Phase 3 Study Evaluating the Efficacy and Safety of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: CheckMate 143. Neuro. Oncol. 2017, 19, iii21. [Google Scholar] [CrossRef]
- Lillehei, K.O.; Kalkanis, S.N.; Liau, L.M.; Mydland, D.E.; Olson, J.; Paleologos, N.A.; Ryken, T.; Johnson, T.; Scullin, E. Rationale and design of the 500-patient, 3-year, and prospective Vigilant ObservatIon of GlIadeL WAfer ImplaNT registry. CNS Oncol. 2018, 7, CNS08. [Google Scholar] [CrossRef] [PubMed]
- Ashby, L.S.; Smith, K.A.; Stea, B. Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: A systematic literature review. World J. Surg. Oncol. 2016, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Bregy, A.; Shah, A.H.; Diaz, M.V.; Pierce, H.E.; Ames, P.L.; Diaz, D.; Komotar, R.J. The role of Gliadel wafers in the treatment of high-grade gliomas. Expert Rev. Anticancer Ther. 2013, 13, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, S.A.; Ryken, T.; Newton, H.B. Survival outcomes and safety of carmustine wafers in the treatment of high-grade gliomas: A meta-analysis. J. Neurooncol. 2015, 122, 367–382. [Google Scholar] [CrossRef]
- Nagpal, S. The Role of BCNU Polymer Wafers (Gliadel) in the Treatment of Malignant Glioma. Neurosurg. Clin. 2012, 23, 289–295. [Google Scholar] [CrossRef]
- Xing, W.; Shao, C.; Qi, Z.; Yang, C.; Wang, Z. The role of Gliadel wafers in the treatment of newly diagnosed GBM: A meta-analysis. Drug Des. Dev. Ther. 2015, 9, 3341–3348. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection with Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Almenawer, S.A.; Badhiwala, J.H.; Alhazzani, W.; Greenspoon, J.; Farrokhyar, F.; Yarascavitch, B.; Algird, A.; Kachur, E.; Cenic, A.; Sharieff, W.; et al. Biopsy versus partial versus gross total resection in older patients with high-grade glioma: A systematic review and meta-analysis. Neuro Oncol. 2015, 17, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Fang, C.; Chen, G.; Sun, C. Relationship between the extent of resection and the survival of patients with low-grade gliomas: A systematic review and meta-analysis. BMC Cancer 2018, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- Cata, J.P.; Hagan, K.B.; Bhavsar, S.D.O.; Arunkumar, R.; Grasu, R.; Dang, A.; Carlson, R.; Arnold, B.; Potylchansky, Y.; Lipski, I.; et al. The use of isoflurane and desflurane as inhalational agents for glioblastoma surgery. A survival analysis. J. Clin. Neurosci. 2017, 35, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Kazda, T.; Dziacky, A.; Burkon, P.; Pospisil, P.; Slavik, M.; Rehak, Z.; Jancalek, R.; Slampa, P.; Slaby, O.; Lakomy, R. Radiotherapy of glioblastoma 15 years after the landmark Stupp’s trial: More controversies than standards? Radiol. Oncol. 2018, 52, 121–128. [Google Scholar] [CrossRef]
- Skardelly, M.; Dangel, E.; Gohde, J.; Noell, S.; Behling, F.; Lepski, G.; Borchers, C.; Koch, M.; Schittenhelm, J.; Bisdas, S.; et al. Prolonged Temozolomide Maintenance Therapy in Newly Diagnosed Glioblastoma. Oncologist 2017, 22, 570–575. [Google Scholar] [CrossRef]
- Blumenthal, D.T.; Gorlia, T.; Gilbert, M.R.; Kim, M.M.; Burt Nabors, L.; Mason, W.P.; Hegi, M.E.; Zhang, P.; Golfinopoulos, V.; Perry, J.R.; et al. Is more better? The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma: A secondary analysis of EORTC and NRG Oncology/RTOG. Neuro Oncol. 2017, 19, 1119–1126. [Google Scholar] [CrossRef]
- Gramatzki, D.; Kickingereder, P.; Hentschel, B.; Felsberg, J.; Herrlinger, U.; Schackert, G.; Tonn, J.-C.; Westphal, M.; Sabel, M.; Schlegel, U.; et al. Limited role for extended maintenance temozolomide for newly diagnosed glioblastoma. Neurology 2017, 88, 1422–1430. [Google Scholar] [CrossRef]
- Xu, W.; Li, T.; Gao, L.; Zheng, J.; Shao, A.; Zhang, J. Efficacy and safety of long-term therapy for high-grade glioma with temozolomide: A meta-analysis. Oncotarget 2017, 8, 51758–51765. [Google Scholar] [CrossRef]
- Deutsch, M.B.; Panageas, K.S.; Lassman, A.B.; DeAngelis, L.M. Steroid management in newly diagnosed glioblastoma. J. Neurooncol. 2013, 113, 111–116. [Google Scholar] [CrossRef]
- Ly, K.I.; Wen, P.Y. Clinical Relevance of Steroid Use in Neuro-Oncology. Curr. Neurol. Neurosci. Rep. 2017, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Hohwieler Schloss, M.; Freidberg, S.R.; Heatley, G.J.; Lo, T.C. Glucocorticoid dependency as a prognostic factor in radiotherapy for cerebral gliomas. Acta Oncol. 1989, 28, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Watne, K.; Hannisdal, E.; Nome, O.; Hager, B.; Hirschberg, H. Prognostic factors in malignant gliomas with special reference to intra-arterial chemotherapy. Acta Oncol. 1993, 32, 307–310. [Google Scholar] [CrossRef]
- Tieu, M.T.; Lovblom, L.E.; McNamara, M.G.; Mason, W.; Laperriere, N.; Millar, B.-A.; Ménard, C.; Kiehl, T.-R.; Perkins, B.A.; Chung, C. Impact of glycemia on survival of glioblastoma patients treated with radiation and temozolomide. J. Neurooncol. 2015, 124, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, S.R.; Christensen, I.J.; Grunnet, K.; Stockhausen, M.-T.; Broholm, H.; Kosteljanetz, M.; Poulsen, H.S. Clinical variables serve as prognostic factors in a model for survival from glioblastoma multiforme: An observational study of a cohort of consecutive non-selected patients from a single institution. BMC Cancer 2013, 13, 402. [Google Scholar] [CrossRef] [PubMed]
- Shields, L.B.E.; Shelton, B.J.; Shearer, A.J.; Chen, L.; Sun, D.A.; Parsons, S.; Bourne, T.D.; LaRocca, R.; Spalding, A.C. Dexamethasone administration during definitive radiation and temozolomide renders a poor prognosis in a retrospective analysis of newly diagnosed glioblastoma patients. Radiat. Oncol. 2015, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, O.N.; Mohammad, A.-A.; Bartek, J.; Winter, J.; Jung, M.; Stragliotto, G.; Söderberg-Nauclér, C.; Landázuri, N. Glucocorticoids promote a glioma stem cell-like phenotype and resistance to chemotherapy in human glioblastoma primary cells: Biological and prognostic significance. Int. J. Cancer 2017. [Google Scholar] [CrossRef] [PubMed]
- Díez Valle, R.; Becerra Castro, V.; Marigil Sánchez, M.; Gállego Pérez-Larraya, J.; Núñez-Córdoba, J.M.; Tejada Solis, S. Results of a Policy of Fast Tapering of Steroids After Resection Surgery in Glioblastoma. World Neurosurg. 2018, 109, e845–e852. [Google Scholar] [CrossRef]
