Geometric Parameter Optimization of 3D-Printed Microneedle Arrays Based on Comprehensive Mechanical Testing and Failure Analysis
Abstract
1. Introduction
2. Experimental Methodology
2.1. Sample Preparation and Design Parameters
2.1.1. Material Selection and Biocompatibility
2.1.2. Geometric Design Matrix
2.1.3. Array Configuration Categories
2.2. Manufacturing Process and Quality Control
2.2.1. Stereolithography Fabrication Protocol
2.2.2. Post-Processing and Curing
2.2.3. Quality Control and Acceptance Criteria
2.3. Mechanical Testing and Statistical Analysis
Statistical Analysis Methods
2.4. Simulation Analysis
2.4.1. Model Geometry and Material Properties
2.4.2. Boundary Conditions and Loading
- 1 × 1 configuration → 1 N–50 N total per needle;
- 5 × 5 configuration → 2 N per needle (50 N total);
- 10 × 10 configuration → 0.5 N per needle (50 N total).
2.4.3. Meshing and Solver Settings
- 1 × 1 model: ≈9.6 × 105 elements (1.3 × 106 nodes);
- 5 × 5 model: ≈3.8 × 104 elements (6.5 × 104 nodes);
- 10 × 10 model: ≈7.3 × 104 elements (1.1 × 105 nodes).
2.4.4. Output Parameters
- Maximum Von Mises stress (σmax);
- Maximum displacement (δmax) at the needle tip;
- Equivalent strain (εeq);
- Safety Factor (SF = σy/σmax).
3. Results and Discussion
3.1. Manufacturing Success Rate Analysis
- Statistical Analysis of Manufacturing Success Rates:
- 1 × 1 vs. 5 × 5: Δ = 19.6%, 95% CI [14.2%, 25.0%], p < 0.001
- 1 × 1 vs. 10 × 10: Δ = 23.1%, 95% CI [17.9%, 28.3%], p < 0.001
- 5 × 5 vs. 10 × 10: Δ = 3.5%, 95% CI [−1.2%, 8.2%], p = 0.182
3.2. Integrated Mechanical Performance: Experimental, Analytical, and FEA Comparison
3.3. Analysis of Simulation vs. Experimental Results
3.3.1. Discrepancies in Displacement Measurements
- Single Needle Configurations (1 × 1):
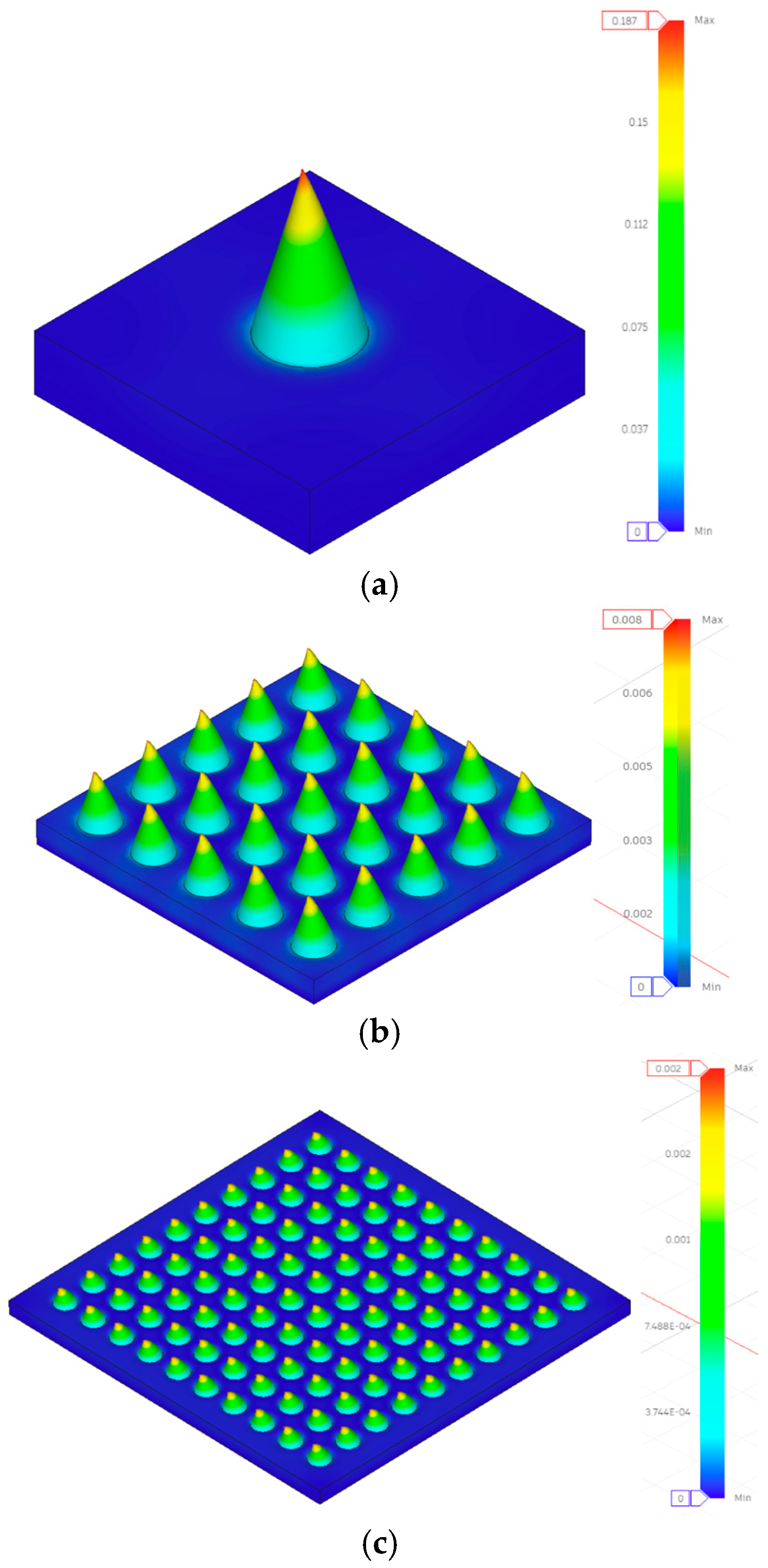
- Design 1: Experimental = 0.349 mm, FEA = 0.187 mm (86% higher experimental);
- Design 3: Experimental = 0.570 mm, FEA = 0.358 mm (59% higher experimental);
- Mean discrepancy across all 1 × 1 configurations: 67% ± 15%.
