Calcium Independent Effect of Orai1 and STIM1 in Non-Hodgkin B Cell Lymphoma Dissemination
Abstract
1. Introduction
2. Results
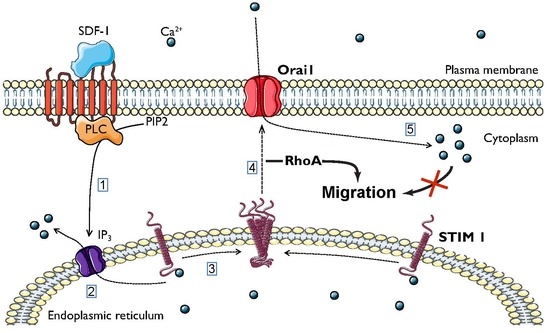
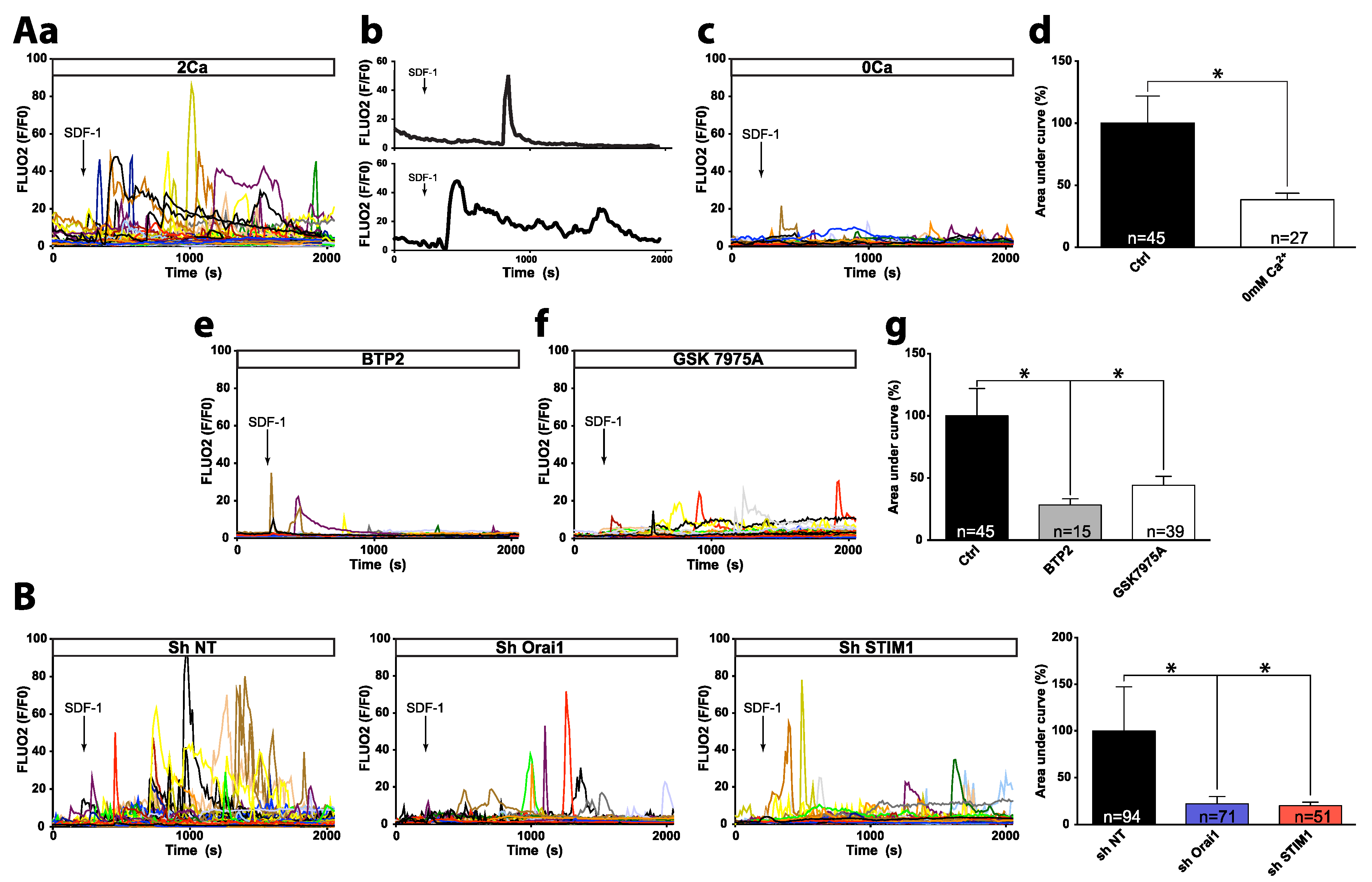
2.1. SDF-1 Induces Ca2+ Responses Involving Orai1/STIM1-Dependent Ca2+ Entry
2.2. Calcium Independent Involvement of Orai1 and STIM1 in DLBCL Migration
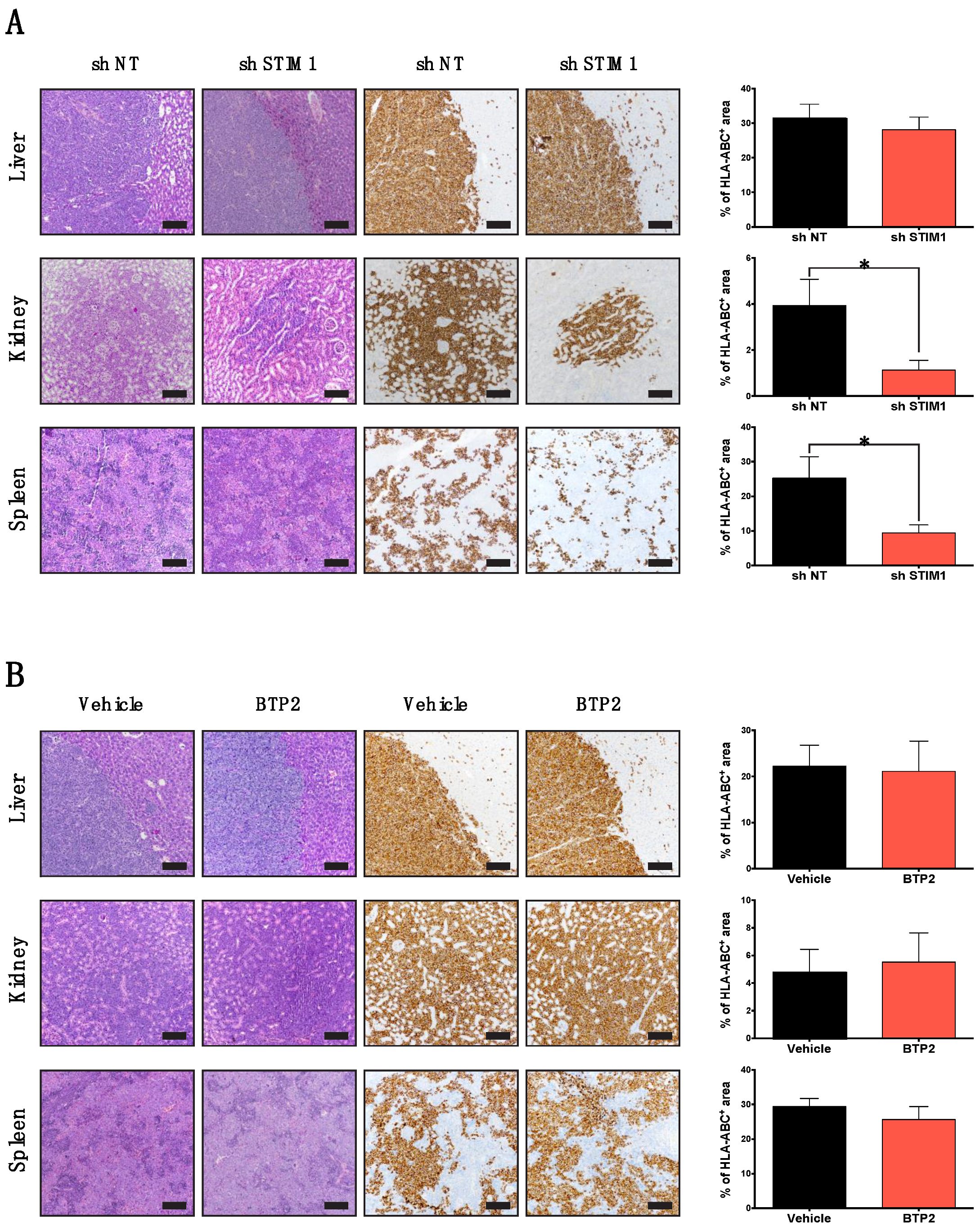
2.3. STIM1 Knock-Down Impaired DLBCL Dissemination In Vivo
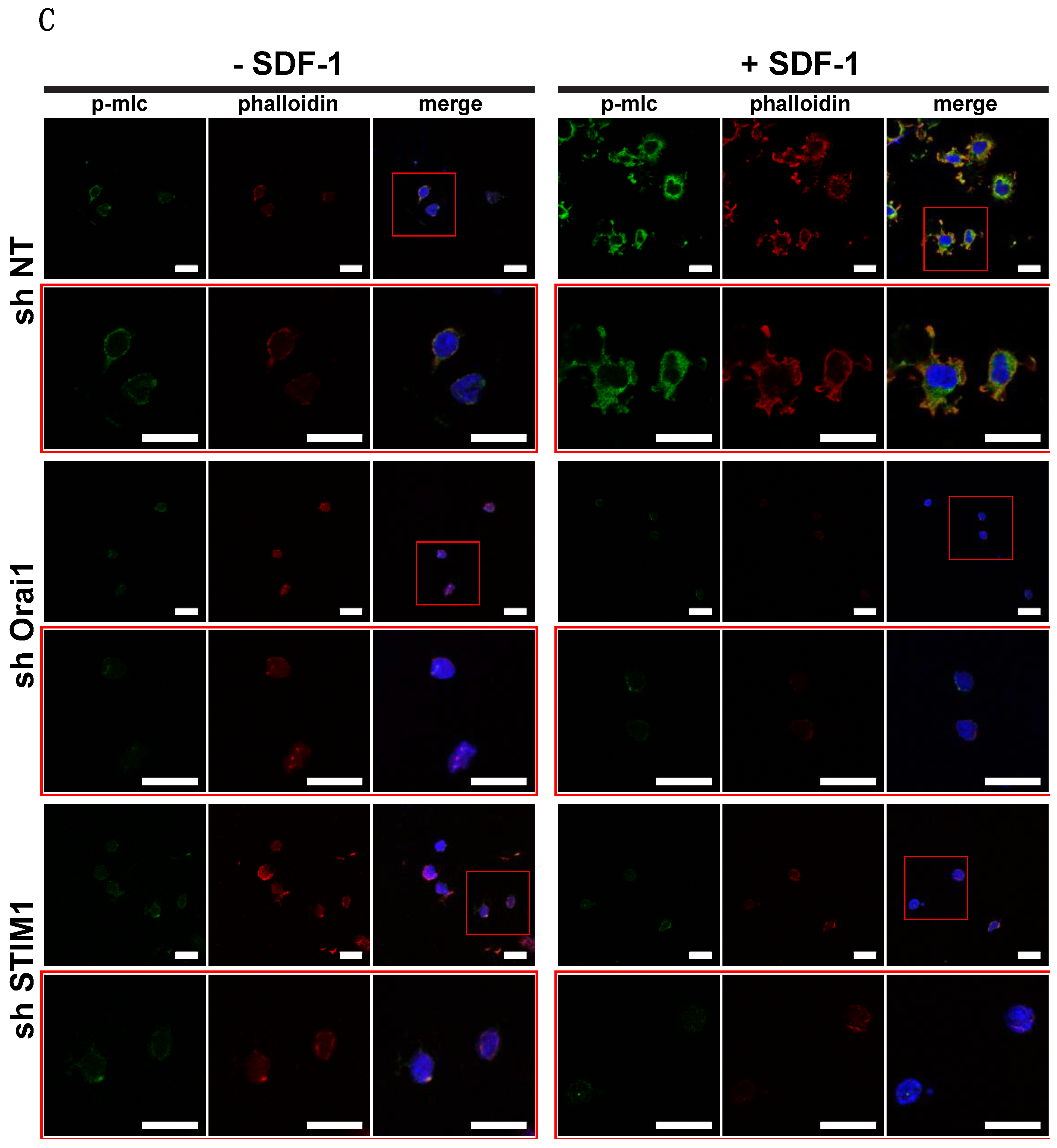
2.4. Molecular Mechanisms Mediating Orai1/STIM1 Dependent DLBCL Migration
2.5. STIM1 and Orai1 Expression in Diffuse Large B Cell Lymphomas
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Lines
4.3. Calcium Imaging
4.4. Transwell Assay
4.5. In Vivo Experiments
4.6. RhoA Activation Assay
4.7. Immunohistochemistry
4.8. Flow Cytometry Analysis
4.9. Tissue Microarrays
4.10. Western Blot
4.11. Confocal Microscopy
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Møller, M.B.; Pedersen, N.T.; Christensen, B.E. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation—A population-based study of 1575 cases. Br. J. Haematol. 2004, 124, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Zerdes, I.; Vassou, A.; Bareta, E.; Seraj, E.; Papoudou-Bai, A.; Pavlidis, N.; Batistatou, A.; Pentheroudakis, G. Extranodal diffuse large B-cell lymphomas: A retrospective case series and review of the literature. Hematol. Rep. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Pals, S.T.; de Gorter, D.J.J.; Spaargaren, M. Lymphoma dissemination: the other face of lymphocyte homing. Blood 2007, 110, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Broxmeyer, H.E. Chemokines in hematopoiesis. Curr. Opin. Hematol. 2008, 15, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Waite, J.; Brewer, F.; Sunshine, M.-J.; Littman, D.R.; Zou, Y.-R. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J. Exp. Med. 2004, 200, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Cyster, J.G. Homing of antibody secreting cells. Immunol. Rev. 2003, 194, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Tokoyoda, K.; Egawa, T.; Sugiyama, T.; Choi, B.