Characterization and Integration of Terahertz Technology within Microfluidic Platforms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Microfluidic Platform Fabrication
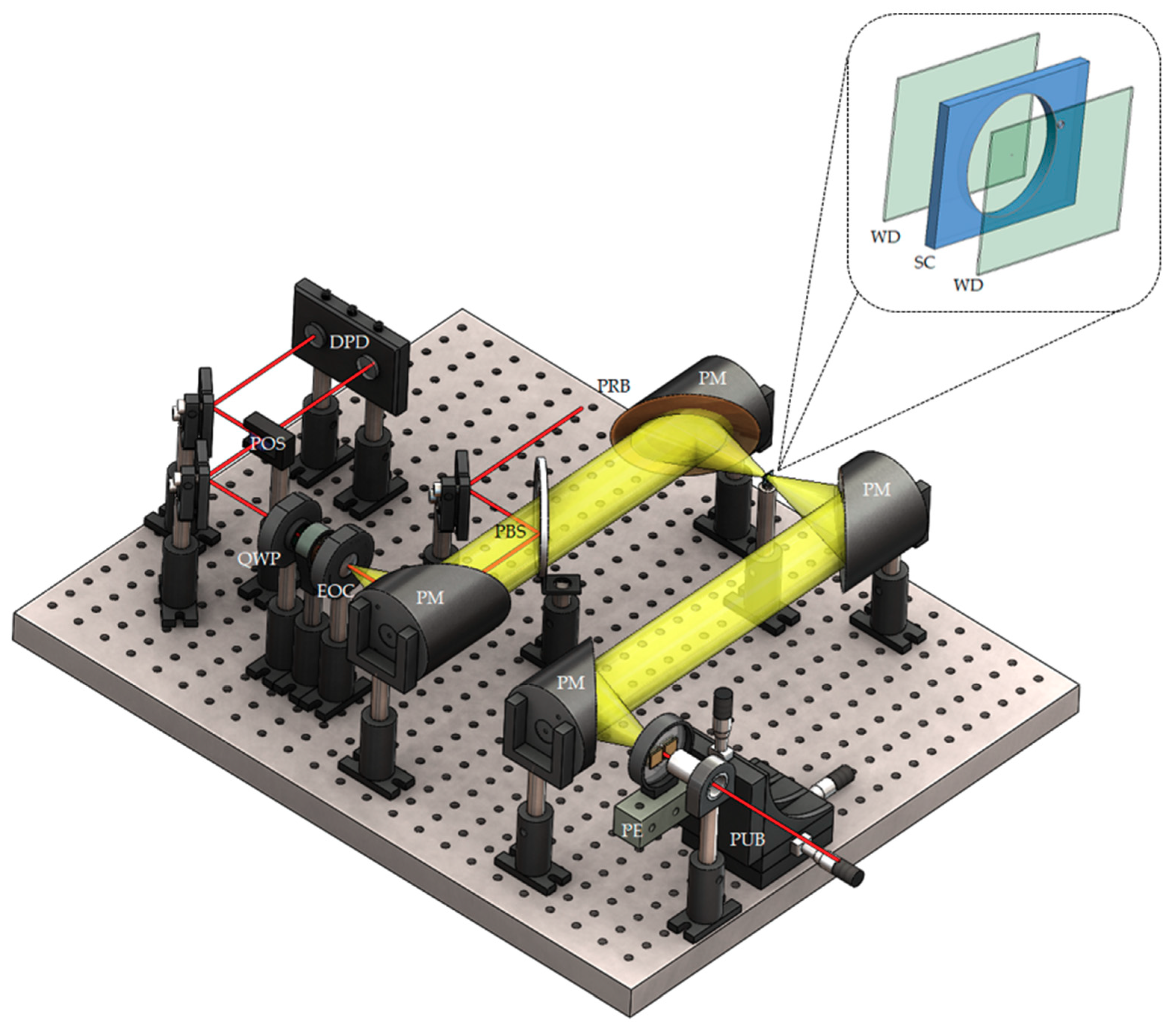
2.3. Terahertz Time-Domain Spectroscopy
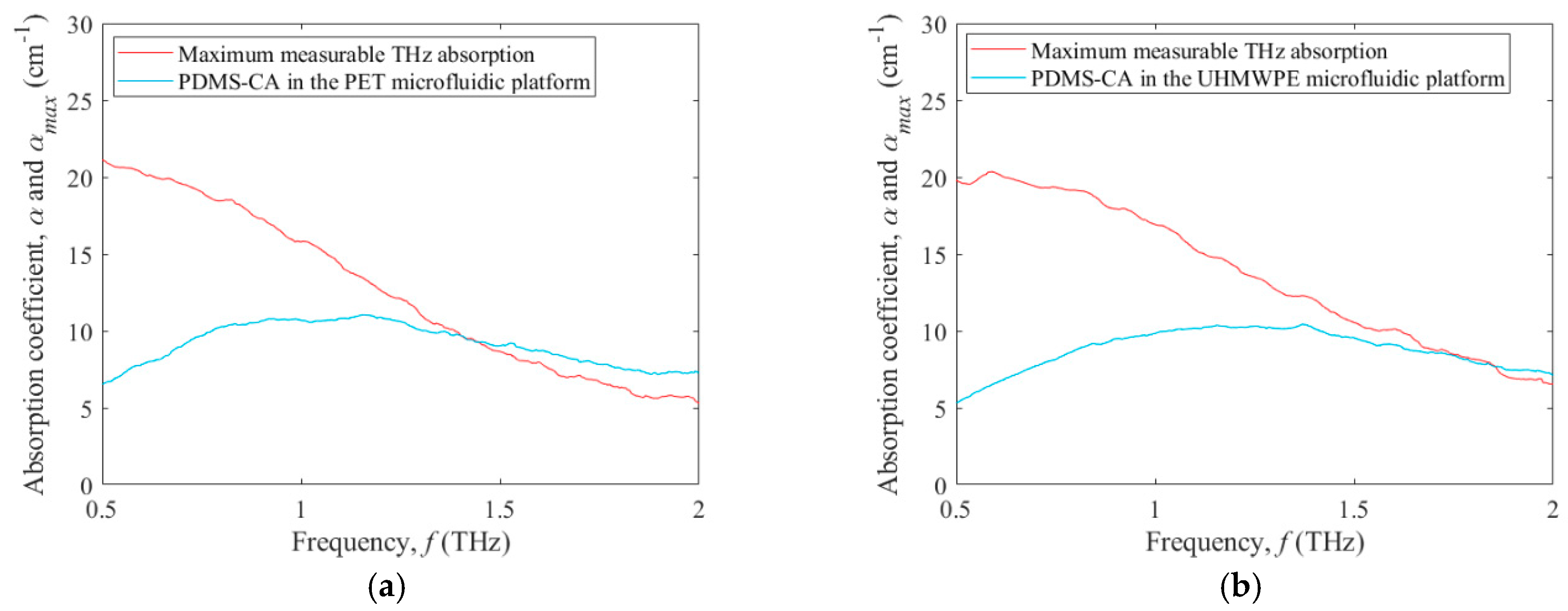
3. Results and Discussion
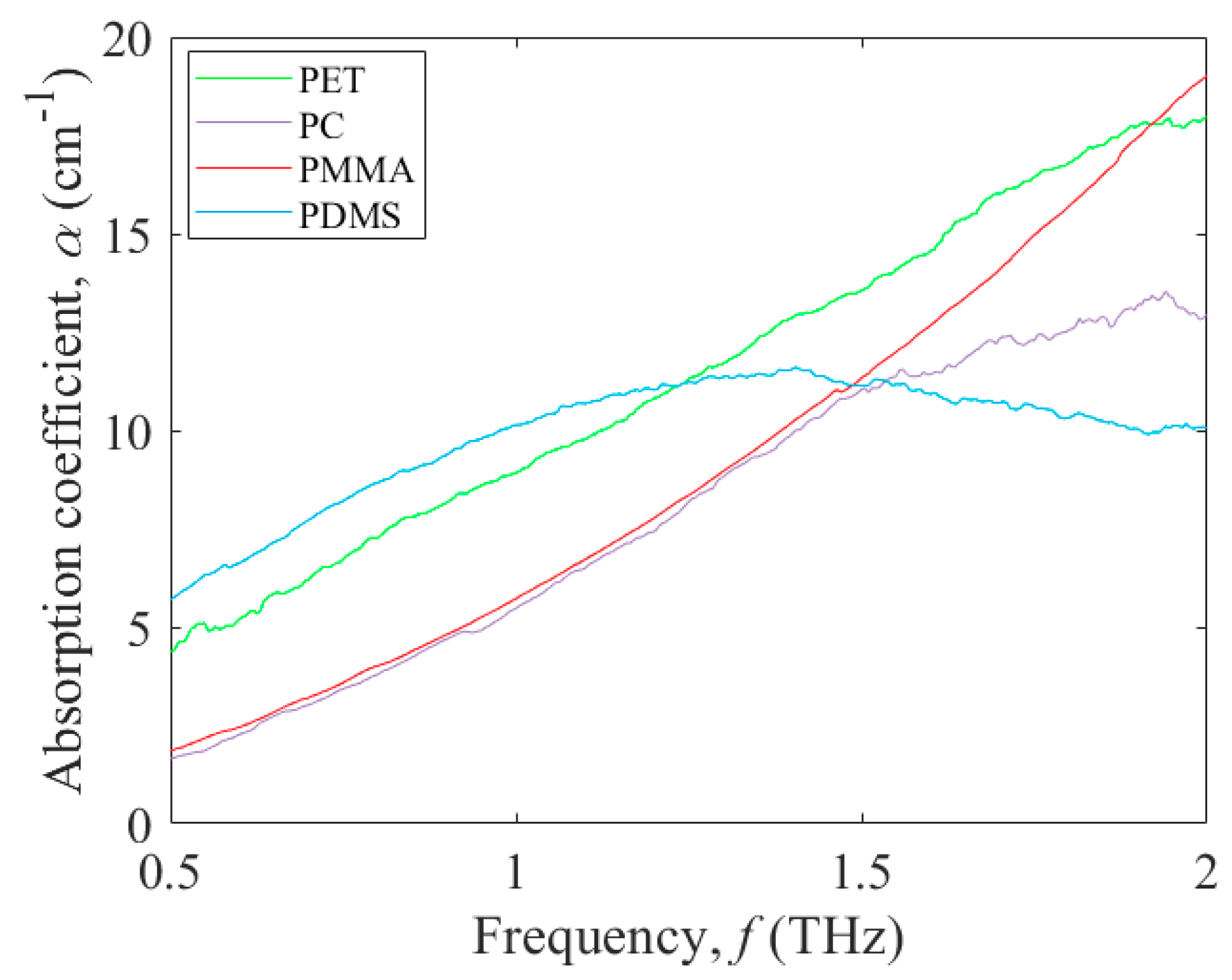
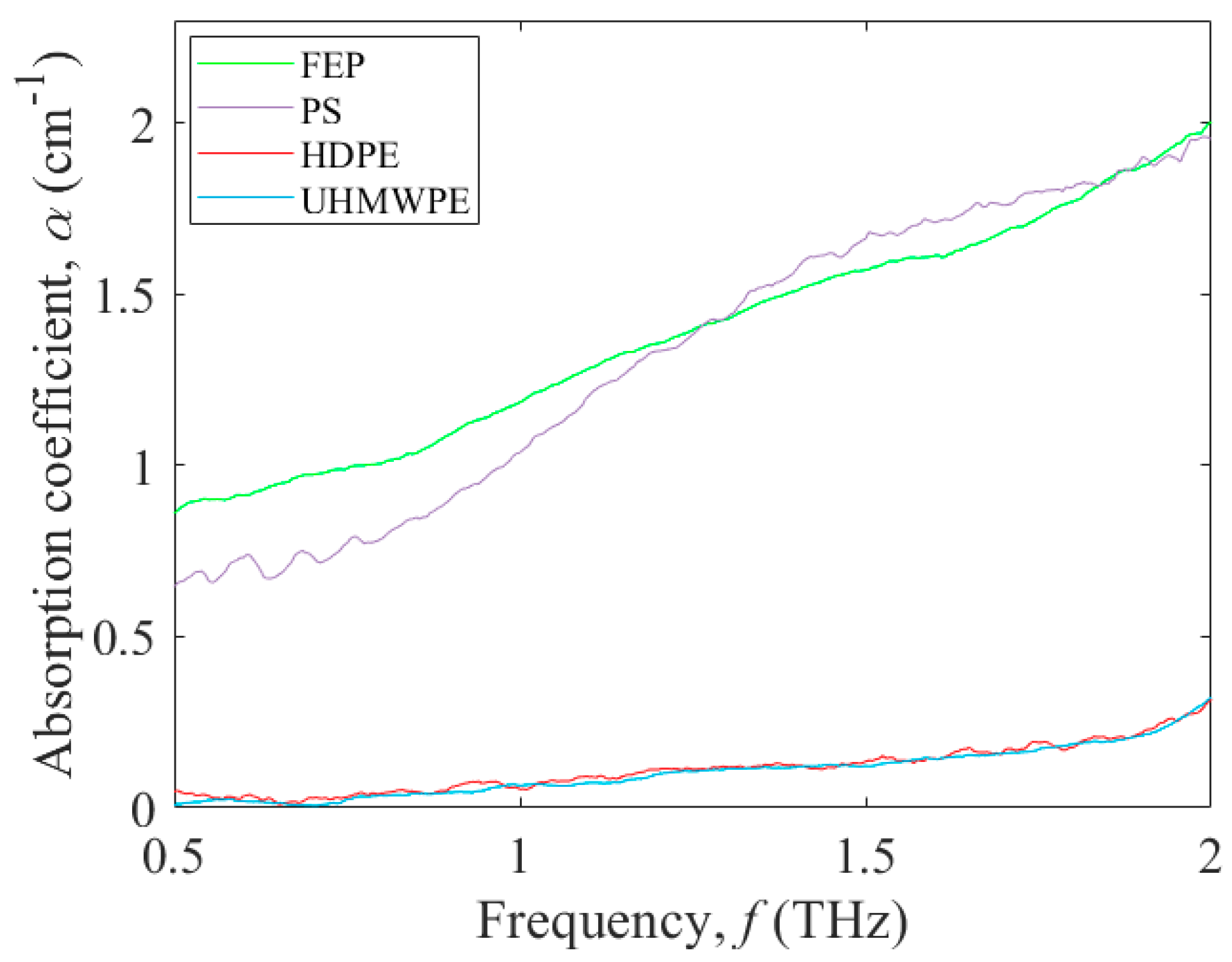
3.1. Terahertz Absorption of Potential Polymers for Microfluidic Platforms
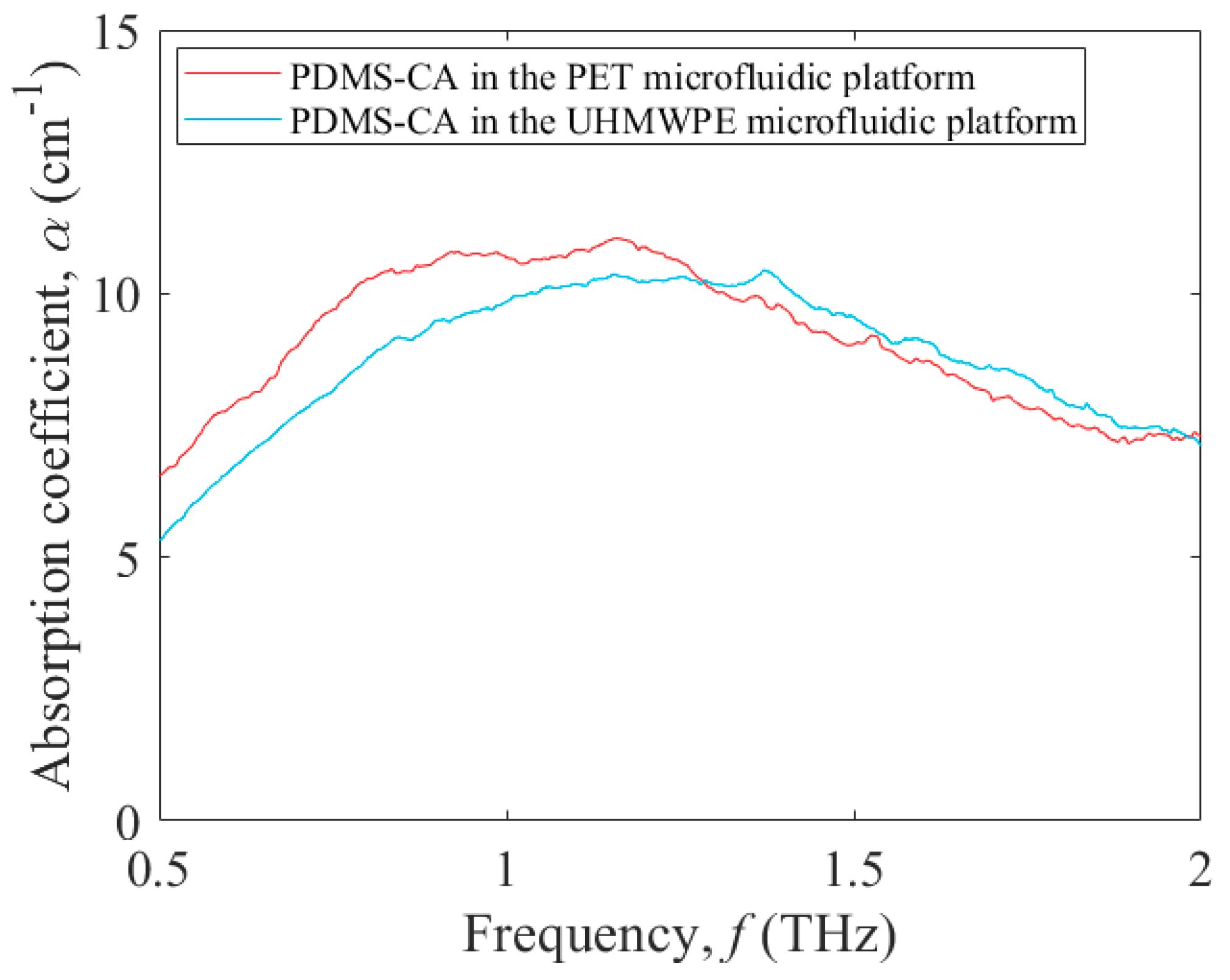
3.2. Terahertz Absorption of a Test Fluid within Microfluidic Platforms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Soon, J.H. Terahertz electromagnetic interactions with biological matter and their applications. J. Appl. Phys. 2009, 105, 102033. [Google Scholar] [CrossRef]
- Choudhury, B.; Rakesh, A.M.; Jha, M. Active terahertz metamaterial for biomedical applications. In Active Terahertz Metamaterial for Biomedical Applications Singapore, 1st ed.; Springer: Singapore, 2016; pp. 1–49. [Google Scholar]
