Micro-Dumbbells—A Versatile Tool for Optical Tweezers
Abstract
:1. Introduction
2. Materials and Methods
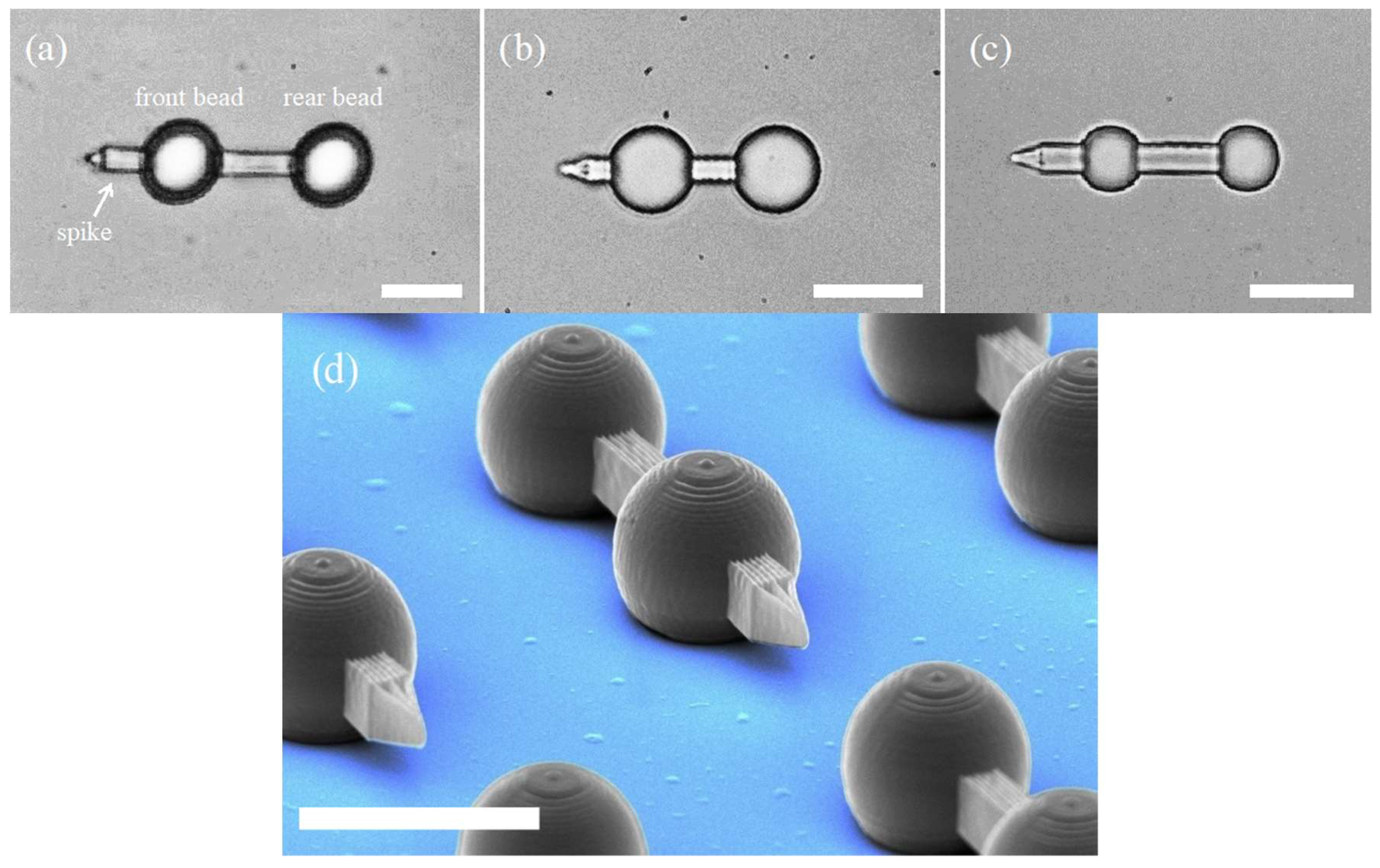