- Array Configurations (10 × 10):
- Design 1: Experimental = 0.281 mm, FEA = 0.002 mm (14,050% higher experimental);
- Design 5: Experimental = 0.578 mm, FEA = 0.002 mm (28,800% higher experimental).
- Critical Assessment:
- Linear elastic material model: Photopolymers exhibit time-dependent viscoelastic behavior and nonlinear stress–strain relationships, neither of which was captured in the FEA model;
- Idealized boundary conditions: Perfect fixation assumption vs. realistic fixture compliance and rotation;
- Contact mechanics simplification: Point loading assumption vs. distributed contact with finite compressive plate stiffness;
- Manufacturing variability: Geometric deviations and material property variations not reflected in nominal CAD models.
- Implications for Model Utility:
- Visualizing stress distribution patterns and concentration regions;
- Understanding relative performance trends between configurations;
- Identifying critical stress locations for design refinement.
- Future Model Improvements:
3.3.2. Safety Factor Analysis
3.3.3. FEA-Revealed Load Distribution Mechanisms
3.4. Individual Configuration Performance Analysis
3.4.1. 1 × 1 Microneedle Array Performance
3.4.2. 5 × 5 Microneedle Array Performance
3.4.3. 10 × 10 Microneedle Array Performance
3.5. Comparative Analysis of Array Configurations
3.5.1. Designs Optimized for High-Density Arrays (Designs 1, 5, 6)
3.5.2. Designs Optimized for Single Needle Configuration (Designs 2, 3, 4)