- Joseph, C.S.; Yaroslavsky, A.N.; Neel, V.A.; Goyette, T.M.; Giles, R.H. Continuous-wave terahertz reflection imaging of ex vivo nonmelanoma skin cancers. In Proceedings of the 2012 SPIE OPTO Conference, San Francisco, CA, USA, 28 January–2 February 2012. [Google Scholar]
- Haddad, J.E.; Bousquet, B.; Canioni, L.; Mounaix, P. Review in terahertz spectral analysis. TrAC Trends Anal. Chem. 2013, 44, 98–105. [Google Scholar] [CrossRef]
- Xie, J.; Zhu, X.; Zang, X.; Cheng, Q.; Chen, L.; Zhu, Y. Metamaterial-enhanced terahertz vibrational spectroscopy for thin film detection. Opt. Mater. Express 2018, 8, 128–135. [Google Scholar] [CrossRef]
- Smye, S.W.; Chamberlain, J.M.; Fitzgerald, A.J.; Berry, E. The interaction between terahertz radiation and biological tissue. Phys. Med. Biol. 2001, 49, R101–R112. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, X.; Yang, K.; Liu, Y.; Fu, W.; Luo, Y. Biomedical applications of terahertz spectroscopy and imaging. Trends Biotechnol. 2016, 34, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Baxter, J.B.; Guglietta, G.W. Terahertz Spectroscopy. Anal. Chem. 2011, 83, 4342–4368. [Google Scholar] [CrossRef] [PubMed]
- Tsurkan, V.; Balbekin, N.S.; Sobakinskaya, E.A.; Panin, A.N.; Vaks, V.L. Terahertz spectroscopy of DNA. Opt. Spectrosc. 2013, 114, 894–898. [Google Scholar] [CrossRef]