- Pitter, K.L.; Tamagno, I.; Alikhanyan, K.; Hosni-Ahmed, A.; Pattwell, S.S.; Donnola, S.; Dai, C.; Ozawa, T.; Chang, M.; Chan, T.A.; et al. Corticosteroids compromise survival in glioblastoma. Brain 2016, 139, 1458–1471. [Google Scholar] [CrossRef]
- Van Linde, M.E.; Brahm, C.G.; de Witt Hamer, P.C.; Reijneveld, J.C.; Bruynzeel, A.M.E.; Vandertop, W.P.; van de Ven, P.M.; Wagemakers, M.; van der Weide, H.L.; Enting, R.H.; et al. Treatment outcome of patients with recurrent glioblastoma multiforme: A retrospective multicenter analysis. J. Neurooncol. 2017. [Google Scholar] [CrossRef]
- Duerinck, J.; Du Four, S.; Bouttens, F.; Andre, C.; Verschaeve, V.; Van Fraeyenhove, F.; Chaskis, C.; D’Haene, N.; Le Mercier, M.; Rogiers, A.; et al. Randomized phase II trial comparing axitinib with the combination of axitinib and lomustine in patients with recurrent glioblastoma. J. Neurooncol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.T.; Lok, E.; Gautam, S.; Swanson, K.D. Dexamethasone exerts profound immunologic interference on treatment efficacy for recurrent glioblastoma. Br. J. Cancer 2015, 113, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Zen, M.; Canova, M.; Campana, C.; Bettio, S.; Nalotto, L.; Rampudda, M.; Ramonda, R.; Iaccarino, L.; Doria, A. The kaleidoscope of glucorticoid effects on immune system. Autoimmun. Rev. 2011, 10, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Thaci, B.; Crawford, A.C.; Sampath, P. Interleukin-13 Receptor Alpha 2-Targeted Glioblastoma Immunotherapy. Biomed. Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, M.P.; Lin, Y.; New, K.C.; Bulur, P.A.; O’Neill, B.P.; Gastineau, D.A.; Dietz, A.B. Systemic immune suppression in glioblastoma: The interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010, 12, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Chitadze, G.; Flüh, C.; Quabius, E.S.; Freitag-Wolf, S.; Peters, C.; Lettau, M.; Bhat, J.; Wesch, D.; Oberg, H.-H.; Luecke, S.; et al. In-depth immunophenotyping of patients with glioblastoma multiforme: Impact of steroid treatment. Oncoimmunology 2017, 6, e1358839. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.M.; McDonnell, A.M.; Lake, R.A.; Nowak, A.K. Dexamethasone co-medication in cancer patients undergoing chemotherapy causes substantial immunomodulatory effects with implications for chemo-immunotherapy strategies. Oncoimmunology 2016, 5, e1066062. [Google Scholar] [CrossRef] [PubMed]
- Knudsen-Baas, K.M.; Engeland, A.; Gilhus, N.E.; Storstein, A.M.; Owe, J.F. Does the choice of antiepileptic drug affect survival in glioblastoma patients? J. Neurooncol. 2016, 129, 461–469. [Google Scholar] [CrossRef]
- Vecht, C.J.; Kerkhof, M.; Duran-Pena, A. Seizure Prognosis in Brain Tumors: New Insights and Evidence-Based Management. Oncologist 2014, 19, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Bruna, J.; Miró, J.; Velasco, R. Epilepsy in glioblastoma patients: Basic mechanisms and current problems in treatment. Expert Rev. Clin. Pharmacol. 2013, 6, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, G.D.; Eljamel, S. Impact of particular antiepileptic drugs on the survival of patients with glioblastoma multiforme. J. Neurosurg. 2013, 118, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Kuwabara, Y.; Suehiro, S.; Yamashita, D.; Tanaka, M.; Tanaka, A.; Ohue, S.; Araki, H. Valproic acid reduces hair loss and improves survival in patients receiving temozolomide-based radiation therapy for high-grade glioma. Eur. J. Clin. Pharmacol. 2017, 73, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-C.; Wei, K.-C.; Tsai, C.-N.; Huang, Y.-C.; Chen, P.-Y.; Chen, S.-M.; Lu, Y.-J.; Lee, S.-T. Effect of valproic acid on the outcome of glioblastoma multiforme. Br. J. Neurosurg. 2012, 26, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, M.; Dielemans, J.C.M.; van Breemen, M.S.; Zwinkels, H.; Walchenbach, R.; Taphoorn, M.J.; Vecht, C.J. Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro Oncol. 2013, 15, 961–967. [Google Scholar] [CrossRef]
- Barker, C.A.; Bishop, A.J.; Chang, M.; Beal, K.; Chan, T.A. Valproic Acid Use During Radiation Therapy for Glioblastoma Associated With Improved Survival. Int. J. Radiat. Oncol. 2013, 86, 504–509. [Google Scholar] [CrossRef]
- Rudà, R.; Pellerino, A.; Soffietti, R. Does valproic acid affect tumor growth and improve survival in glioblastomas? CNS Oncol. 2016, 5, 51–53. [Google Scholar] [CrossRef]
- Weller, M.; Gorlia, T.; Cairncross, J.G.; van den Bent, M.J.; Mason, W.; Belanger, K.; Brandes, A.A.; Bogdahn, U.; Macdonald, D.R.; Forsyth, P.; et al. Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology 2011, 77, 1156–1164. [Google Scholar] [CrossRef]
- Redjal, N.; Reinshagen, C.; Le, A.; Walcott, B.P.; McDonnell, E.; Dietrich, J.; Nahed, B.V. Valproic acid, compared to other antiepileptic drugs, is associated with improved overall and progression-free survival in glioblastoma but worse outcome in grade II/III gliomas treated with temozolomide. J. Neurooncol. 2016, 127, 505–514. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiang, W.; Qing, M.; Yanhui, L.; Jiewen, L.; Yunhe, M. Survival analysis for valproic acid use in adult glioblastoma multiforme: A meta-analysis of individual patient data and a systematic review. Seizure 2014, 23, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Kim, T.; Joo, J.-D.; Han, J.H.; Kim, Y.J.; Kim, I.A.; Yun, C.-H.; Kim, C.-Y. Survival benefit of levetiracetam in patients treated with concomitant chemoradiotherapy and adjuvant chemotherapy with temozolomide for glioblastoma multiforme. Cancer 2015, 121, 2926–2932. [Google Scholar] [CrossRef] [PubMed]
- Valiyaveettil, D.; Malik, M.; Joseph, D.; Ahmed, S.; Kothwal, S.; Vijayasaradhi, M. Effect of valproic acid on survival in glioblastoma: A prospective single-arm study. South Asian J. Cancer 2018, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Felix, F.H.C.; de Araujo, O.L.; da Trindade, K.M.; Trompieri, N.M.; Fontenele, J.B. Survival of children with malignant brain tumors receiving valproate: A retrospective study. Childs. Nerv. Syst. 2013, 29, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Breemen, M.S.M.; Rijsman, R.M.; Taphoorn, M.J.B.; Walchenbach, R.; Zwinkels, H.; Vecht, C.J. Efficacy of anti-epileptic drugs in patients with gliomas and seizures. J. Neurol. 2009, 256, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, S.; Varkila, M.; Kroonen, J.; Seute, T.; Snijders, T.J.; Kauw, F.; Spliet, W.G.M.; Willems, M.; Poulet, C.; Broekman, M.L.; et al. Prognostic relevance of epilepsy at presentation in glioblastoma patients. Neuro Oncol. 2016, 18, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.; Sarria-Estrada, S.; Quintana, M.; Maldonado, X.; Martinez-Ricarte, F.; Rodon, J.; Auger, C.; Salas-Puig, J.; Santamarina, E.; Martinez-Saez, E. Prognostic implications of epilepsy in glioblastomas. Clin. Neurol. Neurosurg. 2015, 139, 166–171. [Google Scholar] [CrossRef]