3.6. Practical Implications and Design Optimization
3.6.1. Best Selection of Configuration
3.6.2. Optimal Configuration Selection
3.6.3. Manufacturing Considerations and Array Configuration Recommendations
3.7. Study Scope and Future Research Directions
4. Conclusions
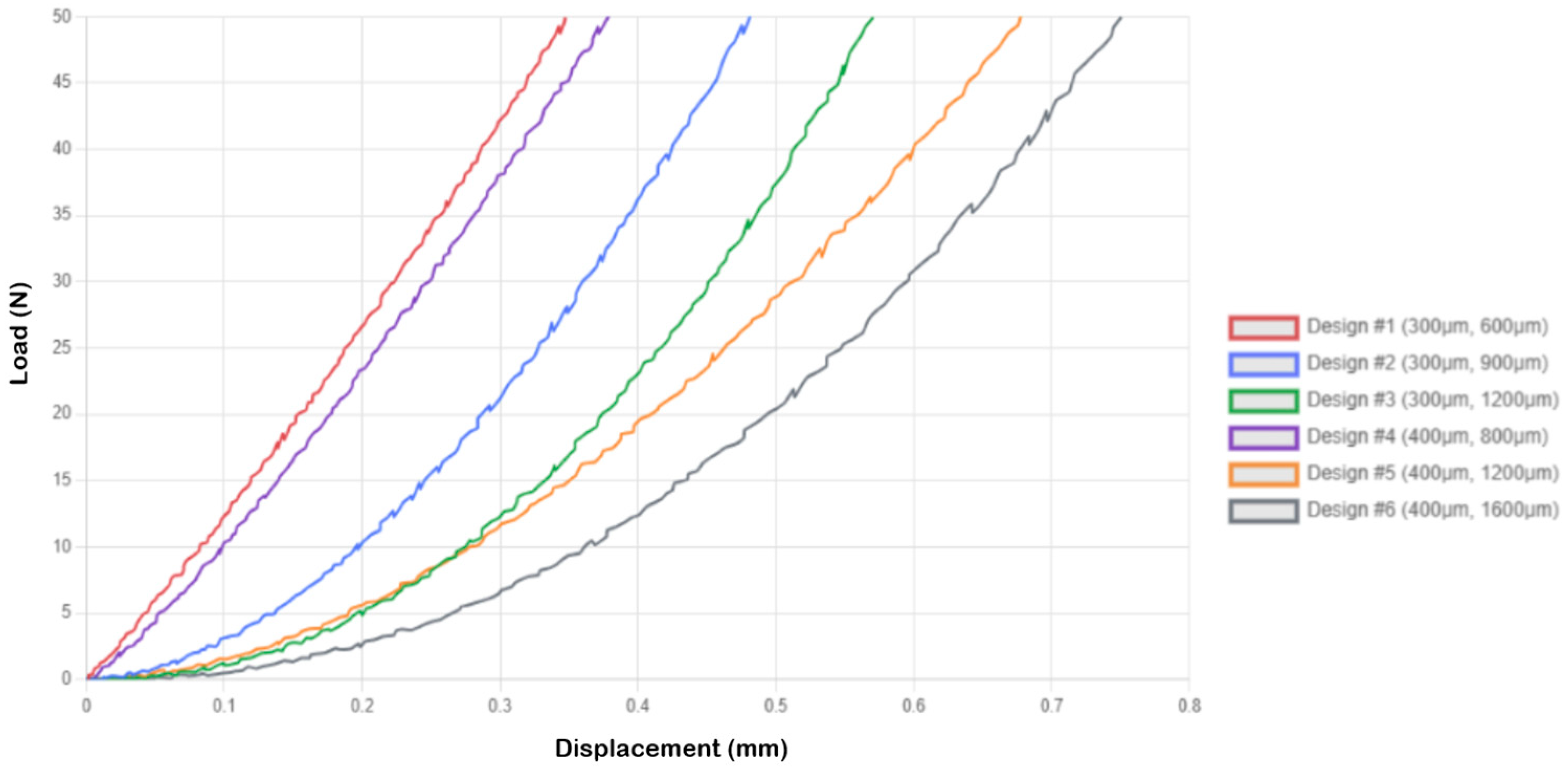
- Compressive displacement at 50 N ranged from 0.281 ± 0.016 mm (Design 1, 10 × 10) to 0.966 ± 0.057 mm (Design 3, 5 × 5), with both geometric design [F(5, 72) = 145.3, p < 0.001, η2 = 0.91] and array configuration [F(2, 72) = 78.2, p < 0.001, η2 = 0.68] showing significant effects;
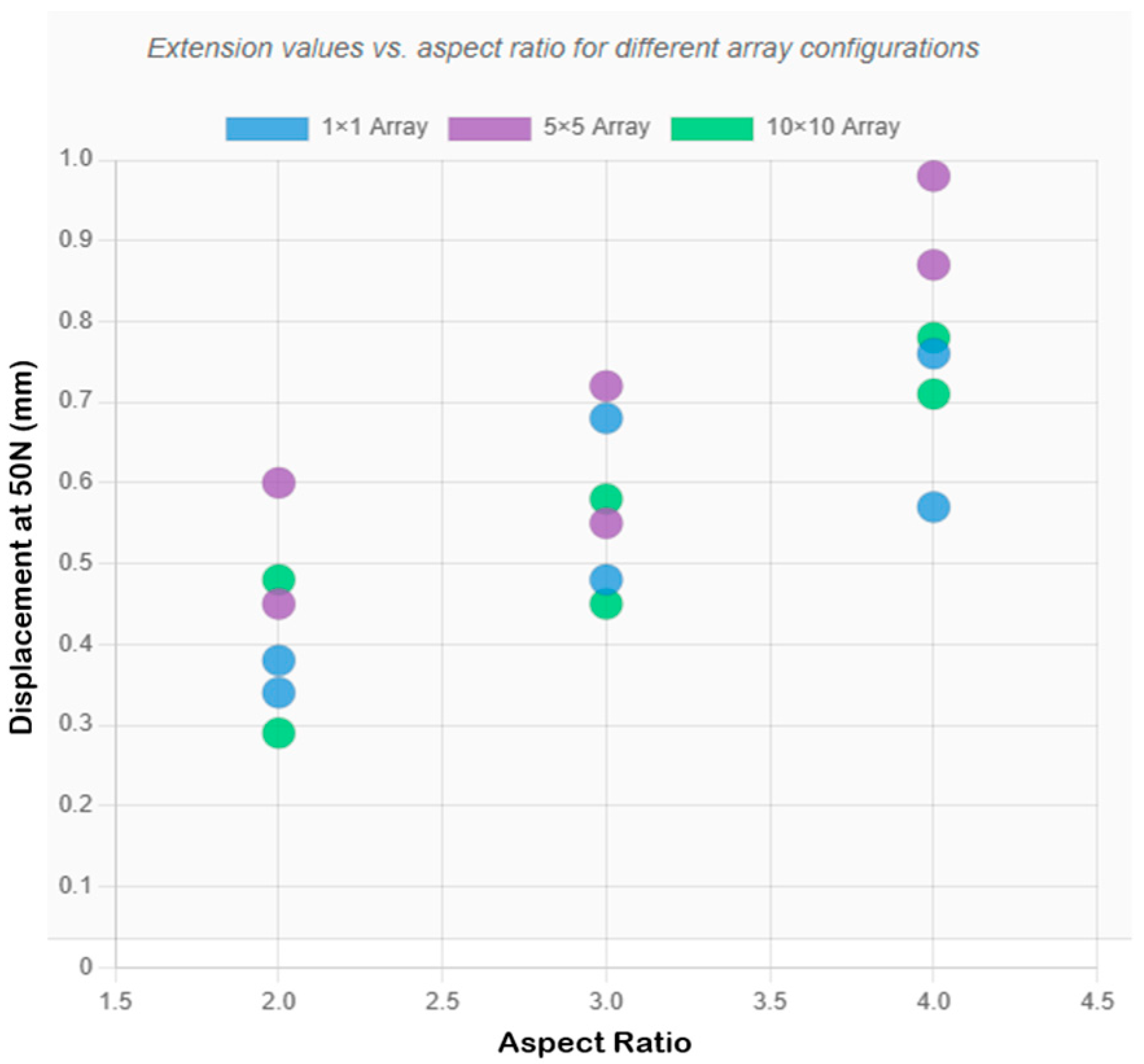
- Low aspect ratio designs (2:1) exhibited superior mechanical stability in high-density arrays (10 × 10), while high aspect ratio designs (≥3:1) performed better as single needles;
- Design 5 (400 μm diameter, 3:1 aspect ratio, 10 × 10) demonstrated optimal balance: controlled displacement (0.578 ± 0.036 mm), high safety factor (SF = 13.32), and low stress (3.979 MPa).
- FEA revealed load distribution mechanisms: stress reduced 100-fold in 10 × 10 arrays through both load division (each needle bears 0.5 N vs. 50 N) and structural interaction (mutual lateral support reduces bending stress by ~20%);
- Safety factor improvement (SF = 0.075 → 7.493 for Design 1) confirms that the array configuration transforms critically stressed single needles into robust platforms.