-I.; Nagasawa, T. Cellular Niches Controlling B Lymphocyte Behavior within Bone Marrow during Development. Immunity 2004, 20, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu-Monette, Z.Y.; Deng, L.; Shen, Q.; Manyam, G.C.; Martinez-Lopez, A.; Zhang, L.; Montes-Moreno, S.; Visco, C.; Tzankov, A.; et al. Dysregulated CXCR4 expression promotes lymphoma cell survival and independently predicts disease progression in germinal center B-cell-like diffuse large B-cell lymphoma. Oncotarget 2015, 6, 5597–5614. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Fricker, S.P. CXCL12 (SDF-1)/CXCR4 Pathway in Cancer. Clin. Cancer Res. 2010, 16, 2927–2931. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, D.; Wei, D.; Wang, X.; Wang, L.; Zeng, X. RhoA and RhoC are involved in stromal cell-derived factor-1-induced cell migration by regulating F-actin redistribution and assembly. Mol. Cell. Biochem. 2017, 436, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Henschler, R.; Piiper, A.; Bistrian, R.; Möbest, D. SDF-1alpha-induced intracellular calcium transient involves Rho GTPase signalling and is required for migration of hematopoietic progenitor cells. Biochem. Biophys. Res. Commun. 2003, 311, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Zuccolo, E.; Di Buduo, C.; Lodola, F.; Orecchioni, S.; Scarpellino, G.; Kheder, D.A.; Poletto, V.; Guerra, G.; Bertolini, F.; Balduini, A.; et al. Stromal Cell-Derived Factor-1α Promotes Endothelial Colony-Forming Cell Migration Through the Ca2+-Dependent Activation of the Extracellular Signal-Regulated Kinase 1/2 and Phosphoinositide 3-Kinase/AKT Pathways. Stem Cells Dev. 2018, 27, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Iamshanova, O.; Fiorio Pla, A.; Prevarskaya, N. Molecular mechanisms of tumour invasion: Regulation by calcium signals. J. Physiol. 2017, 595, 3063–3075. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.; Wulff, H.; Skolnik, E.Y. Ion channels in innate and adaptive immunity. Annu. Rev. Immunol. 2015, 33, 291–353. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.; Gwack, Y.; Prakriya, M.; Srikanth, S.; Puppel, S.