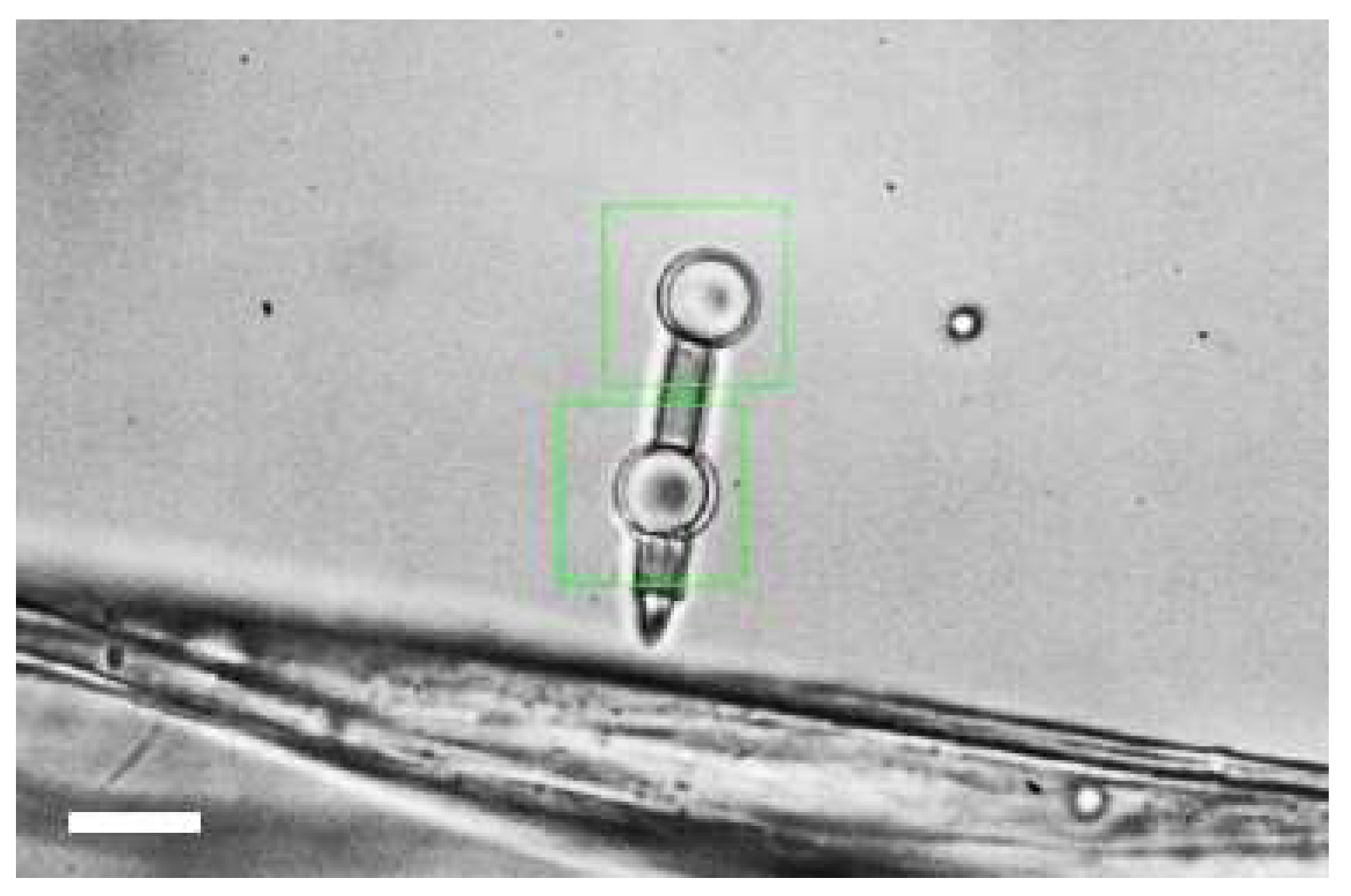
2.1. Optical Micro-Dumbbell
2.2. Fabrication