- Happold, C.; Gorlia, T.; Chinot, O.; Gilbert, M.R.; Nabors, L.B.; Wick, W.; Pugh, S.L.; Hegi, M.; Cloughesy, T.; Roth, P.; et al. Does Valproic Acid or Levetiracetam Improve Survival in Glioblastoma? A Pooled Analysis of Prospective Clinical Trials in Newly Diagnosed Glioblastoma. J. Clin. Oncol. 2016, 34, 731–739. [Google Scholar] [CrossRef]
- Cote, D.J.; Smith, T.R. Venous thromboembolism in brain tumor patients. J. Clin. Neurosci. 2016, 25, 13–18. [Google Scholar] [CrossRef]
- Morgan, E.R.; Mason, W.P.; Maurice, C. A critical balance: Managing coagulation in patients with glioma. Expert Rev. Neurother. 2016, 16, 803–814. [Google Scholar] [CrossRef]
- Streiff, M.B.; Ye, X.; Kickler, T.S.; Desideri, S.; Jani, J.; Fisher, J.; Grossman, S.A. A prospective multicenter study of venous thromboembolism in patients with newly-diagnosed high-grade glioma: Hazard rate and risk factors. J. Neurooncol. 2015, 124, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Taillibert, S.; Taillandier, L.; Le Rhun, E. Venous thrombosis in patients with high-grade glioma. Curr. Opin. Oncol. 2015, 27, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Edwin, N.C.; Khoury, M.N.; Sohal, D.; McCrae, K.R.; Ahluwalia, M.S.; Khorana, A.A. Recurrent venous thromboembolism in glioblastoma. Thromb. Res. 2016, 137, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Schnoor, R.; Maas, S.L.N.; Broekman, M.L.D. Heparin in malignant glioma: Review of preclinical studies and clinical results. J. Neurooncol. 2015, 124, 151–156. [Google Scholar] [CrossRef] [PubMed]
- De Vos, F.Y.; Gijtenbeek, J.M.; Bleeker-Rovers, C.P.; van Herpen, C.M. Pneumocystis jirovecii pneumonia prophylaxis during temozolomide treatment for high-grade gliomas. Crit. Rev. Oncol. Hematol. 2013, 85, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, A.J.; Nguyen, T.M.; Fu, R.; Bubalo, J.; Tyson, R.M.; Lacy, C.; Gahramanov, S.; Nasseri, M.; Barnes, P.D.; Neuwelt, E.A. Incidence of Pneumocystis jirovecii pneumonia after temozolomide for CNS malignancies without prophylaxis. CNS Oncol. 2014, 3, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Wen, P.; Nishikawa, R.; Reardon, D.; Peters, K. Critical review of the addition of tumor treating fields (TTFields) to the existing standard of care for newly diagnosed glioblastoma patients. Crit. Rev. Oncol. Hematol. 2017, 111, 60–65. [Google Scholar] [CrossRef]
- Zhu, P.; Zhu, J.-J. Tumor treating fields: A novel and effective therapy for glioblastoma: Mechanism, efficacy, safety and future perspectives. Chin. Clin. Oncol. 2017, 6, 41. [Google Scholar] [CrossRef]
- Magouliotis, D.E.; Asprodini, E.K.; Svokos, K.A.; Tasiopoulou, V.S.; Svokos, A.A.; Toms, S.A. Tumor-treating fields as a fourth treating modality for glioblastoma: A meta-analysis. Acta Neurochir. (Wien) 2018, 160, 1167–1174. [Google Scholar] [CrossRef]
- Guzauskas, G.F.; Salzberg, M.; Wang, B.C. Estimated lifetime survival benefit of tumor treating fields and temozolomide for newly diagnosed glioblastoma patients. CNS Oncol. 2018. [Google Scholar] [CrossRef]
- Roh, T.H.; Park, H.H.; Kang, S.-G.; Moon, J.H.; Kim, E.H.; Hong, C.-K.; Ahn, S.S.; Choi, H.J.; Cho, J.; Kim, S.H.; et al. Long-term outcomes of concomitant chemoradiotherapy with temozolomide for newly diagnosed glioblastoma patients. Medicine (Baltimore). 2017, 96, e7422. [Google Scholar] [CrossRef] [PubMed]
- Mandel, J.J.; Yust-Katz, S.; Patel, A.J.; Cachia, D.; Liu, D.; Park, M.; Yuan, Y.; Kent, T.A.; de Groot, J.F. Inability of positive phase II clinical trials of investigational treatments to subsequently predict positive phase III clinical trials in glioblastoma. Neuro Oncol. 2018, 20, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Mandel, J.J.; Youssef, M.; Ludmir, E.; Yust-Katz, S.; Patel, A.J.; De Groot, J.F. Highlighting the need for reliable clinical trials in glioblastoma. Expert Rev. Anticancer Ther. 2018, 18, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Go, no-go decision making for phase 3 clinical trials: ACT IV revisited—Authors’ reply. Lancet Oncol. 2017, 18, e708. [Google Scholar] [CrossRef]
- Nguyen, H.T.N.; Grogan, P.; Robins, H.I. Go, no-go decision making for phase 3 clinical trials: ACT IV revisited. Lancet Oncol. 2017, 18, e708. [Google Scholar] [CrossRef]
- Vanderbeek, A.M.; Rahman, R.; Fell, G.; Ventz, S.; Chen, T.; Redd, R.; Parmigiani, G.; Cloughesy, T.F.; Wen, P.Y.; Trippa, L.; et al. The clinical trials landscape for glioblastoma: Is it adequate to develop new treatments? Neuro Oncol. 2018, 20, 1034–1043. [Google Scholar] [CrossRef]
- Porter, K.R.; McCarthy, B.J.; Berbaum, M.L.; Davis, F.G. Conditional survival of all primary brain tumor patients by age, behavior, and histology. Neuroepidemiology 2011, 36, 230–239. [Google Scholar] [CrossRef]
- Darefsky, A.S.; King, J.T.; Dubrow, R. Adult glioblastoma multiforme survival in the temozolomide era: A population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer 2012, 118, 2163–2172. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro Oncol. 2014, 16, iv1–iv63. [Google Scholar] [CrossRef]
- Tykocki, T.; Eltayeb, M. Ten-year survival in glioblastoma. A systematic review. J. Clin. Neurosci. 2018, 54, 7–13. [Google Scholar] [CrossRef]