-H.; Tanasa, B.; Hogan, P.G.; Lewis, R.S.; Daly, M.; Rao, A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006, 441, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Yeromin, A.V.; Zhang, S.L.; Jiang, W.; Yu, Y.; Safrina, O.; Cahalan, M.D. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 2006, 443, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Chiu, W.-T.; Chen, Y.-T.; Lin, P.-Y.; Huang, H.-J.; Chou, C.-Y.; Chang, H.-C.; Tang, M.-J.; Shen, M.-R. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15225–15230. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Chen, Y.-F.; Chiu, W.-T.; Wang, Y.-K.; Chang, H.-C.; Shen, M.-R. The ER Ca2+ sensor STIM1 regulates actomyosin contractility of migratory cells. J. Cell Sci. 2013, 126, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Jardin, I.; Rosado, J.A. STIM and calcium channel complexes in cancer. BBA–Mol. Cell Res. 2016, 1863, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P. Prespecification and plasticity: Shifting mechanisms of cell migration. Curr. Opin. Cell Biol. 2004, 16, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Takemura, H.; Hughes, A.R.; Thastrup, O.; Putney, J.W. Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J. Biol. Chem. 1989, 264, 12266–12271. [Google Scholar] [PubMed]
- Stolwijk, J.A.; Zhang, X.; Gueguinou, M.; Zhang, W.; Matrougui, K.; Renken, C.; Trebak, M. Calcium Signaling is Dispensable for Receptor-Regulation of Endothelial Barrier Function. J. Biol. Chem. 2016, 291. [Google Scholar] [CrossRef] [PubMed]
- Kondratska, K.; Kondratskyi, A.; Yassine, M.; Lemonnier, L.; Lepage, G.; Morabito, A.; Skryma, R.; Prevarskaya, N. Orai1 and STIM1 mediate SOCE and contribute to apoptotic resistance of pancreatic adenocarcinoma. Biochim. Biophys. Acta 2014, 1843, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Andrique, L.; Poglio, S.; Prochazkova-Carlotti, M.; Kadin, M.E.; Giese, A.; Idrissi, Y.; Beylot-Barry, M.; Merlio, J.-P.; Chevret, E. Intrahepatic Xenograft of Cutaneous T-Cell Lymphoma Cell Lines: A Useful Model for Rapid Biological and Therapeutic Evaluation. Am. J. Pathol. 