- Success rates improved significantly from 44.2 ± 3.1% (1 × 1) to 67.3 ± 3.8% (10 × 10) [χ2(2) = 127.4, p < 0.001], with 400 μm designs showing 12.3% higher yield than 300 μm designs [95% CI: 8.7–15.9%];
- Plateau effect observed between 5 × 5 and 10 × 10 densities (Δ = 3.5%, p = 0.182), suggesting manufacturing benefits saturate beyond moderate array densities.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Kim, Y.C.; Park, J.H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Larraneta, E.; Lutton, R.E.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- Singh, T.; Garland, M.; Cassidy, C.; Migalska, K.; Demir, Y.; Abdelghany, S.; Ryan, E.; Woolfson, D.; Donnelly, R. Microporation techniques for enhanced delivery of therapeutic agents. Recent Pat. Drug Deliv. Formul. 2010, 4, 1–17. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Dave, K.; Venuganti, V.V.K. Microneedles in the clinic. J. Control. Release 2017, 260, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Sahm, L.J.; Moore, A.C. The success of microneedle-mediated vaccine delivery into skin. Hum. Vaccines Immunother. 2016, 12, 2975–2983. [Google Scholar] [CrossRef]
- Arya, J.; Prausnitz, M.R. Microneedle patches for vaccination in developing countries. J. Control. Release 2016, 240, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Ceramic microneedles and hollow microneedles for transdermal drug delivery: Two decades of research. J. Drug Deliv. Sci. Technol. 2018, 44, 314–322. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Karande, P.; Jain, A.; Ergun, K.; Kispersky, V.; Mitragotri, S. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc. Natl. Acad. Sci. USA 2005, 102, 4688–4693. [Google Scholar] [CrossRef]
- McAllister, D.V.; Wang, P.M.; Davis, S.P.; Park, J.H.; Canatella, P.J.; Allen, M.G.; Prausnitz, M.R. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA 2003, 100, 13755–13760. [Google Scholar] [CrossRef]
- Park, J.H.; Allen, M.G.; Prausnitz, M.R. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. J. Control. Release 2005, 104, 51–66. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.H.; Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef]
- Quinn, H.L.; Kearney, M.C.; Courtenay, A.J.; McCrudden, M.T.; Donnelly, R.F. The role of microneedles for drug and vaccine delivery. Expert Opin. Drug Deliv. 2014, 11, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Kommineni, N.; Ebhodaghe, S.O.; Bulusu, R.; Jyothi, V.G.S.; Sayed, A.A.; Awaji, A.A.; Germoush, M.O.; Al-Malky, H.S.; Nasrullah, M.Z.; et al. Microneedle-based natural polysaccharide for drug delivery systems (DDS): Progress and challenges. Pharmaceuticals 2022, 15, 190. [Google Scholar] [CrossRef]
- Dharadhar, S.; Majumdar, A.; Dhoble, S.; Patravale, V. Microneedles for transdermal drug delivery: A systematic review. Drug Dev. Ind. Pharm. 2019, 45, 188–201. [Google Scholar] [CrossRef]