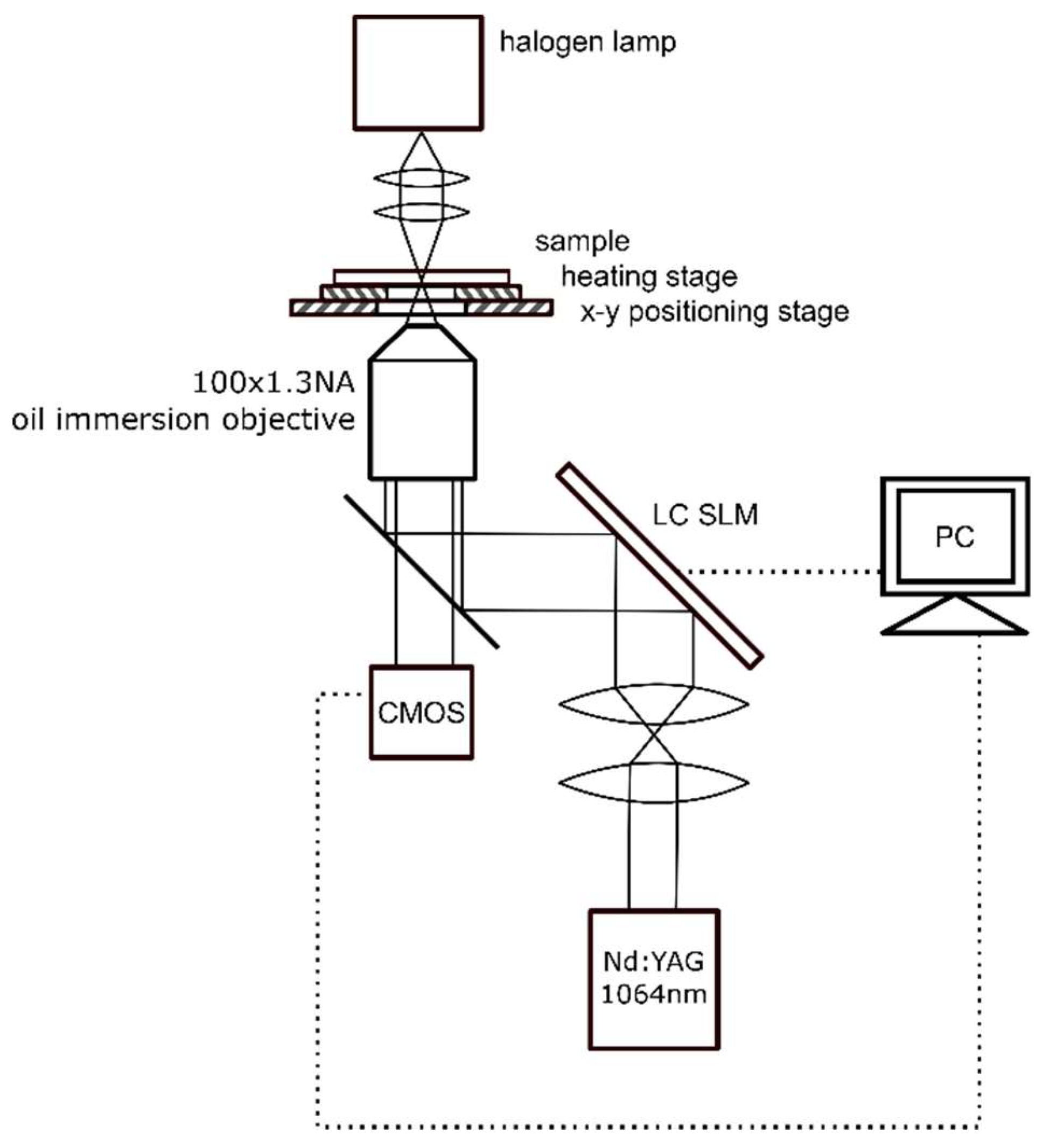
2.3. Experimental Setup
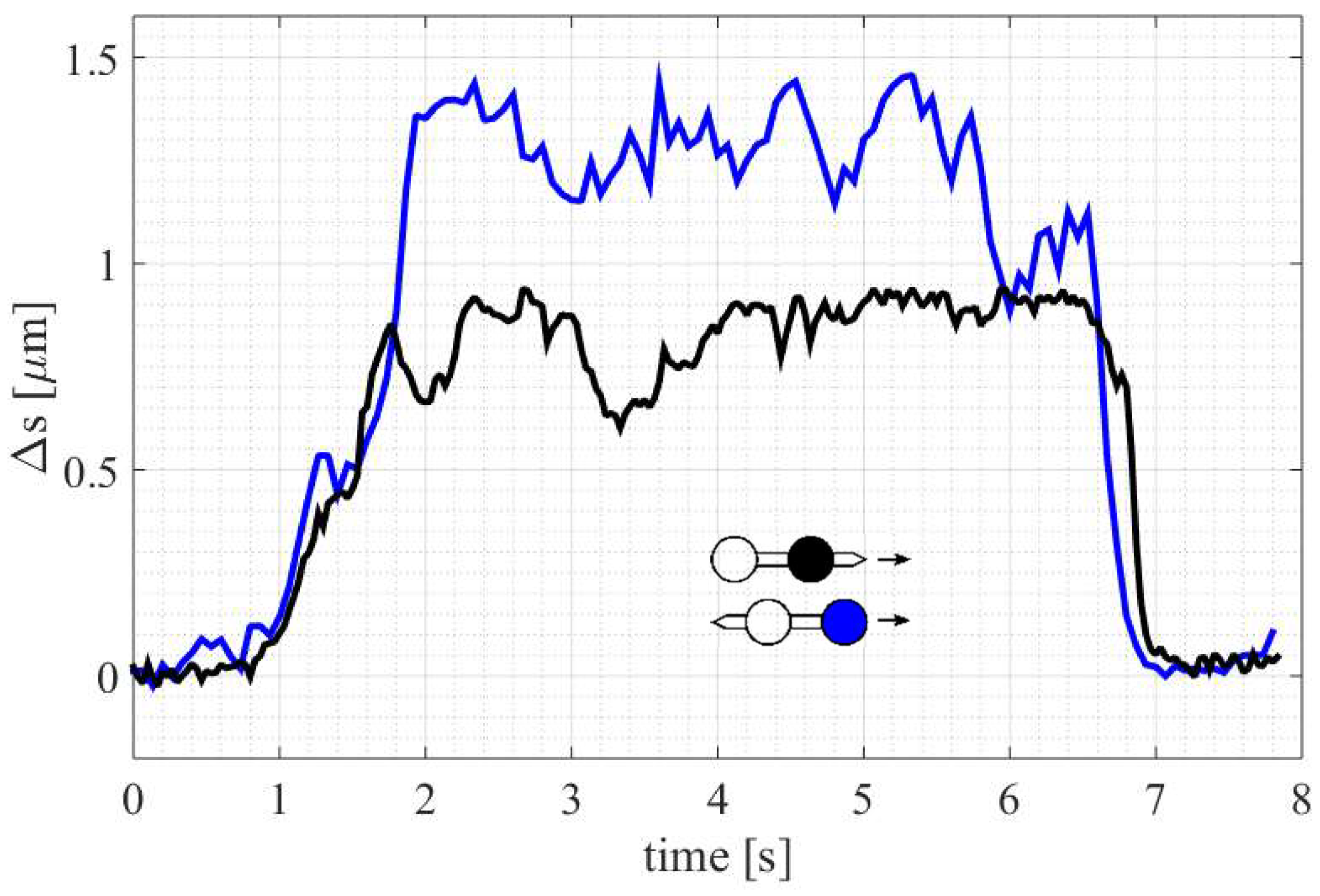