2016, 186, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Vacher, P.; Vacher, A.-M.; Pineau, R.; Latour, S.; Soubeyran, I.; Pangault, C.; Tarte, K.; Soubeyran, P.; Ducret, T.; Bresson-Bepoldin, L. Localized Store-Operated Calcium Influx Represses CD95-Dependent Apoptotic Effects of Rituximab in Non-Hodgkin B Lymphomas. J. Immunol. 2015, 195, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Fourel, L.; Dalonneau, F.; Sadir, R.; Leal, S.; Lortat-Jacob, H.; Weidenhaupt, M.; Albiges-Rizo, C.; Picart, C. Biomaterial-enabled delivery of SDF-1α at the ventral side of breast cancer cells reveals a crosstalk between cell receptors to promote the invasive phenotype. Biomaterials 2017, 127, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Agle, K.A.; Vongsa, R.A.; Dwinell, M.B. Calcium Mobilization Triggered by the Chemokine CXCL12 Regulates Migration in Wounded Intestinal Epithelial Monolayers. J. Biol. Chem. 2010, 285, 16066–16075. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Calcium in tumour metastasis: new roles for known actors. Nat. Rev. Cancer 2011, 11, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.H.; Parker, I.; Miller, M.J.; Cahalan, M.D. A stochastic view of lymphocyte motility and trafficking within the lymph node. Immunol. Rev. 2003, 195, 136–159. [Google Scholar] [CrossRef] [PubMed]
- Cahalan, M.D.; Chandy, K.G. The functional network of ion channels in T lymphocytes. Immunol. Rev. 2009, 231, 59–87. [Google Scholar] [CrossRef] [PubMed]
- Palmesino, E.; Moepps, B.; Gierschik, P.; Thelen, M. Differences in CXCR4-mediated signaling in B cells. Immunobiology 2006, 211, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Brandes, M.; Legler, D.F.; Spoerri, B.; Schaerli, P.; Moser, B. Activation-dependent modulation of B lymphocyte migration to chemokines. Int. Immunol. 2000, 12, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Borowiec, A.