- Wilke, N.; Mulcahy, A.; Ye, S.R.; Morrissey, A. Process optimization and characterization of silicon microneedles fabricated by wet etch technology. Microelectron. J. 2005, 36, 650–656. [Google Scholar] [CrossRef]
- Moon, S.J.; Lee, S.S.; Lee, H.S.; Kwon, T.H. Fabrication of microneedle array using LIGA and hot embossing process. Microsyst. Technol. 2005, 11, 311–318. [Google Scholar] [CrossRef]
- Roxhed, N.; Gasser, T.C.; Griss, P.; Holzapfel, G.A.; Stemme, G. Penetration-enhanced ultrasharp microneedles and prediction on skin interaction for efficient transdermal drug delivery. J. Microelectromech. Syst. 2007, 16, 1429–1440. [Google Scholar] [CrossRef]
- Davis, S.P.; Landis, B.J.; Adams, Z.H.; Allen, M.G.; Prausnitz, M.R. Insertion of microneedles into skin: Measurement and prediction of insertion force and needle fracture force. J. Biomech. 2004, 37, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Stoeber, B.; Liepmann, D. Arrays of hollow out-of-plane microneedles for drug delivery. J. Microelectromech. Syst. 2005, 14, 472–479. [Google Scholar] [CrossRef]
- Chu, L.Y.; Choi, S.O.; Prausnitz, M.R. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: Bubble and pedestal microneedle designs. J. Pharm. Sci. 2010, 99, 4228–4238. [Google Scholar] [CrossRef]
- Sullivan, S.P.; Murthy, N.; Prausnitz, M.R. Minimally invasive protein delivery with rapidly dissolving polymer microneedles. Adv. Mater. 2008, 20, 933–938. [Google Scholar] [CrossRef]
- Indermun, S.; Luttge, R.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Modi, G.; Pillay, V. Current advances in the fabrication of microneedles for transdermal delivery. J. Control. Release 2014, 185, 130–138. [Google Scholar] [CrossRef]
- Lim, S.H.; Ng, J.Y.; Kang, L. Three-dimensional printing of a microneedle array on personalized curved surfaces for dual-pronged treatment of trigger finger. Biofabrication 2017, 9, 015010. [Google Scholar] [CrossRef]
- Pere, C.P.P.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamprou, D.A.; Douroumis, D. 3D-Printed Microneedles for Insulin Skin Delivery. Int. J. Pharm. 2018, 544, 425–432. [Google Scholar] [CrossRef]
- Lu, Y.; Mantha, S.N.; Crowder, D.C.; Chinchilla, S.; Shah, K.N.; Yun, Y.H.; Wicker, R.B.; Choi, J.-W. Microstereolithography and characterization of poly (propylene fumarate)-based drug-loaded microneedle arrays. Biofabrication 2015, 7, 045001. [Google Scholar] [CrossRef]
- Gittard, S.D.; Ovsianikov, A.; Chichkov, B.N.; Doraiswamy, A.; Narayan, R.J. Two-photon polymerization of microneedles for transdermal drug delivery. Expert Opin. Drug Deliv. 2010, 7, 513–533. [Google Scholar] [CrossRef]
- Johnson, A.R.; Caudill, C.L.; Tumbleston, J.R.; Bloomquist, C.J.; AMoga, K.; Ermoshkin, A.; Shirvanyants, D.; Mecham, S.J.; Luft, J.C.; DeSimone, J.M. Single-step fabrication of computationally designed microneedles by continuous liquid interface production. PLoS ONE 2016, 11, e0162518. [Google Scholar] [CrossRef]
