Electrospun Three-Dimensional Nanofibrous Structure via Probe Arrays Inducing
Abstract
:1. Introduction
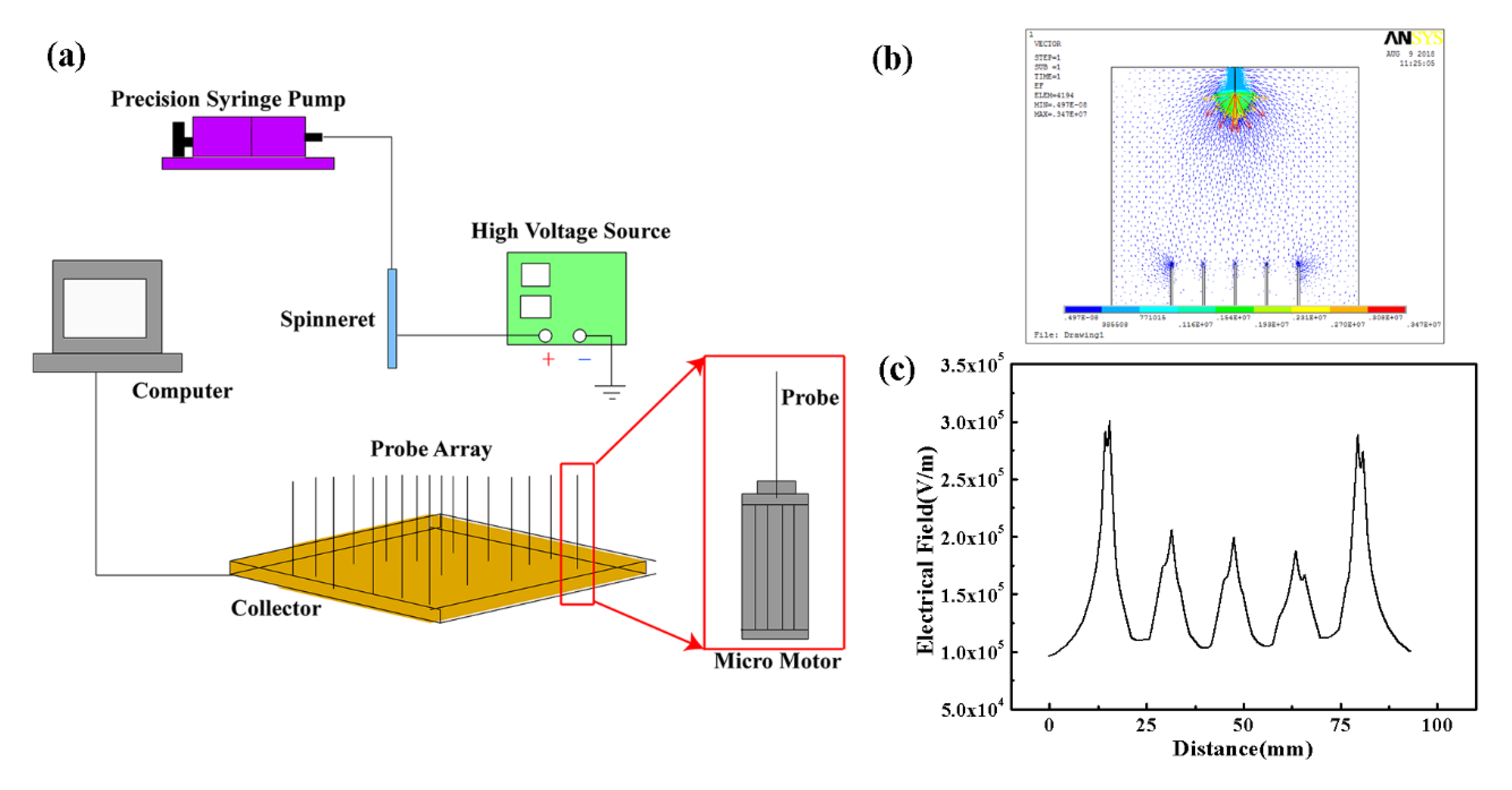
2. Materials and Methods
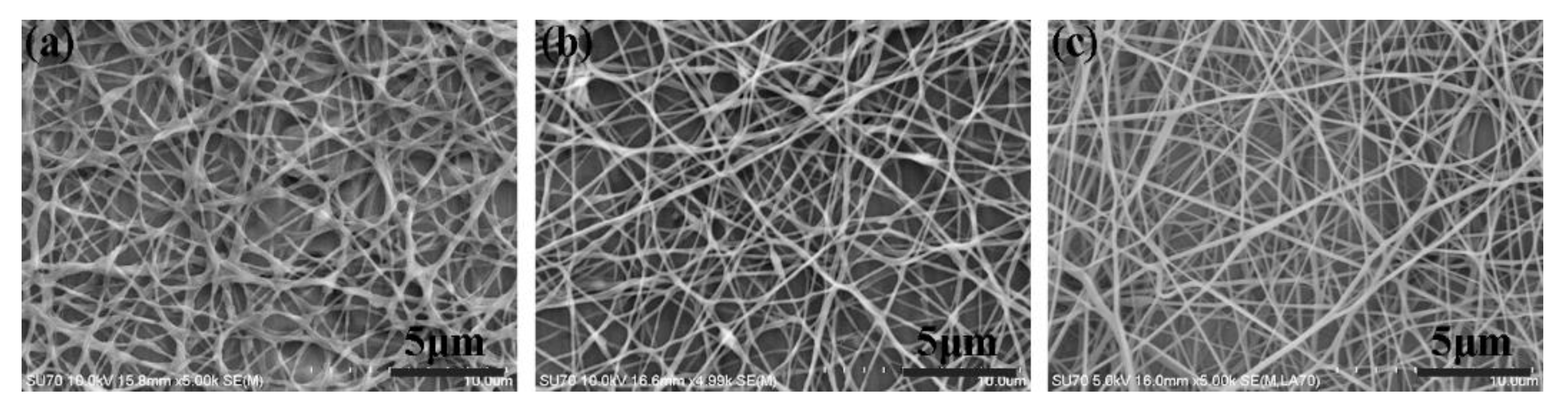
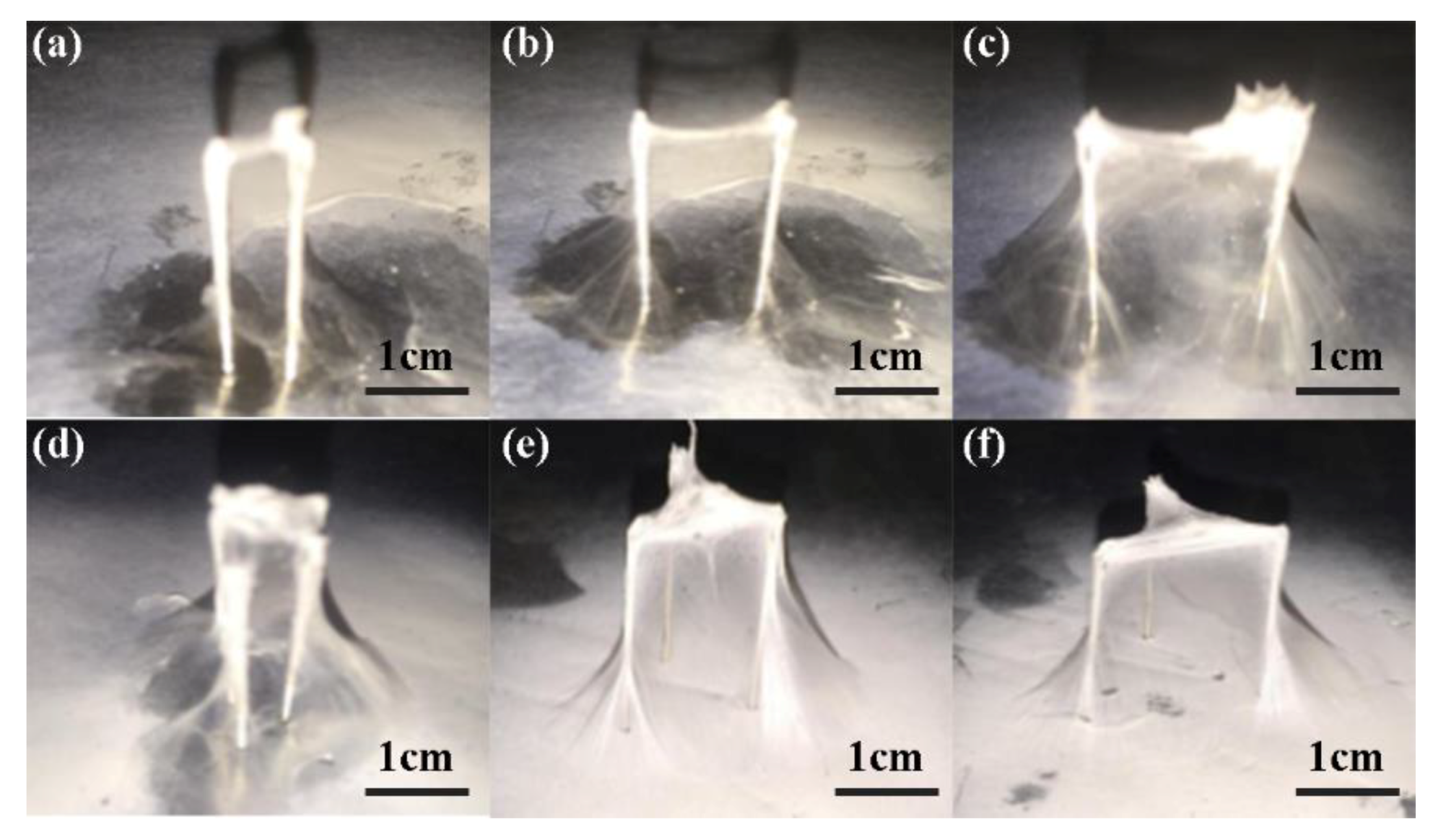
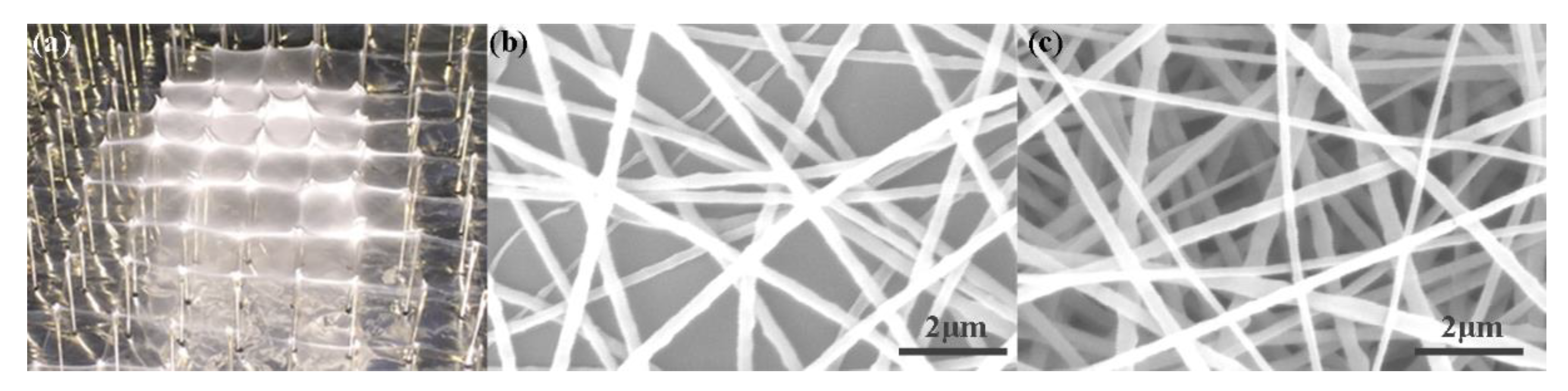
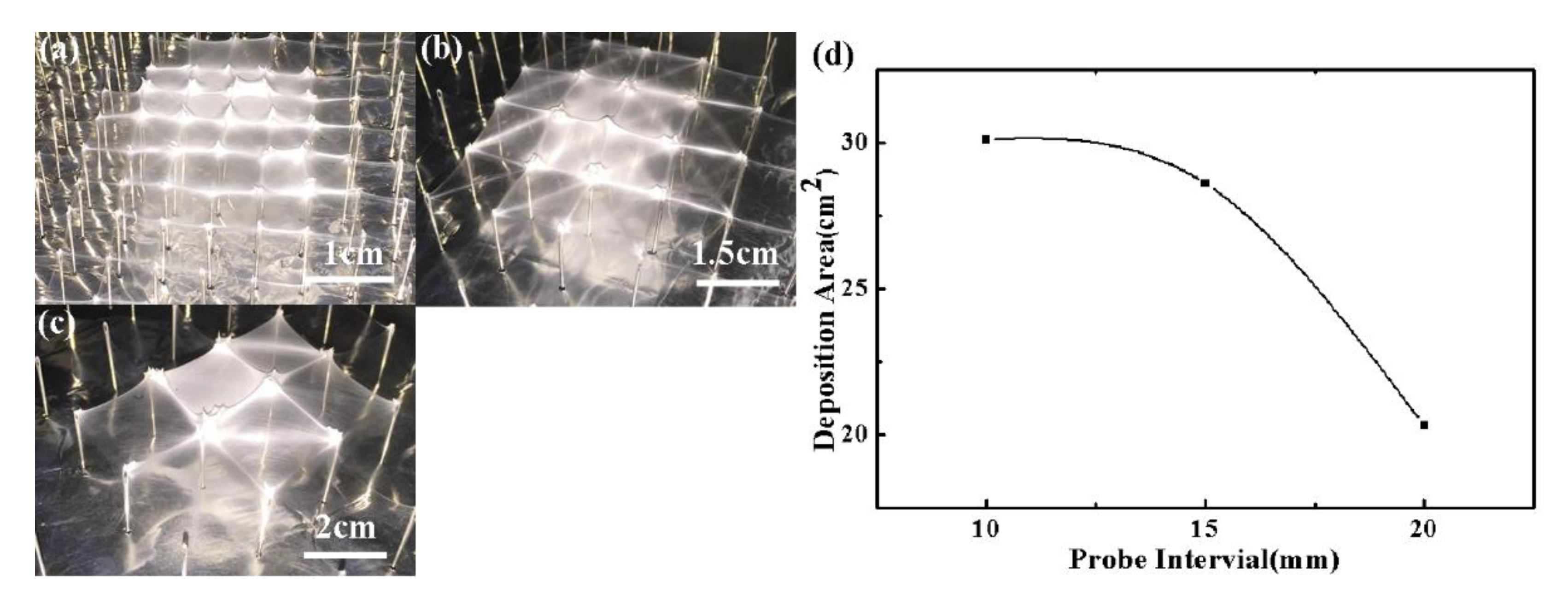
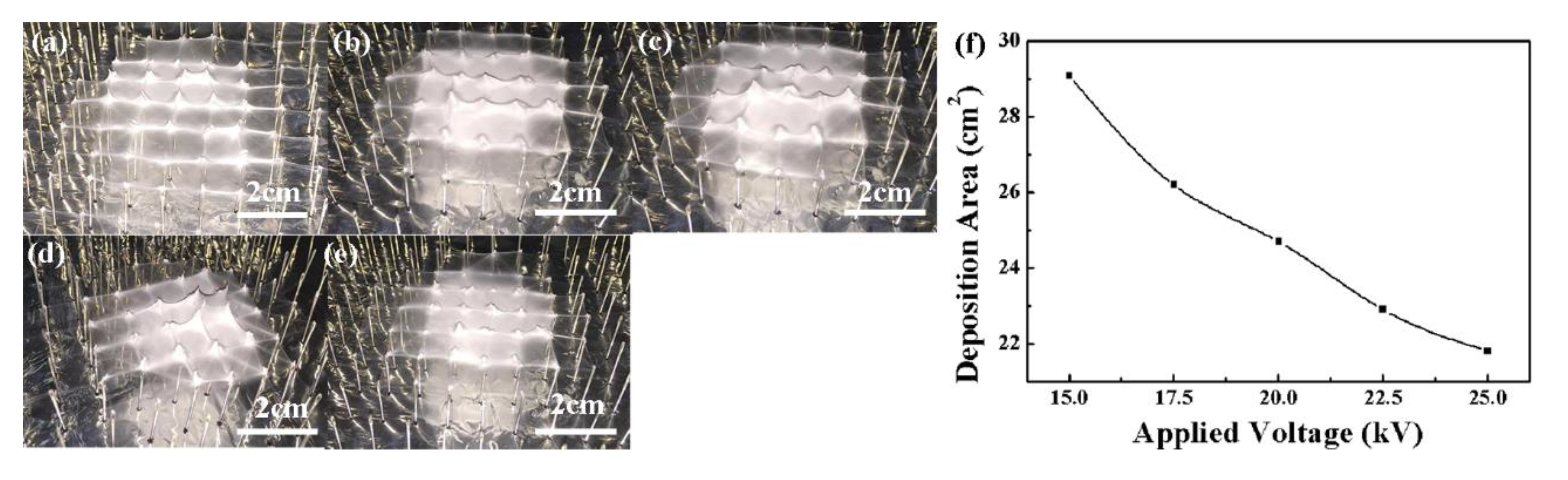
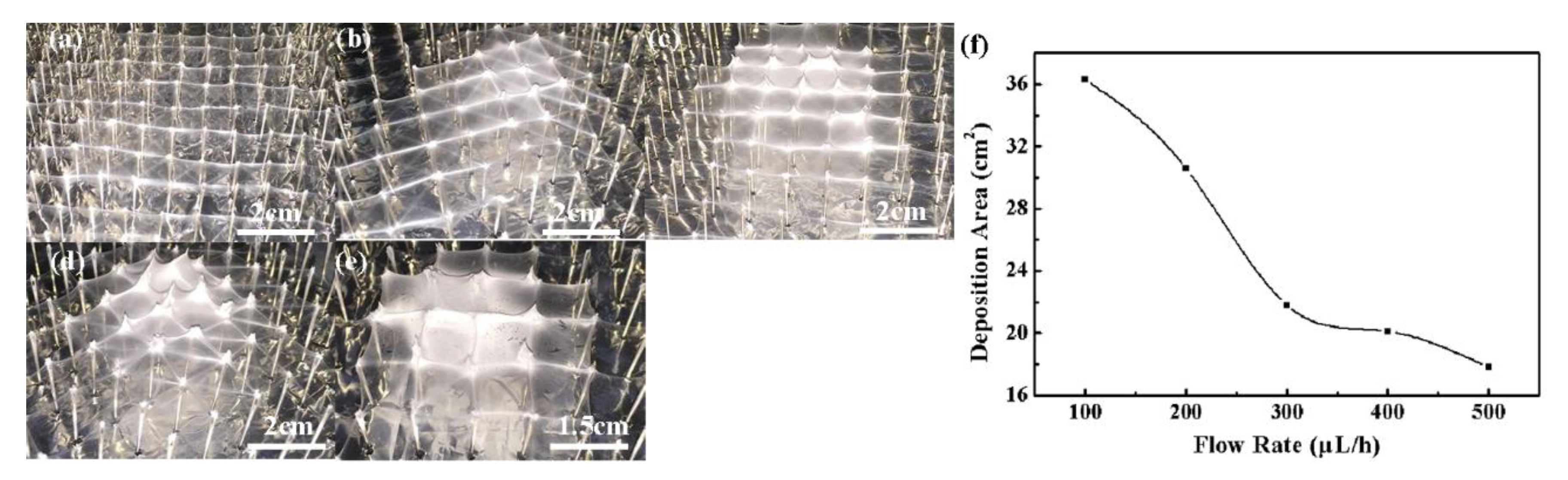
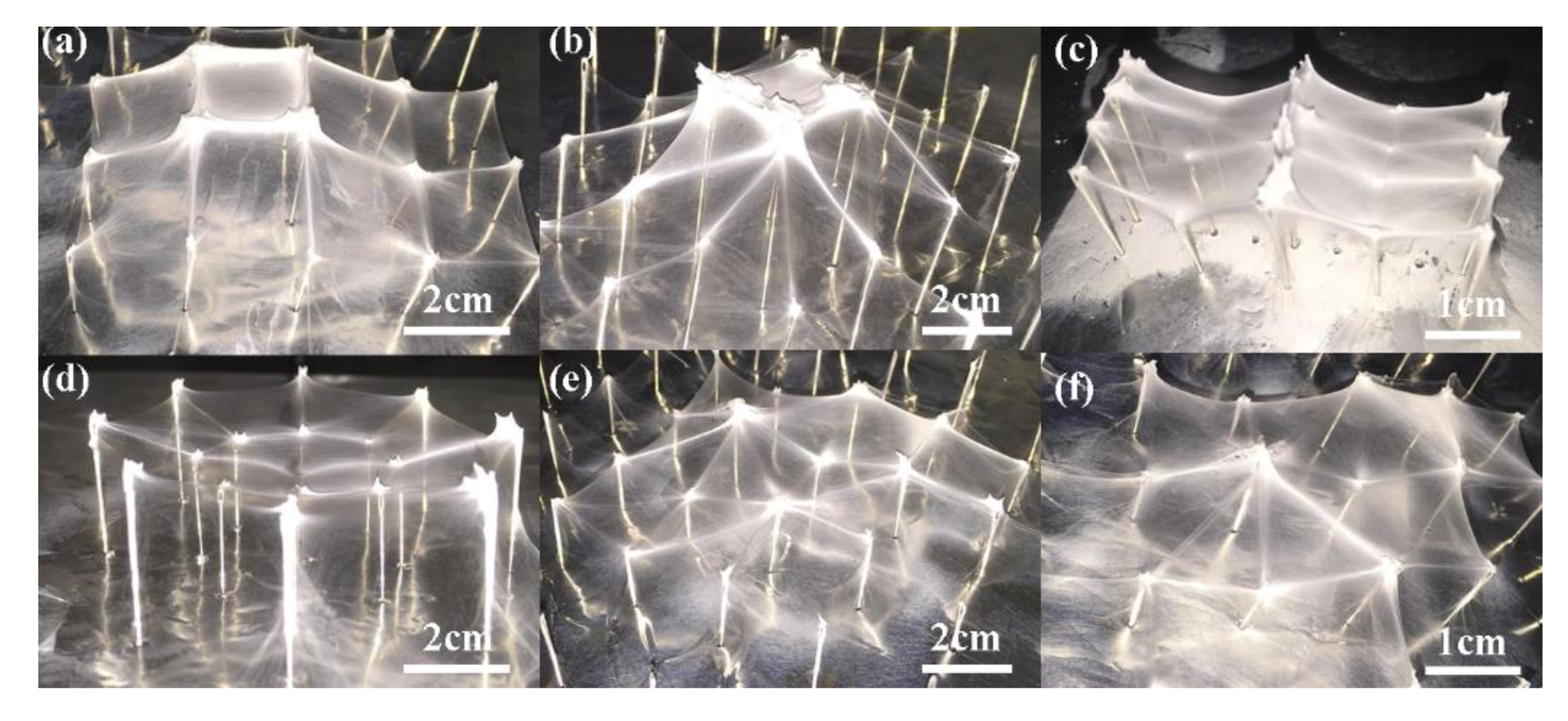
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, X.; Zhang, Y. Tissue engineering applications of three-dimensional bioprinting. Cell Biochem. Biophys. 2015, 72, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Yassen, G.H.; Platt, J.A.; Labban, N.; Windsor, L.J.; Spolnik, K.J.; Bressiani, A.H.A. A novel three-dimensional scaffold for regenerative endodontics: Materials and biological characterizations. J. Tissue Eng. Regen. Med. 2015, 9, E116–E123. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Cosme, J.G.; Xu, T.; Miszuk, J.M.; Picciani, P.H.; Fong, H.; Sun, H. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 2016, 115, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Hamza, S.; Kiani, A. A rapid and easy procedure of conductive 3D nanofibrous structure induced by nanosecond laser processing of Si wafer coated by Au thin-film. Sens. Bio-Sens. Res. 2017, 16, 6–11. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, X.; Xu, C.; Kotov, N.A. Water-rich biomimetic composites with abiotic self-organizing nanofiber network. Adv. Mater. 2018, 30, 1870007. [Google Scholar] [CrossRef]
- Ren, W.; Zheng, Z.; Xu, C.; Niu, C.; Wei, Q.; An, Q.; Zhao, K.; Yan, M.; Qin, M.; Mai, L. Self-sacrificed synthesis of three-dimensional Na3V2(PO4)3 nanofiber network for high-rate sodium–ion full batteries. Nano Energy 2016, 25, 145–153. [Google Scholar] [CrossRef]
- Bai, L.; Jia, L.; Yan, Z.; Liu, Z.; Liu, Y. Plasma-etched electrospun nanofiber membrane as adsorbent for dye removal. Chem. Eng. Res. Des. 2018, 132, 445–451. [Google Scholar] [CrossRef]
- Richner, P.; Kress, S.J.P.; Norris, D.J.; Poulikakos, D. Charge effects and nanoparticle pattern formation in electrohydrodynamic nanodrip printing of colloids. Nanoscale 2016, 8, 6028–6034. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Zheng, G.F.; Jiang, J.X.; Wang, X.; Li, W.W.; Zhong, W.Z.; Guo, S.M. Self-cleaning threaded rod spinneret for high-efficiency needleless electrospinning. Appl. Phys. A-Mater. 2018, 124, 473. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Li, B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.X.; Zheng, G.F.; Li, W.W.; Zhong, L.B.; Zheng, Y.M. Electrospun chitosan nanofiber membrane for adsorption of Cu(II) from aqueous solution: Fabrication, characterization and performance. J. Nanosci. Nanotechnol. 