- Cheon, H.; Yang, H.J.; Son, J.H. Toward clinical cancer imaging using terahertz spectroscopy. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 1–9. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Ma, S.; Wu, X.; Yang, W.; Zhang, W.; Li, X. Quantify glucose level in freshly diabetic’s blood by terahertz time-domain spectroscopy. J. Infrared Millim. Terahertz Waves 2018, 39, 399–408. [Google Scholar] [CrossRef]
- Tang, Q.; Liang, M.; Lu, Y.; Wong, P.K.; Wilmink, P.K.; Zhang, D.D.; Xin, H. Microfluidic devices for terahertz spectroscopy of live cells toward lab-on-a-chip applications. Sensors 2016, 16, 476. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, J.; Fischer, B.M.; Abbott, D. Sensing the hygroscopicity of polymer and copolymer materials using terahertz time-domain spectroscopy. Appl. Opt. 2009, 48, 2262–2266. [Google Scholar] [CrossRef] [PubMed]
- George, P.A.; Hui, W.; Rana, F.; Hawkins, B.G.; Smith, A.E.; Kirby, B.J. Microfluidic devices for terahertz spectroscopy of biomolecules. Opt. Express 2008, 16, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jiang, Z.; Rahman, S.; Shams, I.B.; Jing, B.; Kannegulla, A.; Cheng, L.J. Quasi-optical