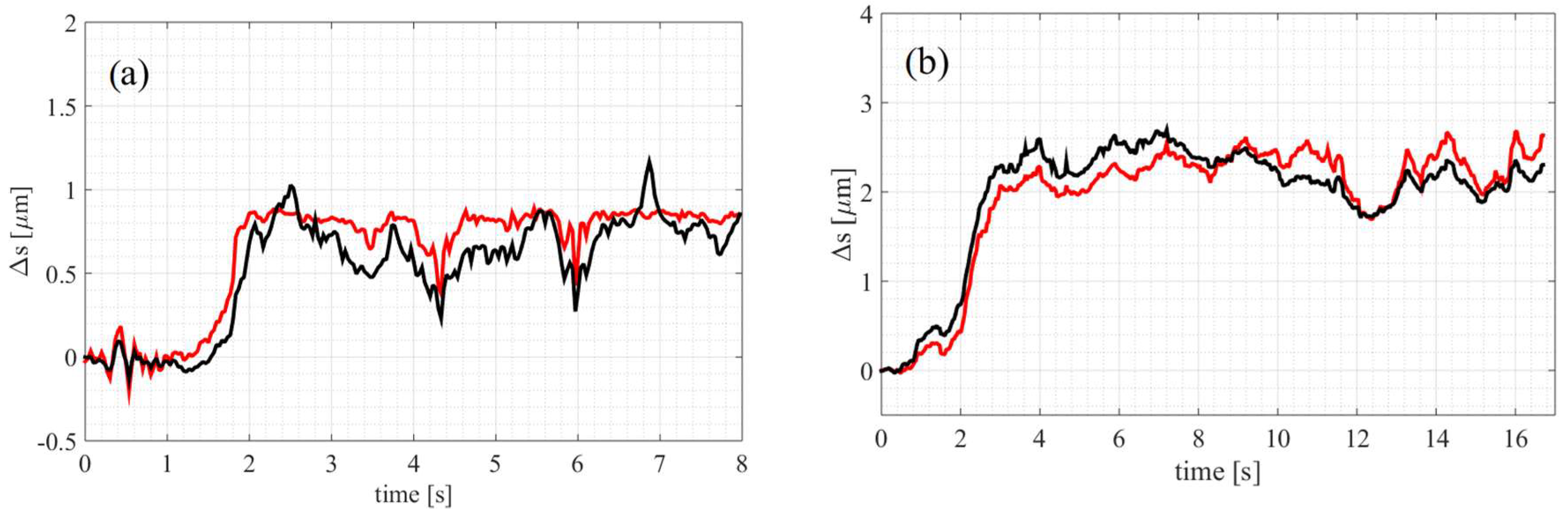
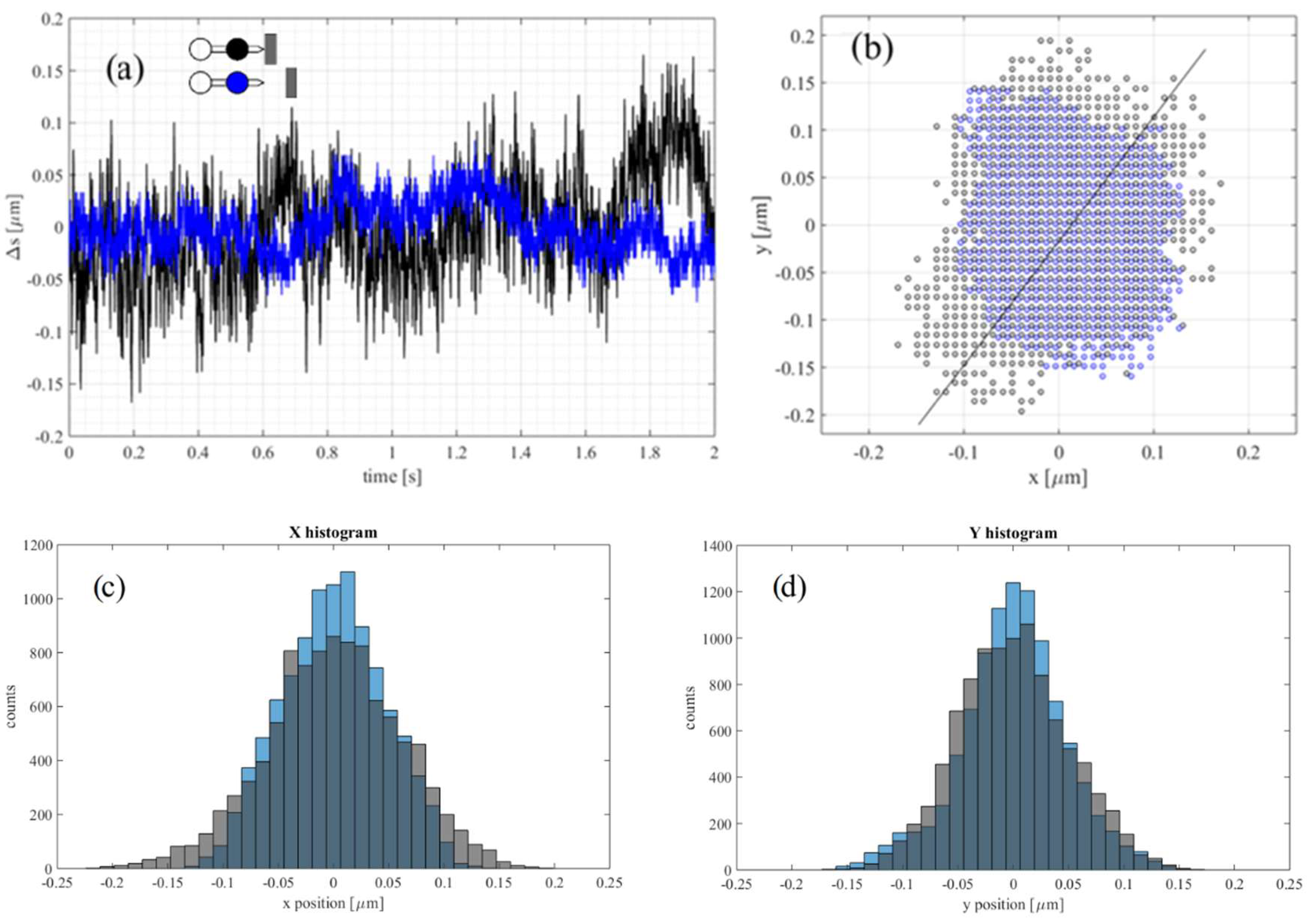