- Cihoric, N.; Tsikkinis, A.; Minniti, G.; Lagerwaard, F.J.; Herrlinger, U.; Mathier, E.; Soldatovic, I.; Jeremic, B.; Ghadjar, P.; Elicin, O.; et al. Current status and perspectives of interventional clinical trials for glioblastoma—Analysis of ClinicalTrials.gov. Radiat. Oncol. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.A.; Pignon, J.-P.; Blanchard, P.; Lefeuvre, D.; Levy, A.; Touat, M.; Louvel, G.; Dhermain, F.; Soria, J.-C.; Deutsch, E.; et al. Systematic review and meta-analysis of phase I/II targeted therapy combined with radiotherapy in patients with glioblastoma multiforme: Quality of report, toxicity, and survival. J. Neurooncol. 2015, 123, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Cai, M.; Li, W.; Hou, B.; He, H.; Ling, C.; Huang, T.; Liu, H.; Guo, Y. Molecularly Targeted Drugs Plus Radiotherapy and Temozolomide Treatment for Newly Diagnosed Glioblastoma: A Meta-Analysis and Systematic Review. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2016, 24, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.-W.; Morgan, E.R.; Mason, W.P. Contemporary management of high-grade gliomas. CNS Oncol. 2018, 7, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.; Cheng, J.; Zhang, X.; Liu, B. The treatment of glioblastomas: A systematic update on clinical Phase III trials. Crit. Rev. Oncol. Hematol. 2013, 87, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Guishard, A.F.; Yakisich, J.S.; Azad, N.; Iyer, A.K.V. Translational gap in ongoing clinical trials for glioma. J. Clin. Neurosci. 2018, 47, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.M.; Trippa, L.; Gaffey, S.C.; Arrillaga, I.; Lee, E.Q.; Tanguturi, S.K.; Ahluwalia, M.S.; Colman, H.; Galanis, E.; De Groot, J.F.; et al. Individualized screening trial of innovative glioblastoma therapy (INSIGhT). J. Clin. Oncol. 2017, 35, TPS2079. [Google Scholar] [CrossRef]
- Prasad, V. Perspective: The precision-oncology illusion. Nature 2016, 537, S63. [Google Scholar] [CrossRef]
- Joyner, M.J.; Paneth, N.; Ioannidis, J.P.A. What Happens When Underperforming Big Ideas in Research Become Entrenched? JAMA 2016, 316, 1355–1356. [Google Scholar] [CrossRef]
- Tannock, I.F.; Hickman, J.A. Limits to Personalized Cancer Medicine. N. Engl. J. Med. 2016, 375, 1289–1294. [Google Scholar] [CrossRef]
- Massard, C.; Michiels, S.; Ferté, C.; Le Deley, M.-C.; Lacroix, L.; Hollebecque, A.; Verlingue, L.; Ileana, E.; Rosellini, S.; Ammari, S.; et al. High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discov. 2017, 7, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Delord, J.-P.; Gonçalves, A.; Gavoille, C.; Dubot, C.; Isambert, N.; Campone, M.; Trédan, O.; Massiani, M.-A.; Mauborgne, C.; et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015, 16, 1324–1334. [Google Scholar] [CrossRef]
- Wirsching, H.-G.; Tabatabai, G.; Roelcke, U.; Hottinger, A.F.; Jörger, F.; Schmid, A.; Plasswilm, L.; Schrimpf, D.; Mancao, C.; Capper, D.; et al. Bevacizumab plus hypofractionated radiotherapy versus radiotherapy alone in elderly patients with glioblastoma: The randomized, open-label, phase II ARTE trial. Ann. Oncol. 2018, 29, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Pambuku, A.; Bellu, L.; Farina, M.; Della Puppa, A.; Denaro, L.; Zagonel, V. Effectiveness of antiangiogenic drugs in glioblastoma patients: A systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Oncol. Hematol. 2017, 111, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xue, Y.-Q.; Zhao, M.-M.; Xu, P. Effectiveness of lomustine and bevacizumab in progressive glioblastoma: A meta-analysis. Onco. Targets Ther. 2018, 11, 3435–3439. [Google Scholar] [CrossRef] [PubMed]
- Taal, W.; Oosterkamp, H.M.; Walenkamp, A.M.E.; Dubbink, H.J.; Beerepoot, L.V.; Hanse, M.C.J.; Buter, J.; Honkoop, A.H.; Boerman, D.; de Vos, F.Y.F.; et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol. 2014, 15, 943–953. [Google Scholar] [CrossRef]
- Kim, M.M.; Umemura, Y.; Leung, D. Bevacizumab and Glioblastoma. Cancer J. 2018, 24, 180–186. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, N.; Zhao, C.; Xue, T.; Wu, X.; Wang, Z. Bevacizumab combined with chemotherapy vs single-agent therapy in recurrent glioblastoma: Evidence from randomized controlled trials. Cancer Manag. Res. 2018, 10, 2193–2205. [Google Scholar] [CrossRef] [PubMed]
- Van den Bent, M.J.; Klein, M.; Smits, M.; Reijneveld, J.C.; French, P.J.; Clement, P.; de Vos, F.Y.F.; Wick, A.; Mulholland, P.J.; Taphoorn, M.J.B.; et al. Bevacizumab and temozolomide in patients with first recurrence of WHO grade II and III glioma, without 1p/19q co-deletion (TAVAREC): A randomised controlled phase 2 EORTC trial. Lancet Oncol. 2018, 19, 1170–1179. [Google Scholar] [CrossRef]
- Yazici, G.; Cengiz, M.; Ozyigit, G.; Eren, G.; Yildiz, F.; Akyol, F.; Gurkaynak, M.; Zorlu, F. Hypofractionated stereotactic reirradiation for recurrent glioblastoma. J. Neurooncol. 2014, 120, 117–123. [Google Scholar] [CrossRef]
- Fogh, S.E.; Andrews, D.W.; Glass, J.; Curran, W.; Glass, C.; Champ, C.; Evans, J.J.; Hyslop, T.; Pequignot, E.; Downes, B.; et al. Hypofractionated Stereotactic Radiation Therapy: An Effective Therapy for Recurrent High-Grade Gliomas. J. Clin. Oncol. 2010, 28, 3048–3053. [Google Scholar] [CrossRef] [PubMed]
- Ciammella, P.; Podgornii, A.; Galeandro, M.; D’Abbiero, N.; Pisanello, A.; Botti, A.; Cagni, E.; Iori, M.; Iotti, C. Hypofractionated stereotactic radiation therapy for recurrent glioblastoma: Single institutional experience. Radiat. Oncol. 2013, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Wacker, U.; Gross, M.W.; Henzel, M.; Encheva, E.; Engenhart-Cabillic, R. Hypofractionated Stereotactic Reirradiation of Recurrent Glioblastomas. Strahlenther. Onkol. 2009, 185, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Fricke, H.; Junge, K.; Kobyakov, G.; Martens, T.; Heese, O.; Wiestler, B.; Schliesser, M.G.; von Deimling, A.; Pichler, J.; et al. A Phase II, Randomized, Study of Weekly APG101+Reirradiation versus Reirradiation in Progressive Glioblastoma. Clin. Cancer Res. 2014, 20, 6304–6313. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Bhamidipati, D.; Song, A.; Eldredge-Hindy, H.B.; Siglin, J.; Dan, T.D.; Champ, C.E.; Zhang, I.; Bar-Ad, V.; Kim, L.; et al. Bevacizumab and re-irradiation for recurrent high grade gliomas: Does sequence matter? J. Neurooncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Filley, A.C.; Henriquez, M.; Dey, M. Recurrent glioma clinical trial, CheckMate-143: The game is not over yet. Oncotarget 2017, 8, 91779–91794. [Google Scholar] [CrossRef]
- Wilcox, J.A.; Ramakrishna, R.; Magge, R. Immunotherapy in Glioblastoma. World Neurosurg. 2018, 116, 518–528. [Google Scholar] [CrossRef]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- Naboush, A.; Roman, C.A.J.; Shapira, I. Immune checkpoint inhibitors in malignancies with mismatch repair deficiency: A review of the state of the current knowledge. J. Investig. Med. 2017, 65, 754–758. [Google Scholar] [CrossRef]
- Mouw, K.W.; Goldberg, M.S.; Konstantinopoulos, P.A.; D’Andrea, A.D. DNA Damage and Repair Biomarkers of Immunotherapy Response. Cancer Discov. 2017, 7, 675–693. [Google Scholar] [CrossRef]