terahertz Microfluidic devices for chemical sensing and imaging. Micromachines 2016, 7, 75. [Google Scholar] [CrossRef]
- Laurette, S.; Treizebre, A.; Bocquet, B. Co-integrated microfluidic and THz functions for biochip devices. J. Micromech. Microeng. 2011, 21, 065029. [Google Scholar] [CrossRef]
- Jo, B.H.; Van Lerberghe, L.M.; Motsegood, K.M.; Beebe, D.J. Three-dimensional micro-channel fabrication in polydimethylsiloxane (PDMS) elastomer. J. Microelectromech. Syst. 2000, 9, 76–81. [Google Scholar] [CrossRef]
- Khosla, A. Nanoparticle-doped electrically-conducting polymers for flexible nano-micro systems. Electrochem. Soc. Interface 2012, 21, 67–70. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly (dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Su, B.; Wu, Y.; Zhang, C. Terahertz spectra of glycerol, water, and their mixture in a microfluidic chip. In Proceedings of the 2014 SPIE COS Photonics Asia Conference, Beijing, China, 11–14 October 2014. [Google Scholar]
- Hong, T.F.; Ju, W.J.; Wu, M.C.; Tai, C.H.; Tsai, C.H.; Fu, L.M. Rapid prototyping of PMMA microfluidic chips utilizing a CO2 laser. Microfluid. Nanofluid. 2010, 9, 1125–1133. [Google Scholar] [CrossRef]