-S.; Bidaux, G.; Tacine, R.; Dubar, P.; Pigat, N.; Delcourt, P.; Mignen, O.; Capiod, T. Are Orai1 and Orai3 channels more important than calcium influx for cell proliferation? BBA–Mol. Cell Res. 2014, 1843, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Vrenken, K.S.; Jalink, K.; van Leeuwen, F.N.; Middelbeek, J. Beyond ion-conduction: Channel-dependent and -independent roles of TRP channels during development and tissue homeostasis. Biochim. Biophys. Acta 2016, 1863, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Genova, T.; Grolez, G.P.; Camillo, C.; Bernardini, M.; Bokhobza, A.; Richard, E.; Scianna, M.; Lemonnier, L.; Valdembri, D.; Munaron, L.; et al. TRPM8 inhibits endothelial cell migration via a non-channel function by trapping the small GTPase Rap1. J. Cell Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.V.; Motiani, R.K.; Zhang, X.; Abdullaev, I.F.; Adam, A.P.; González-Cobos, J.C.; Zhang, W.; Matrougui, K.; Vincent, P.A.; Trebak, M. STIM1 controls endothelial barrier function independently of Orai1 and Ca2+ entry. Sci. Signal. 2013, 6, ra18. [Google Scholar] [CrossRef] [PubMed]
- Mele, S.; Devereux, S.; Ridley, A.J. Rho and Rap guanosine triphosphatase signaling in B cells and chronic lymphocytic leukemia. Leuk. Lymphoma 2014, 55, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Heasman, S.J.; Carlin, L.M.; Cox, S.; Ng, T.; Ridley, A.J. Coordinated RhoA signaling at the leading edge and uropod is required for T cell transendothelial migration. J. Cell Biol. 2010, 190, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Gwack, Y. Orai1, STIM1, and their associating partners. J. Physiol. 2012, 590, 4169–4177. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.J.; Albarran, L.; Gómez, L.J.; Smani, T.; Salido, G.M.; Rosado, J.A. Molecular modulators of store-operated calcium entry. Biochim. Biophys. Acta 2016, 1863, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-R.; Chen, C.-C.; Chan, Y.-T.; Wang, H.-J.; Chien, F.-T.; Chen, Y.-L.; Liu, J.-L.; Yang, M.-H. STAT3-coordinated migration facilitates the dissemination of diffuse large B-cell lymphomas. Nat. Commun. 2018, 9, 3696. [Google Scholar] [CrossRef] [PubMed]