- Economidou, S.N.; Lamprou, D.A.; Douroumis, D. 3D printing applications for transdermal drug delivery. Int. J. Pharm. 2018, 544, 415–424. [Google Scholar] [CrossRef]
- Uddin, M.J.; Scoutaris, N.; Economidou, S.N.; Giraud, C.; Chowdhry, B.Z.; Donnelly, R.F.; Douroumis, D. 3D printed microneedles for anticancer therapy of skin tumours. Mater. Sci. Eng. C 2020, 107, 110248. [Google Scholar] [CrossRef]
- Mathew, E.; Pitzanti, G.; Larraneta, E.; Lamprou, D.A. Three-dimensional printing of pharmaceuticals and drug delivery devices. Pharmaceutics 2021, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Ausaf, T.; Rajaraman, S. 3D printing, ink casting, and laminated micromanufacturing techniques for 3D microneedle arrays. Micromachines 2018, 9, 635. [Google Scholar]
- Caudill, C.; Perry, J.L.; Iliadis, K.; Tessema, A.T.; Lee, B.J.; Mecham, B.S.; Tian, S.; DeSimone, J.M. Transdermal vaccination via 3D-printed microneedles induces potent humoral and cellular immunity. Proc. Natl. Acad. Sci. USA 2018, 115, 10313–10318. [Google Scholar] [CrossRef] [PubMed]
- Krieger, K.J.; Bertollo, N.; Dangol, M.; Sheridan, J.T.; Lowery, M.M.; O’Cearbhaill, E.D. Simple and customizable method for fabrication of high-aspect ratio microneedle molds using low-cost 3D printing. Microsyst. Nanoeng. 2019, 5, 1–14. [Google Scholar] [CrossRef]
- Luzuriaga, M.A.; Berry, D.R.; Reagan, J.C.; Smaldone, R.A.; Gassensmith, J.J. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab A Chip 2018, 18, 1223–1230. [Google Scholar] [CrossRef]
- Camović, M.; Biščević, A.; Brčić, I.; Borčak, K.; Bušatlić, S.; Ćenanović, N.; Dedović, A.; Mulalić, A.; Osmanlić, M.; Sirbubalo, M.; et al. Coated 3D Printed PLA Microneedles as Transdermal Drug Delivery Systems. In CMBEBIH 2019. CMBEBIH 2019. IFMBE Proceedings; Badnjevic, A., Škrbić, R., Gurbeta Pokvić, L., Eds.; Springer: Cham, Switzerland, 2020; Volume 73. [Google Scholar] [CrossRef]
- Park, J.H.; Allen, M.G.; Prausnitz, M.R. Polymer microneedles for controlled-release drug delivery. Pharm. Res. 2006, 23, 1008–1019. [Google Scholar] [CrossRef]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef]
- Khanna, P.; Strom, J.A.; Malone, J.I.; Bhansali, S. Microneedle-based automated therapy for diabetes mellitus. J. Diabetes Sci. Technol. 2008, 2, 1122–1129. [Google Scholar] [CrossRef]
- van der Maaden, K.; Luttge, R.; Vos, P.J.; Bouwstra, J.; Kersten, G.; Ploemen, I. Microneedle-based drug and vaccine delivery via nanoporous microneedle arrays. Drug Deliv. Transl. Res. 2015, 5, 397–406. [Google Scholar] [CrossRef]
- Haq, M.I.; Smith, E.; John, D.N.; Kalavala, M.; Edwards, C.; Anstey, A.; Morrissey, A.; Birchall, J.C. Clinical administration of microneedles: Skin puncture, pain and sensation. Biomed. Microdevices 2009, 11, 35–47. [Google Scholar] [CrossRef]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of Microneedle Design on Pain in Human Subjects. Clin. J. Pain 2008, 24, 585. [Google Scholar] [CrossRef]