- Jena, R.K.; Yue, C.Y. Cyclic olefin copolymer based microfluidic devices for biochip applications: Ultraviolet surface grafting using 2-metha153cryloyloxyethyl phosphorylcholine. Biomicrofluidics 2012, 6, 012822. [Google Scholar] [CrossRef] [PubMed]
- Toossi, A.; Moghadas, H.; Daneshmand, M.; Sameoto, D. Bonding PMMA microfluidics using commercial microwave ovens. J. Micromech. Microeng. 2015, 25, 085008. [Google Scholar] [CrossRef]
- Withayachumnankul, W.; Fischer, B.M.; Abbott, D. Material thickness optimization for transmission-mode terahertz time-domain spectroscopy. Opt. Express 2008, 16, 7382–7396. [Google Scholar] [CrossRef] [PubMed]
- Alfihed, S.; Bergen, M.H.; Holzman, J.F.; Foulds, I.G. A detailed investigation on the terahertz absorption characteristics of polydimethylsiloxane (PDMS). Polymer 2018, 153, 325–330. [Google Scholar] [CrossRef]
- Gallot, G.; Grischkowsky, D. Electro-optic detection of terahertz radiation. JOSA B 1999, 16, 1204–1212. [Google Scholar] [CrossRef]
- Naftaly, M.; Miles, R.E. Terahertz Time-Domain Spectroscopy for Material Characterization. Proc. IEEE 2007, 95, 1658–1665. [Google Scholar] [CrossRef]
- Naftaly, M.; Dudley, R. Methodologies for determining the dynamic ranges and signal-to-noise ratios of terahertz time-domain spectrometers. Opt. Lett. 2009, 34, 1213–1215. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, P.U.; Fischer, B.M. Dynamic range in terahertz time-domain transmission and reflection spectroscopy. Opt. Lett. 2005, 30, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.N.; Willms, R.B.; Gray, G.T.; Rae, P.J.; Cady, C.M.; Vecchio, K.S.; Flowers, J.; Martinez, M.Y. Influence of molecular conformation on the constitutive response of polyethylene: A comparison of HDPE, UHMWPE, and PEX. Exp. Mech. 2007, 47, 381–393. [Google Scholar] [CrossRef]
- Nagai, N.; Fukasawa, R. Abnormal dispersion of polymer films in the THz frequency region. Chem. Phys. Lett. 2004, 388, 479–482. [Google Scholar] [CrossRef]
| Polymer | Thickness (mm) | Preparation | |
|---|---|---|---|
| Polar Polymers | PET | 0.65 ± 0.1 | Thermal bonding |
| PC | 1.00 ± 0.1 | Bulk material | |
| PMMA | 1.00 ± 0.1 | Bulk material | |
| PDMS | 1.70 ± 0.3 | Mixing and curing | |