- Middle, S.; Coupland, S.E.; Taktak, A.; Kidgell, V.; Slupsky, J.R.; Pettitt, A.R.; Till, K.J. Immunohistochemical analysis indicates that the anatomical location of B-cell non-Hodgkin’s lymphoma [is determined by differentially expressed chemokine receptors, sphingosine-1-phosphate receptors and integrins. Exp. Hematol. Oncol. 2015, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-L.; Su, Y.-C.; Chao, T.-Y.; Lin, C.-W.; Chou, S.-C.; Yao, M.; Kuo, S.-H.; Yu, S.-C. Intralymphatic Spread is a Rare Finding Associated with Poor Prognosis in Diffuse Large B-Cell Lymphoma with Extranodal Involvements. Am. J. Surg. Pathol. 2018, 42, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Faustin, B.; Mahouche, I.; Ruggieri, R.; Brulard, C.; Ichas, F.; Soubeyran, I.; Lartigue, L.; De Giorgi, F. The MAP3K ZAK, a novel modulator of ERK-dependent migration, is upregulated in colorectal cancer. Oncogene 2016, 35, 3190–3200. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latour, S.; Mahouche, I.; Cherrier, F.; Azzi-Martin, L.; Velasco, V.; Soubeyran, P.; Merlio, J.-P.; Poglio, S.; Bresson-Bepoldin, L. Calcium Independent Effect of Orai1 and STIM1 in Non-Hodgkin B Cell Lymphoma Dissemination. Cancers 2018, 10, 402. https://doi.org/10.3390/cancers10110402
Latour S, Mahouche I, Cherrier F, Azzi-Martin L, Velasco V, Soubeyran P, Merlio J-P, Poglio S, Bresson-Bepoldin L. Calcium Independent Effect of Orai1 and STIM1 in Non-Hodgkin B Cell Lymphoma Dissemination. Cancers. 2018; 10(11):402. https://doi.org/10.3390/cancers10110402
Chicago/Turabian StyleLatour, Simon, Isabelle Mahouche, Floriane Cherrier, Lamia Azzi-Martin, Valérie Velasco, Pierre Soubeyran, Jean-Philippe Merlio, Sandrine Poglio, and Laurence Bresson-Bepoldin. 2018. "Calcium Independent Effect of Orai1 and STIM1 in Non-Hodgkin B Cell Lymphoma Dissemination" Cancers 10, no. 11: 402. https://doi.org/10.3390/cancers10110402
APA StyleLatour, S., Mahouche, I., Cherrier, F., Azzi-Martin, L., Velasco, V., Soubeyran, P., Merlio, J.-P., Poglio, S., & Bresson-Bepoldin, L. (2018). Calcium Independent Effect of Orai1 and STIM1 in Non-Hodgkin B Cell Lymphoma Dissemination. Cancers, 10(11), 402. https://doi.org/10.3390/cancers10110402