- Viale, G.; Trapani, D.; Curigliano, G. Mismatch Repair Deficiency as a Predictive Biomarker for Immunotherapy Efficacy. Biomed. Res. Int. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Johanns, T.M.; Miller, C.A.; Dorward, I.G.; Tsien, C.; Chang, E.; Perry, A.; Uppaluri, R.; Ferguson, C.; Schmidt, R.E.; Dahiya, S.; et al. Immunogenomics of Hypermutated Glioblastoma: A Patient with Germline POLE Deficiency Treated with Checkpoint Blockade Immunotherapy. Cancer Discov. 2016, 6, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Bouffet, E.; Larouche, V.; Campbell, B.B.; Merico, D.; de Borja, R.; Aronson, M.; Durno, C.; Krueger, J.; Cabric, V.; Ramaswamy, V.; et al. Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. J. Clin. Oncol. 2016, 34, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Hodges, T.R.; Ott, M.; Xiu, J.; Gatalica, Z.; Swensen, J.; Zhou, S.; Huse, J.T.; de Groot, J.; Li, S.; Overwijk, W.W.; et al. Mutational burden, immune checkpoint expression, and mismatch repair in glioma: Implications for immune checkpoint immunotherapy. Neuro Oncol. 2017, 19, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, H.-Y.; Zhang, F.-C.; Sun, Y.; Xiong, Z.-Y.; Jiang, X.-B. Dendritic Cell-Based Vaccine for the Treatment of Malignant Glioma: A Systematic Review. Cancer Investig. 2014, 32, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-X.; Zhang, X.-Y.; Liu, J.-L.; Li, D.; Li, J.-L.; Liu, Y.-S.; Wang, M.; Xu, B.-L.; Wang, H.-B.; Wang, Z.-X. Clinical efficacy of tumor antigen-pulsed DC treatment for high-grade glioma patients: Evidence from a meta-analysis. PLoS ONE 2014, 9, e107173. [Google Scholar] [CrossRef] [PubMed]
- Artene, S.-A.; Turcu-Stiolica, A.; Ciurea, M.E.; Folcuti, C.; Tataranu, L.G.; Alexandru, O.; Purcaru, O.S.; Tache, D.E.; Boldeanu, M.V.; Silosi, C.; et al. Comparative effect of immunotherapy and standard therapy in patients with high grade glioma: A meta-analysis of published clinical trials. Sci. Rep. 2018, 8, 11800. [Google Scholar] [CrossRef] [PubMed]
- Hanaei, S.; Afshari, K.; Hirbod-Mobarakeh, A.; Mohajer, B.; Amir Dastmalchi, D.; Rezaei, N. Therapeutic efficacy of specific immunotherapy for glioma: A systematic review and meta-analysis. Rev. Neurosci. 2018, 29, 443–461. [Google Scholar] [CrossRef]
- Sokratous, G.; Polyzoidis, S.; Ashkan, K. Immune infiltration of tumor microenvironment following immunotherapy for glioblastoma multiforme. Hum. Vaccin. Immunother. 2017, 13, 2575–2582. [Google Scholar] [CrossRef]
- Wick, W.; van den Bent, M.J. First results on the DCVax phase III trial: Raising more questions than providing answers. Neuro Oncol. 2018, 20, 1283–1284. [Google Scholar] [CrossRef]
- Sampson, J.H.; Aldape, K.D.; Archer, G.E.; Coan, A.; Desjardins, A.; Friedman, A.H.; Friedman, H.S.; Gilbert, M.R.; Herndon, J.E.; McLendon, R.E.; et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011, 13, 324–333. [Google Scholar] [CrossRef]
- Schuster, J.; Lai, R.K.; Recht, L.D.; Reardon, D.A.; Paleologos, N.A.; Groves, M.D.; Mrugala, M.M.; Jensen, R.; Baehring, J.M.; Sloan, A.; et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: The ACT III study. Neuro Oncol. 2015, 17, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Desjardins, A.; Schuster, J.; Tran, D.D.; Fink, K.L.; Nabors, L.B.; Li, G.; Bota, D.A.; Lukas, R.V.; Ashby, L.S.; et al. IMCT-08ReACT: long-term survival from a randomized phase ii study of rindopepimut (CDX-110) plus bevacizumab in relapsed glioblastoma. Neuro Oncol. 2015, 17. [Google Scholar] [CrossRef]
- Wen, P.; Reardon, D.; Phuphanich, S.; Aiken, R.; Landolfi, J.; Curry, W.; Zhu, J.-J.; Glantz, M.; Peereboom, D.; Markert, J.; et al. A randomized, double-blind, placebo-controlled phase 2 trial of dendritic cell (DC) vaccination with ICT-107 in newly diagnosed glioblastoma (GBM) patients. J. Clin. Oncol. 2014, 32, 2005. [Google Scholar]
- Buchroithner, J.; Erhart, F.; Pichler, J.; Widhalm, G.; Preusser, M.; Stockhammer, G.; Nowosielski, M.; Iglseder, S.; Freyschlag, C.; Oberndorfer, S.; et al. Audencel Immunotherapy Based on Dendritic Cells Has No Effect on Overall and Progression-Free Survival in Newly Diagnosed Glioblastoma: A Phase II Randomized Trial. Cancers 2018, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.-Y.; Yang, W.-K.; Lee, H.-C.; Hsu, D.-M.; Lin, H.-L.; Lin, S.-Z.; Chen, C.-C.; Harn, H.-J.; Liu, C.-L.; Lee, W.-Y.; et al. Adjuvant immunotherapy with whole-cell lysate dendritic cells vaccine for glioblastoma multiforme: A phase II clinical trial. World Neurosurg. 2012, 77, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Inogés, S.; Tejada, S.; de Cerio, A.L.-D.; Gállego Pérez-Larraya, J.; Espinós, J.; Idoate, M.A.; Domínguez, P.D.; de Eulate, R.G.; Aristu, J.; Bendandi, M.; et al. A phase II trial of autologous dendritic cell vaccination and radiochemotherapy following fluorescence-guided surgery in newly diagnosed glioblastoma patients. J. Transl. Med. 2017, 15, 104. [Google Scholar] [CrossRef]
- Yao, Y.; Luo, F.; Tang, C.; Chen, D.; Qin, Z.; Hua, W.; Xu, M.; Zhong, P.; Yu, S.; Chen, D.; et al. Molecular subgroups and B7-H4 expression levels predict responses to dendritic cell vaccines in glioblastoma: An exploratory randomized phase II clinical trial. Cancer Immunol. Immunother. 2018. [Google Scholar] [CrossRef]
- Bota, D.A.; Chung, J.; Dandekar, M.; Carrillo, J.A.; Kong, X.-T.; Fu, B.D.; Hsu, F.P.; Schönthal, A.H.; Hofman, F.M.; Chen, T.C.; et al. Phase II study of ERC1671 plus bevacizumab versus bevacizumab plus placebo in recurrent glioblastoma: Interim results and correlations with CD4+ T-lymphocyte counts. CNS Oncol. 2018, cns-2018-0009. [Google Scholar] [CrossRef]
- Dillman, R.O.; Duma, C.M.; Ellis, R.A.; Cornforth, A.N.; Schiltz, P.M.; Sharp, S.L.; DePriest, M.C. Intralesional lymphokine-activated killer cells as adjuvant therapy for primary glioblastoma. J. Immunother. 2009, 32, 914–919. [Google Scholar] [CrossRef]
- Dutoit, V.; Migliorini, D.; Patrikidou, A.; Mayer-Mokler, A.; Hilf, N.; Walker, P.R.; Dietrich, P.-Y. IMA950 multipeptide vaccine adjuvanted with poly-ICLC in combination with standard therapy in newly diagnosed HLA-A2 glioblastoma patients. Ann. Oncol. 2017, 28, B148. [Google Scholar] [CrossRef]
- Prins, R.M.; Soto, H.; Konkankit, V.; Odesa, S.K.; Eskin, A.; Yong, W.H.; Nelson, S.F.; Liau, L.M. Gene Expression Profile Correlates with T-Cell Infiltration and Relative Survival in Glioblastoma Patients Vaccinated with Dendritic Cell Immunotherapy. Clin. Cancer Res. 2011, 17, 1603–1615. [Google Scholar] [CrossRef] [PubMed]