- Aggarwal, P.; Johnston, C.R. Geometrical effects in the mechanical characterizing of microneedle for biomedical applications. Sens. Actuators B Chem. 2004, 102, 226–234. [Google Scholar] [CrossRef]
- Davis, S.P.; Martanto, W.; Allen, M.G.; Prausnitz, M.R. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans. Biomed. Eng. 2005, 52, 909–915. [Google Scholar] [CrossRef]
- Olatunji, O.; Das, D.B.; Garland, M.J.; Belaid, L.; Donnelly, R.F. Influence of array interspacing on the force required for successful microneedle skin penetration: Theoretical and practical approaches. J. Pharm. Sci. 2013, 102, 1209–1221. [Google Scholar] [CrossRef]
- Yang, M.; Zahn, J.D. Microneedle insertion force reduction using vibratory actuation. Biomed. Microdevices 2004, 6, 177–182. [Google Scholar] [CrossRef]
- ISO 10993-1:2018; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process. International Organization for Standardization: Geneva, Switzerland, 2018.

| Design | Diameter (mm) | Aspect Ratio | Height (mm) |
|---|---|---|---|
| 1 | 0.3 | 2:1 | 0.6 |
| 2 | 0.3 | 3:1 | 0.9 |
| 3 | 0.3 | 4:1 | 1.2 |
| 4 | 0.4 | 2:1 | 0.8 |
| 5 | 0.4 | 3:1 | 1.2 |
| 6 | 0.4 | 4:1 | 1.6 |
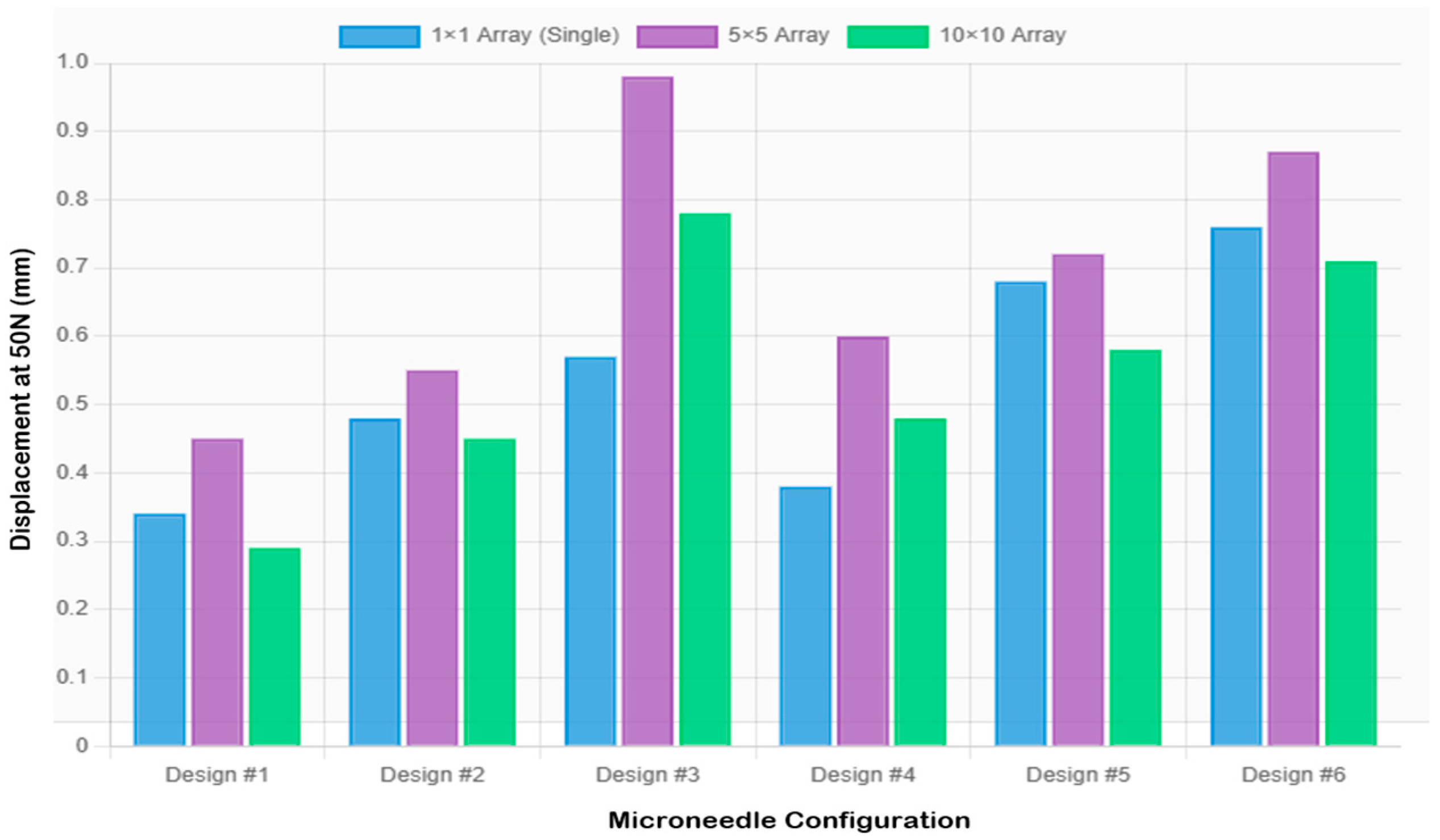
| Design | Config | Diameter (mm) | Height (mm) | Load/Needle (N) | Experimental δ (mm) | FEA SF | FEA δ (mm) |
|---|---|---|---|---|---|---|---|
| 1 | 1 × 1 | 0.3 | 0.6 | 50 | 0.349 ± 0.024 | 0.075 | 0.1872 |
| 1 | 5 × 5 | 0.3 | 0.6 | 2 | 0.456 ± 0.035 | 1.873 | 0.0082 |
| 1 | 10 × 10 | 0.3 | 0.6 | 0.5 | 0.281 ± 0.016 | 7.493 | 0.0018 |
| 2 | 1 × 1 | 0.3 | 0.9 | 50 | 0.481 ± 0.031 | 0.075 | 0.2687 |
| 2 | 5 × 5 | 0.3 | 0.9 | 2 | 0.552 ± 0.041 | 1.873 | 0.0107 |
| 2 | 10 × 10 | 0.3 | 0.9 | 0.5 | 0.448 ± 0.027 | 7.493 | 0.0027 |
| 3 | 1 × 1 | 0.3 | 1.2 | 50 | 0.570 ± 0.028 | 0.075 | 0.3583 |
| 3 | 5 × 5 | 0.3 | 1.2 | 2 | 0.966 ± 0.057 | 1.873 | 0.0143 |
| 3 | 10 × 10 | 0.3 | 1.2 | 0.5 | 0.782 ± 0.045 | 7.493 | 0.0036 |
| 4 | 1 × 1 | 0.4 | 0.8 | 50 | 0.378 ± 0.033 | 0.133 | 0.1344 |
| 4 | 5 × 5 | 0.4 | 0.8 | 2 | 0.598 ± 0.038 | 3.33 | 0.0054 |
| 4 | 10 × 10 | 0.4 | 0.8 | 0.5 | 0.483 ± 0.029 | 13.32 | 0.0013 |
| 5 | 1 × 1 | 0.4 | 1.2 | 50 | 0.680 ± 0.042 | 0.133 | 0.2015 |
| 5 | 5 × 5 | 0.4 | 1.2 | 2 | 0.724 ± 0.045 | 3.33 | 0.0081 |
| 5 | 10 × 10 | 0.4 | 1.2 | 0.5 | 0.578 ± 0.036 | 13.32 | 0.002 |
| 6 | 1 × 1 | 0.4 | 1.6 | 50 | 0.750 ± 0.038 | 0.133 | 0.2687 |
| 6 | 5 × 5 | 0.4 | 1.6 | 2 | 0.874 ± 0.052 | 3.33 | 0.0107 |
| 6 | 10 × 10 | 0.4 | 1.6 | 0.5 | 0.715 ± 0.041 | 13.32 | 0.0027 |
| Source | df | Sum of Squares | Mean Square | F-Statistic | p-Value | Partial η2 |
|---|---|---|---|---|---|---|
| Design (D) | 5 | 2.847 | 0.569 | 145.3 | <0.001 | 0.91 |
| Array Configuration (A) | 2 | 0.612 | 0.306 | 78.2 | <0.001 | 0.685 |
| D × A Interaction | 10 | 0.485 | 0.048 | 12.4 | <0.001 | 0.633 |
| Residual | 72 | 0.282 | 0.004 | — | — | — |
| Total | 89 | 4.226 | — | — | — | — |
| Comparison | Mean Difference (mm) | 95% CI | p-Value | Cohen’s d |
|---|---|---|---|---|
| Design Effects (10 × 10 arrays): | ||||