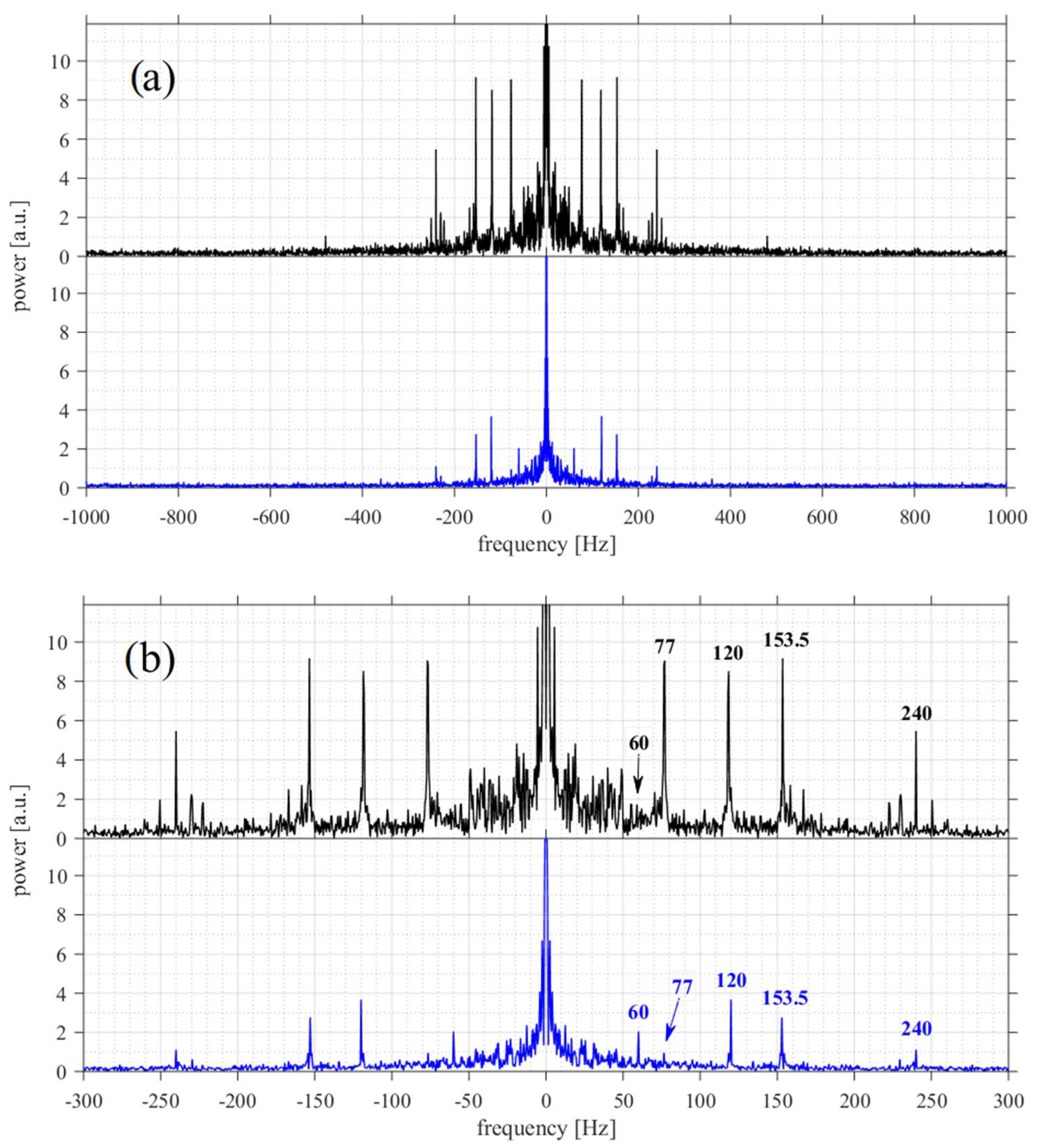
3. Results