- Jie, X.; Hua, L.; Jiang, W.; Feng, F.; Feng, G.; Hua, Z. Clinical application of a dendritic cell vaccine raised against heat-shocked glioblastoma. Cell Biochem. Biophys. 2012, 62, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Vik-Mo, E.O.; Nyakas, M.; Mikkelsen, B.V.; Moe, M.C.; Due-Tønnesen, P.; Suso, E.M.I.; Sæbøe-Larssen, S.; Sandberg, C.; Brinchmann, J.E.; Helseth, E.; et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol. Immunother. 2013, 62, 1499–1509. [Google Scholar] [CrossRef]
- Ardon, H.; Van Gool, S.W.; Verschuere, T.; Maes, W.; Fieuws, S.; Sciot, R.; Wilms, G.; Demaerel, P.; Goffin, J.; Van Calenbergh, F.; et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: Results of the HGG-2006 phase I/II trial. Cancer Immunol. Immunother. 2012, 61, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Homma, J.; Yajima, N.; Tsuchiya, N.; Sano, M.; Kobayashi, T.; Yoshida, S.; Abe, T.; Narita, M.; Takahashi, M.; et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: Results of a clinical phase I/II trial. Clin. Cancer Res. 2005, 11, 4160–4167. [Google Scholar] [CrossRef] [PubMed]
- De Vleeschouwer, S.; Fieuws, S.; Rutkowski, S.; Van Calenbergh, F.; Van Loon, J.; Goffin, J.; Sciot, R.; Wilms, G.; Demaerel, P.; Warmuth-Metz, M.; et al. Postoperative Adjuvant Dendritic Cell-Based Immunotherapy in Patients with Relapsed Glioblastoma Multiforme. Clin. Cancer Res. 2008, 14, 3098–3104. [Google Scholar] [CrossRef]
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016, 375, 2561–2569. [Google Scholar] [CrossRef]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor–Modified Virus-Specific T Cells for Progressive Glioblastoma. JAMA Oncol. 2017, 3, 1094. [Google Scholar] [CrossRef]
- Migliorini, D.; Dietrich, P.-Y.; Stupp, R.; Linette, G.P.; Posey, A.D.; June, C.H. CAR T-Cell Therapies in Glioblastoma: A First Look. Clin. Cancer Res. 2018, 24, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Prinzing, B.L.; Gottschalk, S.M.; Krenciute, G. CAR T-cell therapy for glioblastoma: Ready for the next round of clinical testing? Expert Rev. Anticancer Ther. 2018, 18, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Eissa, I.; Bustos-Villalobos, I.; Ichinose, T.; Matsumura, S.; Naoe, Y.; Miyajima, N.; Morimoto, D.; Mukoyama, N.; Zhiwen, W.; Tanaka, M.; et al. The Current Status and Future Prospects of Oncolytic Viruses in Clinical Trials against Melanoma, Glioma, Pancreatic, and Breast Cancers. Cancers 2018, 10, 356. [Google Scholar] [CrossRef] [PubMed]
- Fueyo, J.; Alemany, R.; Gomez-Manzano, C.; Fuller, G.N.; Khan, A.; Conrad, C.A.; Liu, T.-J.; Jiang, H.; Lemoine, M.G.; Suzuki, K.; et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J. Natl. Cancer Inst. 2003, 95, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.F.; Tran, N.D.; Puduvalli, V.K.; Elder, J.B.; Fink, K.L.; Conrad, C.A.; Yung, W.K.A.; Penas-Prado, M.; Gomez-Manzano, C.; Peterkin, J.; et al. Phase 1b open-label randomized study of the oncolytic adenovirus DNX-2401 administered with or without interferon gamma for recurrent glioblastoma. J. Clin. Oncol. 2017, 35, 2002. [Google Scholar] [CrossRef]
- Chiocca, E.A.; Yu, J.; Phuphanich, S.; Lukas, R.V.; Kumthekar, P.; Yang, Y.; Zhou, Q.; Buck, J.Y.; Deary, A.; Cai, H.; Barrett, J.A.; et al. Expanded phase I study of intratumoral Ad-RTS-hIL-12 plus oral veledimex: Tolerability and survival in recurrent glioblastoma. J. Clin. Oncol. 2017, 35, 2044. [Google Scholar] [CrossRef]
- Chiocca, E.A.; Nassiri, F.; Wang, J.; Peruzzi, P.; Zadeh, G. Viral and other therapies for recurrent GBM: Is a 24-month durable response unusual? Neuro Oncol. 2018. [Google Scholar] [CrossRef]
- Rudra, S.; Hui, C.; Rao, Y.J.; Samson, P.; Lin, A.J.; Chang, X.; Tsien, C.; Fergus, S.; Mullen, D.; Yang, D.; et al. Effect of Radiation Treatment Volume Reduction on Lymphopenia in Patients Receiving Chemoradiotherapy for Glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 217–225. [Google Scholar] [CrossRef]
- Ellsworth, S.G. Field size effects on the risk and severity of treatment-induced lymphopenia in patients undergoing radiation therapy for solid tumors. Adv. Radiat. Oncol. 2018, 3, 512–519. [Google Scholar] [CrossRef]
- Yovino, S.; Kleinberg, L.; Grossman, S.A.; Narayanan, M.; Ford, E. The Etiology of Treatment-related Lymphopenia in Patients with Malignant Gliomas: Modeling Radiation Dose to Circulating Lymphocytes Explains Clinical Observations and Suggests Methods of Modifying the Impact of Radiation on Immune Cells. Cancer Investig. 2013, 31. [Google Scholar] [CrossRef]
- Gupta, T.; Mohanty, S.; Moiyadi, A.; Jalali, R. Factors predicting temozolomide induced clinically significant acute hematologic toxicity in patients with high-grade gliomas: A clinical audit. Clin. Neurol. Neurosurg. 2013, 115, 1814–1819. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, E.; Yamamoto, T.; Sakamoto, N.; Nakai, K.; Akutsu, H.; Tsuboi, K.; Takano, S.; Matsumura, A. Low peripheral lymphocyte count before focal radiotherapy plus concomitant temozolomide predicts severe lymphopenia during malignant glioma treatment. Neurol. Med. Chir. (Tokyo) 2010, 50, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Gerber, D.E.; Grossman, S.A.; Zeltzman, M.; Parisi, M.A.; Kleinberg, L. The impact of thrombocytopenia from temozolomide and radiation in newly diagnosed adults with high-grade gliomas1. Neuro Oncol. 2007, 9, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Mendez, J.S.; Govindan, A.; Leong, J.; Gao, F.; Huang, J.; Campian, J.L. Association between treatment-related lymphopenia and overall survival in elderly patients with newly diagnosed glioblastoma. J. Neurooncol. 2016, 127, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.; Catalano, P.J.; Arvold, N.D.; Aizer, A.A.; Weiss, S.E.; Pinnell, N.; Horvath, M.C.; Christianson, L.; Reardon, D.A.; Lee, E.Q.; et al. Chemoradiation-Related Lymphopenia Is Common Among Glioblastoma Patients and Is Associated with Worse Progression-Free and Overall Survival. Int. J. Radiat. Oncol. 2016, 96, E123. [Google Scholar] [CrossRef]
- Mariucci, S.; Rovati, B.; Manzoni, M.; Della Porta, M.G.; Comolli, G.; Delfanti, S.; Danova, M. Lymphocyte subpopulation and dendritic cell phenotyping during antineoplastic therapy in human solid tumors. Clin. Exp. Med. 2011, 11, 199–210. [Google Scholar] [CrossRef]
- Sengupta, S.; Marrinan, J.; Frishman, C.; Sampath, P. Impact of temozolomide on immune response during malignant glioma chemotherapy. Clin. Dev. Immunol. 2012, 2012, 831090. [Google Scholar] [CrossRef]