| Design 1 vs. Design 5 | −0.297 | [−0.349, −0.245] | <0.001 | 8.24 (large) |
| Design 3 vs. Design 5 | 0.204 | [0.156, 0.252] | <0.001 | 4.71 (large) |
| Design 4 vs. Design 5 | −0.095 | [−0.143, −0.047] | <0.001 | 2.64 (large) |
| Array Configuration Effects (Design 5): | ||||
| 1 × 1 vs. 10 × 10 | 0.102 | [0.062, 0.142] | <0.001 | 2.41 (large) |
| 5 × 5 vs. 10 × 10 | 0.146 | [0.106, 0.186] | <0.001 | 3.26 (large) |
| 1 × 1 vs. 5 × 5 | −0.044 | [−0.084, −0.004] | 0.028 | 1.04 (large) |
| Design | Configuration | Compressive Displacement at 50 N (mm) |
|---|---|---|
| Design 1 | 1 × 1 (0.3 mm × 0.6 mm) | 0.349 ± 0.024 |
| Design 2 | 1 × 1 (0.3 mm × 0.9 mm) | 0.481 ± 0.031 |
| Design 3 | 1 × 1 (0.3 mm × 1.2 mm) | 0.570 ± 0.028 |
| Design 4 | 1 × 1 (0.4 mm × 0.8 mm) | 0.378 ± 0.033 |
| Design 5 | 1 × 1 (0.4 mm × 1.2 mm) | 0.680 ± 0.042 |
| Design 6 | 1 × 1 (0.4 mm × 1.6 mm) | 0.750 ± 0.038 |
| Design | Configuration | Load/Needle (N) | Compressive Displacement at 50 N (mm) |
|---|---|---|---|
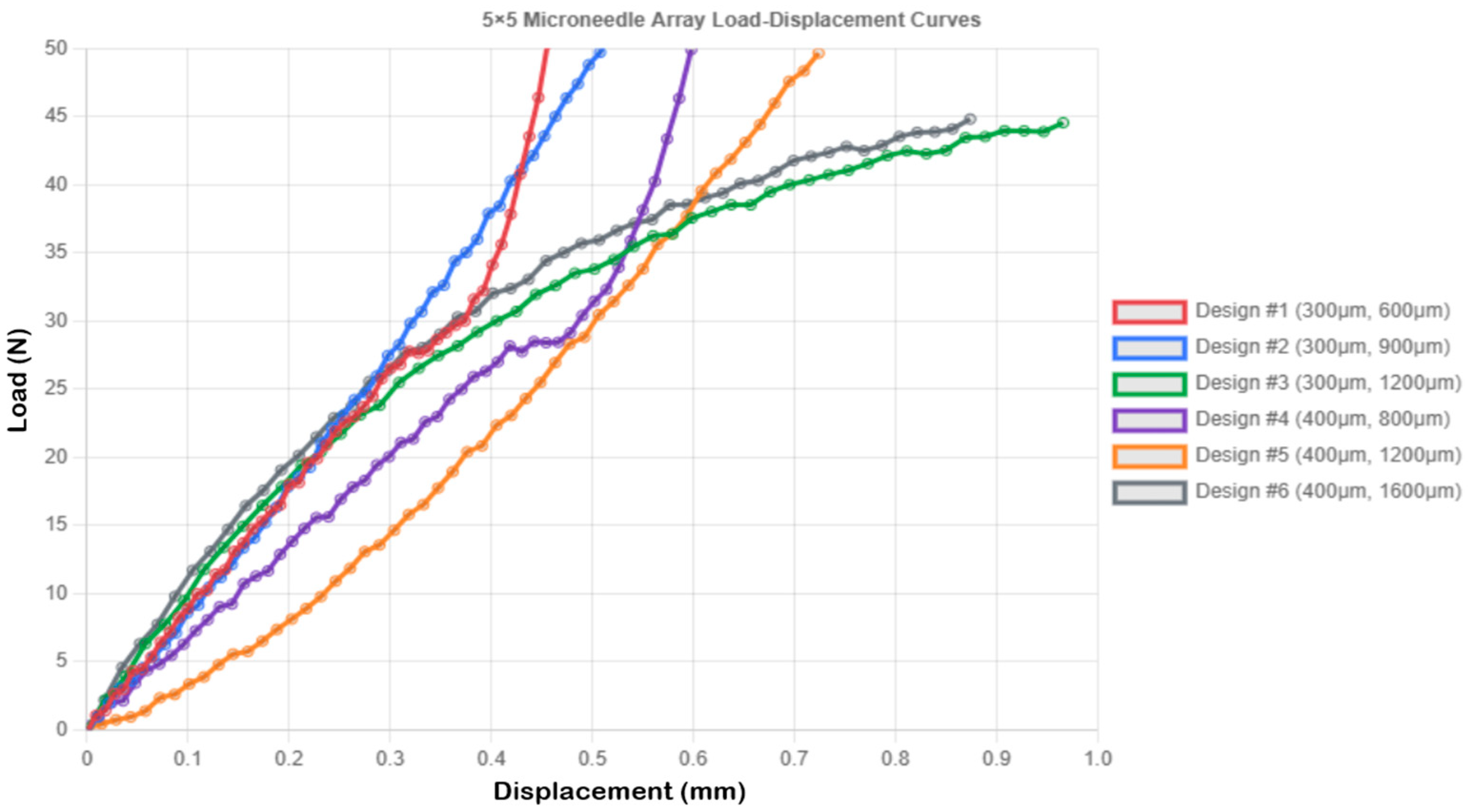
| Design 1 | 5 × 5 (0.3 mm × 0.6 mm) | 2.0 | 0.456 ± 0.035 |
| Design 2 | 5 × 5 (0.3 mm × 0.9 mm) | 2.0 | 0.552 ± 0.041 |
| Design 3 | 5 × 5 (0.3 mm × 1.2 mm) | 2.0 | 0.966 ± 0.057 |
| Design 4 | 5 × 5 (0.4 mm × 0.8 mm) | 2.0 | 0.598 ± 0.038 |
| Design 5 | 5 × 5 (0.4 mm × 1.2 mm) | 2.0 | 0.724 ± 0.045 |
| Design 6 | 5 × 5 (0.4 mm × 1.6 mm) | 2.0 | 0.874 ± 0.052 |
| Design | Configuration | Load/Needle (N) | Compressive Displacement at 50 N (mm) |
|---|---|---|---|
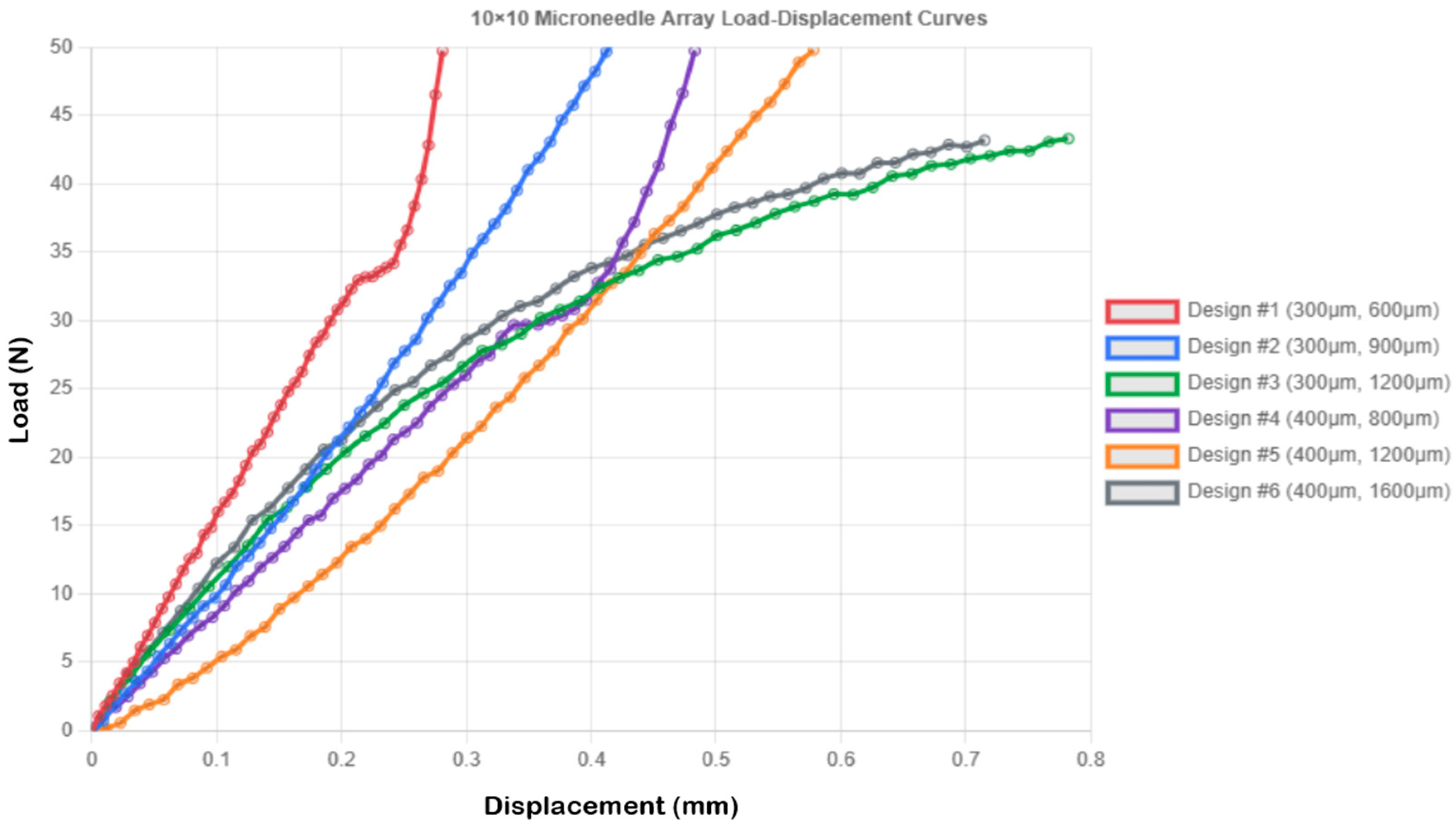
| Design 1 | 10 × 10 (0.3 mm × 0.6 mm) | 0.5 | 0.281 ± 0.016 |
| Design 2 | 10 × 10 (0.3 mm × 0.9 mm) | 0.5 | 0.448 ± 0.027 |
| Design 3 | 10 × 10 (0.3 mm × 1.2 mm) | 0.5 | 0.782 ± 0.045 |
| Design 4 | 10 × 10 (0.4 mm × 0.8 mm) | 0.5 | 0.483 ± 0.029 |
| Design 5 | 10 × 10 (0.4 mm × 1.2 mm) | 0.5 | 0.578 ± 0.036 |
| Design 6 | 10 × 10 (0.4 mm × 1.6 mm) | 0.5 | 0.715 ± 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldawood, F.K.; Abualkhair, H.F. Geometric Parameter Optimization of 3D-Printed Microneedle Arrays Based on Comprehensive Mechanical Testing and Failure Analysis. Micromachines 2025, 16, 1377. https://doi.org/10.3390/mi16121377
Aldawood FK, Abualkhair HF. Geometric Parameter Optimization of 3D-Printed Microneedle Arrays Based on Comprehensive Mechanical Testing and Failure Analysis. Micromachines. 2025; 16(12):1377. https://doi.org/10.3390/mi16121377
Chicago/Turabian StyleAldawood, Faisal Khaled, and Hussain F. Abualkhair. 2025. "Geometric Parameter Optimization of 3D-Printed Microneedle Arrays Based on Comprehensive Mechanical Testing and Failure Analysis" Micromachines 16, no. 12: 1377. https://doi.org/10.3390/mi16121377
APA StyleAldawood, F. K., & Abualkhair, H. F. (2025). Geometric Parameter Optimization of 3D-Printed Microneedle Arrays Based on Comprehensive Mechanical Testing and Failure Analysis. Micromachines, 16(12), 1377. https://doi.org/10.3390/mi16121377