| Nonpolar polymers | FEP | 4.30 ± 0.2 | Thermal bonding |
| PS | 4.80 ± 0.2 | Thermal bonding | |
| HDPE | 25.4 ± 0.1 | Bulk material | |
| UHMWPE | 25.6 ± 0.2 | Thermal bonding | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfihed, S.; Bergen, M.H.; Ciocoiu, A.; Holzman, J.F.; Foulds, I.G. Characterization and Integration of Terahertz Technology within Microfluidic Platforms. Micromachines 2018, 9, 453. https://doi.org/10.3390/mi9090453
Alfihed S, Bergen MH, Ciocoiu A, Holzman JF, Foulds IG. Characterization and Integration of Terahertz Technology within Microfluidic Platforms. Micromachines. 2018; 9(9):453. https://doi.org/10.3390/mi9090453
Chicago/Turabian StyleAlfihed, Salman, Mark H. Bergen, Antonia Ciocoiu, Jonathan F. Holzman, and Ian G. Foulds. 2018. "Characterization and Integration of Terahertz Technology within Microfluidic Platforms" Micromachines 9, no. 9: 453. https://doi.org/10.3390/mi9090453
APA StyleAlfihed, S., Bergen, M. H., Ciocoiu, A., Holzman, J. F., & Foulds, I. G. (2018). Characterization and Integration of Terahertz Technology within Microfluidic Platforms. Micromachines, 9(9), 453. https://doi.org/10.3390/mi9090453