- Hsu, M.; Sedighim, S.; Wang, T.; Antonios, J.P.; Everson, R.G.; Tucker, A.M.; Du, L.; Emerson, R.; Yusko, E.; Sanders, C.; et al. TCR Sequencing Can Identify and Track Glioma-Infiltrating T Cells after DC Vaccination. Cancer Immunol. Res. 2016, 4, 412–418. [Google Scholar] [CrossRef]
- Tysome, J.R.; Li, X.; Wang, S.; Wang, P.; Gao, D.; Du, P.; Chen, D.; Gangeswaran, R.; Chard, L.S.; Yuan, M.; et al. A novel therapeutic regimen to eradicate established solid tumors with an effective induction of tumor-specific immunity. Clin. Cancer Res. 2012, 18, 6679–6689. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Wang, J.; Gao, D.; Li, Y.; Li, H.; Chu, Y.; Zhang, Z.; Liu, H.; Jiang, G.; et al. Re-designing Interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nat. Commun. 2017, 8, 1395. [Google Scholar] [CrossRef]
- Kleijn, A.; van den Bossche, W.; Haefner, E.S.; Belcaid, Z.; Burghoorn-Maas, C.; Kloezeman, J.J.; Pas, S.D.; Leenstra, S.; Debets, R.; de Vrij, J.; et al. The Sequence of Delta24-RGD and TMZ Administration in Malignant Glioma Affects the Role of CD8(+)T Cell Anti-tumor Activity. Mol. Ther. Oncol. 2017, 5, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Koks, C.A.; Garg, A.D.; Ehrhardt, M.; Riva, M.; Vandenberk, L.; Boon, L.; De Vleeschouwer, S.; Agostinis, P.; Graf, N.; Van Gool, S.W. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int. J. Cancer 2015, 136, E313–E325. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade. Cancer Cell 2017, 32, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Fritzell, S.; Sandén, E.; Eberstål, S.; Visse, E.; Darabi, A.; Siesjö, P. Intratumoral temozolomide synergizes with immunotherapy in a T cell-dependent fashion. Cancer Immunol. Immunother. 2013, 62, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Curtin, J.F.; Liu, N.; Candolfi, M.; Xiong, W.; Assi, H.; Yagiz, K.; Edwards, M.R.; Michelsen, K.S.; Kroeger, K.M.; Liu, C.; et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009, 6, e10. [Google Scholar] [CrossRef] [PubMed]
- Curtin, J.F.; Candolfi, M.; Fakhouri, T.M.; Liu, C.; Alden, A.; Edwards, M.; Lowenstein, P.R.; Castro, M.G. Treg depletion inhibits efficacy of cancer immunotherapy: Implications for clinical trials. PLoS ONE 2008, 3, e1983. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, P.; Li, H.; Du, X.; Liu, M.; Huang, Q.; Wang, Y.; Wang, S. The Efficacy of Oncolytic Adenovirus Is Mediated by T-cell Responses against Virus and Tumor in Syrian Hamster Model. Clin. Cancer Res. 2017, 23, 239–249. [Google Scholar] [CrossRef]
- Pellegatta, S.; Eoli, M.; Cuccarini, V.; Anghileri, E.; Pollo, B.; Pessina, S.; Frigerio, S.; Servida, M.; Cuppini, L.; Antozzi, C.; et al. Survival gain in glioblastoma patients treated with dendritic cell immunotherapy is associated with increased NK but not CD8+ T cell activation in the presence of adjuvant temozolomide. Oncoimmunology 2018, 7, e1412901. [Google Scholar] [CrossRef]
- Fadul, C.E.; Fisher, J.L.; Gui, J.; Hampton, T.H.; Cote, A.L.; Ernstoff, M.S. Immune modulation effects of concomitant temozolomide and radiation therapy on peripheral blood mononuclear cells in patients with glioblastoma multiforme. Neuro Oncol. 2011, 13, 393–400. [Google Scholar] [CrossRef]
- Ellsworth, S.; Balmanoukian, A.; Kos, F.; Nirschl, C.J.; Nirschl, T.R.; Grossman, S.A.; Luznik, L.; Drake, C.G. Sustained CD4 + T cell-driven lymphopenia without a compensatory IL-7/IL-15 response among high-grade glioma patients treated with radiation and temozolomide. Oncoimmunology 2014, 3, e27357. [Google Scholar] [CrossRef]
| Trial | Number of Patients in the Control Arm | Median Overall Survival in the Landmark Stupp Study and in the Control Standard Therapy Arms/Cohorts of the Follow-Up Phase III Trials | Year of Publication | References |
|---|---|---|---|---|
| TMZ + RT versus RT | 287 | 14.6 months; 12.6 months (MGMT-unmethylated subgroup), 23.4 months (MGMT-methylated subgroup) | 2009 | [40] |
| Dose-dense TMZ versus standard TMZ | 411 | 16.6 months; 14.6 months (MGMT-unmethylated subgroup); 21.4 months (MGMT-methylated subgroup) | 2013 | [21] |
| Cilengitide + ST versus ST | 273 | 26.3 months (MGMT methylated cohort) | 2014 | [22] |
| Bevacizumab + ST versus ST + placebo | 463 | 16.7 months | 2014 | [23] |
| Bevacizumab + ST versus ST + placebo | 317 | 16.1 months; in the pooled analysis of both arms (n = 621): 14.3 months (MGMT-unmethylated cohort); 23.2 months (MGMT-methylated cohort) | 2014 | [24] |
| Nimotuzumab + ST versus ST | 71 | 19.6 months; 15.5 months (MGMT-unmethylated subgroup); 33.8 months (MGMT-methylated subgroup) | 2015 | [3] |
| Rindopepimut/GM-CSF + TMZ versus KLH + TMZ | 374 | 20.0 months | 2017 | [2] |
| TTFields + TMZ versus TMZ | 229 | 19.8 months (16.0 months from randomization plus median time from diagnosis to randomization 3.8 months) | 2017 | [1] |
| Patient Groups/Registries | 2-Year OS Rate | 3-Year OS Rate | 5-Year OS Rate | 10-Year OS Rate | References |
|---|---|---|---|---|---|
| RT-TMZ group versus RT only group | 27.2% vs. 10.9% | 16.0% vs. 4.4% | 9.8% vs. 1.9% | [40] | |
| RT-TMZ group versus RT only group in MGMT-unmethylated subgroup | 14.8% vs. 1.8% | 11.1% vs. 0% | 8.3% vs. 0% | ||
| RT-TMZ group versus RT only group in MGMT-methylated subgroup | 48.9% vs. 23.9% | 27.6% vs. 7.8% | 13.8% vs. 5.2% | ||
| RT-TMZ MGMT-methylated group | 56% | [22] | |||
| RT-TMZ group (exploratory analysis) | 31% | 16% | 5% | [1] | |
| RT-TMZ plus placebo group | 30.1% | [23] | |||
| The National Cancer Institute’s SEER Program (1985–2005, n = 5991) | 9.5% | 5.4% | 3.6% | 2.9% | [107] |
| The National Cancer Institute’s SEER Program (2005–2007) | 24% | [108] | |||
| The Central Brain Tumor Registry of the United States (CBTRUS) (1995–2011, n = 30611) | 14.8% | 8.7% | 5% | 2.6% | [109] |
| Systematic reviews | 2-5% | <1% | [110] |
| Investigational Treatment versus Comparator Treatment | N of Patients | Newly Diagnosed/Recurrent | Results for Primary Outcome | ClinicalTrials.gov Identifier | References |
|---|---|---|---|---|---|
| Phase III trials | |||||
| Rindopepimut * plus GM-CSF and TMZ versus KLH plus TMZ | 745 | Newly diagnosed | mOS: 20.1 versus 20.0 months (HR 1.01, 95% CI 0.79–1.30; p = 0.93) | NCT01480479 | [2] |
| Autologous cytokine-induced killer cells plus ST versus ST | 180 | Newly diagnosed | mOS: 22.5 versus 16.9 months (p = 0.5237) mPFS: 8.1 versus 5.4 months (HR 0.693, 90% CI 0.512–0.937, p = 0.0218) | NA | [25] |
| Autologous DC * vaccine versus autologous PBMC | 331 | Newly diagnosed | Pending | NCT00045968 | [20] |