2018, 18, 5624–5635. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.H.; Wu, T.; Mao, J.; Chen, T.; Li, Y.T.; Li, M.; Xu, Y.T.; Zeng, B.R.; Luo, W.A.; Yu, L.K.; et al. Predictable particle engineering: Programming the energy level, carrier generation, and conductivity of core-shell particles. J. Am. Chem. Soc. 2018, 140, 7629–7636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; He, J.; Li, X.; Xu, F.; Li, D. Micro/nanoscale electrohydrodynamic printing: From 2D to 3D. Nanoscale 2016, 8, 15376. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Long, Y.Z.; Zhang, H.D.; Li, M.M.; Duvail, J.L.; Jiang, X.Y.; Yin, H.L. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Ju, H.W.; Lee, J.M.; Bo, M.M.; Park, H.J.; Lee, O.J.; Kim, J.H.; Kim, D.K.; Chan, H.P. 3D electrospun silk fibroin nanofibers for fabrication of artificial skin. Nanomed. Nanotechnol. 2015, 11, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Capulli, A.K.; Macqueen, L.A.; Sheehy, S.P.; Parker, K.K. Fibrous scaffolds for building hearts and heart parts. Adv. Drug Deliv. Rev. 2016, 96, 83–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2015, 10, 715–738. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Teh, K.S.; Liu, Y.; Zang, X.; Wen, Z.; Lin, L. Direct-write, self-aligned electrospinning on paper for controllable fabrication of three-dimensional structures. ACS Appl. Mater. Interfaces 2015, 7, 27765. [Google Scholar] [CrossRef] [PubMed]
- Shim, I.K.; Suh, W.H.; Lee, S.Y.; Lee, S.H.; Heo, S.J.; Lee, M.C.; Lee, S.J. Chitosan nano-/microfibrous double-layered membrane with rolled-up three-dimensional structures for chondrocyte cultivation. J. Biomed. Mater. Res. A 2009, 