| Phase II trials | |||||
| Rindopepimut plus GM-CSF and standard or dose-intensified TMZ versus a historical control | 22 | Newly diagnosed | mOS: 23.6 versus 15.0 months (HR = 0.23; 95% CI 0.07–0.79; p = 0.019); mPFS: 15.2 versus 6.3 months (HR = 0.35, 95% CI 0.14–0.87; p = 0.024) | NCT00643097 | [151] |
| Rindopepimut plus GM-CSF and adjuvant TMZ | 65 | Newly diagnosed | mOS: 21.8 months; mPFS: 9.2 months | NCT00458601 | [152] |
| Rindopepimut and GM-CSF plus bevacizumab versus KLH plus bevacizumab | 73 | Recurrent | mOS: 11.6 versus 9.3 months (HR = 0.57, 95% CI 0.33–0.98, p = 0.039) | NCT01498328 | [153] |
| ICT-107 * versus unpulsed autologous DC vaccine | 124 | Newly diagnosed | mOS: 18.3 versus 16.7 months (p > 0.05); PFS: 11.2 versus 9.0 months (p = 0.010) | NCT01280552 | [154] |
| Autologous DC vaccine plus ST versus ST | 76 | Newly diagnosed | mOS: 564 versus 568 days (p = 0.99); PFS: 204 versus 210 days (p = 0.83) | EudraCT number 2009-015979-27 | [155] |
| Autologous DC vaccine plus ST versus ST | 34 | Newly diagnosed | mOS: 31.9 versus 15.0 months (p < 0.002); mPFS: 8.5 versus 8.0 months (p = 0.075) | NA | [156] |
| Autologous DC vaccine plus ST | 27 | Newly diagnosed | mOS: 23.4 months; mPFS: 12.7 months | NCT01006044 | [157] |
| Autologous DC vaccine plus ST versus ST plus placebo | 43 | Newly diagnosed or recurrent | mOS: 13.7 versus 10.7 months (p = 0.05); mPFS: 7.7 versus 6.9 months (p = 0.75) | NA | [158] |
| HSPPC-96 * plus TMZ | 46 | Newly diagnosed | mOS: 23.8 months; mPFS: 18.0 months | NCT00905060 | [17] |
| ERC1671 */GM-CSF/cyclophosphamide plus bevacizumab versus placebo plus bevacizumab | 9 | Recurrent | Interim mOS: 12.0 versus 7.5 months | NCT01903330 | [159] |
| LAK cells * | 33 | Newly diagnosed | mOS: 20.5 months | NCT00331526 | [160] |
| Phase I and I/II trials | |||||
| IMA950 * vaccine with poly ICLC plus ST | 16 | Newly diagnosed | mOS: 21.2 months | NCT01920191 | [161] |
| Autologous DC vaccine plus ST | 23 | Newly diagnosed | mOS: 31.4 months | NA | [162] |
| Autologous DC vaccine plus ST versus ST | 25 | Newly diagnosed | mOS: 17.0 versus 10.5 months (p < 0.05) | NA | [163] |
| Autologous DC vaccine plus ST versus a historical control | 11 | Newly diagnosed | mOS: 759 days versus 585 days | NCT00846456 | [164] |
| Autologous DC vaccine plus ST | 77 | Newly diagnosed | mOS: 18.3 months in ITT analysis; mPFS: 10.4 months in the ITT group versus 20.4 months in the PP group | NA | [165] |
| Autologous DC vaccine versus RT plus nitrosourea | 45 | Recurrent | mOS: 480 versus 400 days (p = 0.010) | NA | [166] |
| Autologous DC vaccine | 56 | Recurrent | mOS: 9.6 months; mPFS: 3 months | NA | [167] |
| Autologous DC vaccine pulsed with pp65 RNA plus tetanus/diphtheria (Td) toxoid or unpulsed autologous DCs | 12 | Newly diagnosed | mOS: 18.5 months; mPFS: 10.8 months | NA | [8] |
| Autologous DC vaccine pulsed with pp65 RNA plus GM-CSF and dose-intensified TMZ | 11 | Newly diagnosed | mOS: 41.1 months; mPFS: 25.3 months | NA | [19] |
| HSPPC-96 vaccine plus ST | 20 | Newly diagnosed | mOS: 31.4 months | NA | [18] |
| ClinicalTrials.gov Identifier | Trial Title | Estimated Sample Size | Satus |
|---|---|---|---|
| NCT03395587 | Phase II multicenter open label, randomized trial of vaccination with lysate-loaded, mature dendritic cells integrated into standard therapy in newly diagnosed glioblastoma (GlioVax) | 136 | Recruiting |
| NCT02465268 | A phase II randomized, blinded, and placebo-controlled trial of CMV RNA-pulsed dendritic cells with tetanus-diphtheria toxoid vaccine in patients with newly diagnosed glioblastoma (ATTAC-II) | 150 | Recruiting |
| NCT02366728 | A randomized phase II study of evaluation of overcoming limited migration and enhancing cytomegalovirus (CMV)-specific dendritic cell vaccines with adjuvant tetanus pre-conditioning in patients with newly diagnosed glioblastoma | 100 | Active, not recruiting |
| NCT03548571 | Open label randomized phase II/III trial of dendritic cell immunotherapy against cancer stem cells in glioblastoma patients receiving standard therapy (DEN-STEM) | 60 | Recruiting |
| NCT03018288 | A randomized, double blind phase II trial of surgery, RT plus TMZ and pembrolizumab with and without heat shock protein-peptide complex-96 (HSPPC-96) in newly diagnosed glioblastoma | 108 | Recruiting |
| NCT01814813 | A phase II randomized trial comparing the efficacy of heat shock protein–peptide complex-96 (HSPPC-96) vaccine given with bevacizumab versus bevacizumab alone in the treatment of surgically resectable recurrent glioblastoma | 90 | Active, not recruiting |
| NCT02455557 | A phase II Study of the safety and efficacy of SVN53-67/M57-KLH (SurVaxM) peptide vaccine in survinin-positive newly diagnosed glioblastoma | 64 | Active, not recruiting |
| NCT01204684 | A phase II clinical trial evaluating autologous dendritic cells pulsed with tumor lysate antigen +/− toll-like receptor agonists for the treatment of malignant glioma | 60 | Active, not recruiting |
| NCT02799238 | An open label, randomized, phase II study to investigate the efficacy and safety of autologous lymphoid effector cells specific against tumor (ALECSAT) treatment as an add-on therapy to RT-TMZ in patients with newly diagnosed glioblastoma | 87 | Recruiting |
| NCT03650257 | A large-scale research for immunotherapy of glioblastoma with autologous heat shock protein gp96 | 150 | Not yet recruiting |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanenko, A.A.; Chekhonin, V.P. Recent Advances in Oncolytic Virotherapy and Immunotherapy for Glioblastoma: A Glimmer of Hope in the Search for an Effective Therapy? Cancers 2018, 10, 492. https://doi.org/10.3390/cancers10120492
Stepanenko AA, Chekhonin VP. Recent Advances in Oncolytic Virotherapy and Immunotherapy for Glioblastoma: A Glimmer of Hope in the Search for an Effective Therapy? Cancers. 2018; 10(12):492. https://doi.org/10.3390/cancers10120492
Chicago/Turabian StyleStepanenko, Aleksei A., and Vladimir P. Chekhonin. 2018. "Recent Advances in Oncolytic Virotherapy and Immunotherapy for Glioblastoma: A Glimmer of Hope in the Search for an Effective Therapy?" Cancers 10, no. 12: 492. https://doi.org/10.3390/cancers10120492
APA StyleStepanenko, A. A., & Chekhonin, V. P. (2018). Recent Advances in Oncolytic Virotherapy and Immunotherapy for Glioblastoma: A Glimmer of Hope in the Search for an Effective Therapy? Cancers, 10(12), 492. https://doi.org/10.3390/cancers10120492