90A, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.; Lakshmanan, A.; Ni, M.; Toh, L.L.; Wang, S.; Hauser, C.A.E. Peptide bioink: Self-assembling nanofibrous scaffolds for three-dimensional organotypic cultures. Nano Lett. 2015, 15, 6919–6925. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Yu, Z.; Zhuang, M.; Wei, W.; Zhao, Y.; Zheng, J.; Sun, D. Electrohydrodynamic direct-writing of three-dimensional multi-loop nanofibrous coils. Appl. Phys. A-Mater. 2014, 116, 171–177. [Google Scholar] [CrossRef]
- Tong, H.-W.; Wang, M. A novel technique for the fabrication of 3D nanofibrous scaffolds using simultaneous positive voltage electrospinning and negative voltage electrospinning. Mater. Lett. 2013, 94, 116–120. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, J. Electrospinning of three-dimensional nanofibrous tubes with controllable architectures. Nano Lett. 2008, 8, 3283–3287. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Kong, B.; Wu, Z.; Sun, W.; Xu, Y.; Su, X. A novel electrospinning setup for the fabrication of thickness-controllable 3D scaffolds with an ordered nanofibrous structure. Mater. Lett. 2015, 160, 343–346. [Google Scholar] [CrossRef]
- Zhu, P.; Lin, A.; Tang, X.; Lu, X.; Zheng, J.; Zheng, G.; Lei, T. Fabrication of three-dimensional nanofibrous macrostructures by electrospinning. AIP Adv. 2016, 6, 055304. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Xiao, Z.; Teh, K.S.; Han, Z.; Luo, G.; Shi, C.; Sun, D.; Zhao, J.; Lin, L. High-throughput rod-induced electrospinning. J. Phys. D Appl. Phys. 2016, 49, 365302. [Google Scholar] [CrossRef]
- Song, Z.; Chiang, S.W.; Chu, X.; Du, H.; Li, J.; Gan, L.; Xu, C.; Yao, Y.; He, Y.; Li, B. Effects of solvent on structures and properties of electrospun poly(ethylene oxide) nanofibers. J. Appl. Polym. Sci. 2017, 135, 45787. [Google Scholar] [CrossRef]
- Zheng, G.; Li, W.; Wang, X.; Wu, D.; Sun, D.; Lin, L. Precision deposition of a nanofibre by near-field electrospinning. J. Phys. D Appl. Phys. 2010, 43, 415501. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, R.; Wang, X.; Jiang, J.; Li, W.; Liu, J.; Guo, S.; Zheng, G. Electrospun Three-Dimensional Nanofibrous Structure via Probe Arrays Inducing. Micromachines 2018, 9, 427. https://doi.org/10.3390/mi9090427
Liu Y, Liu R, Wang X, Jiang J, Li W, Liu J, Guo S, Zheng G. Electrospun Three-Dimensional Nanofibrous Structure via Probe Arrays Inducing. Micromachines. 2018; 9(9):427. https://doi.org/10.3390/mi9090427
Chicago/Turabian StyleLiu, Yifang, Ruimin Liu, Xiang Wang, Jiaxin Jiang, Wenwang Li, Juan Liu, Shumin Guo, and Gaofeng Zheng. 2018. "Electrospun Three-Dimensional Nanofibrous Structure via Probe Arrays Inducing" Micromachines 9, no. 9: 427. https://doi.org/10.3390/mi9090427