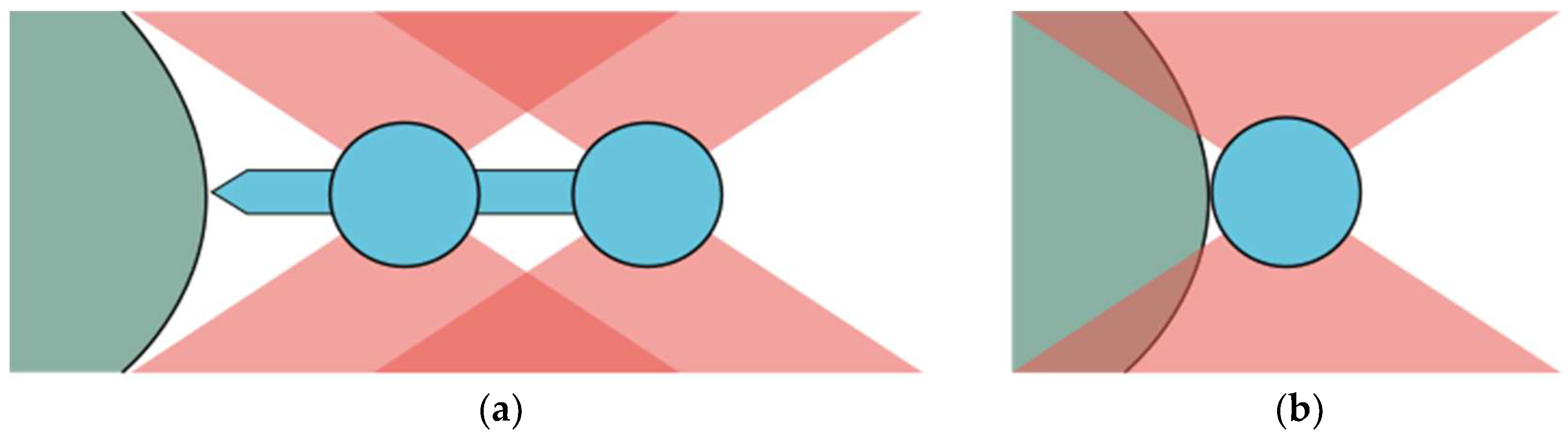
3.1. Streamlined Shape
3.2. Fluid Viscosity Comparison
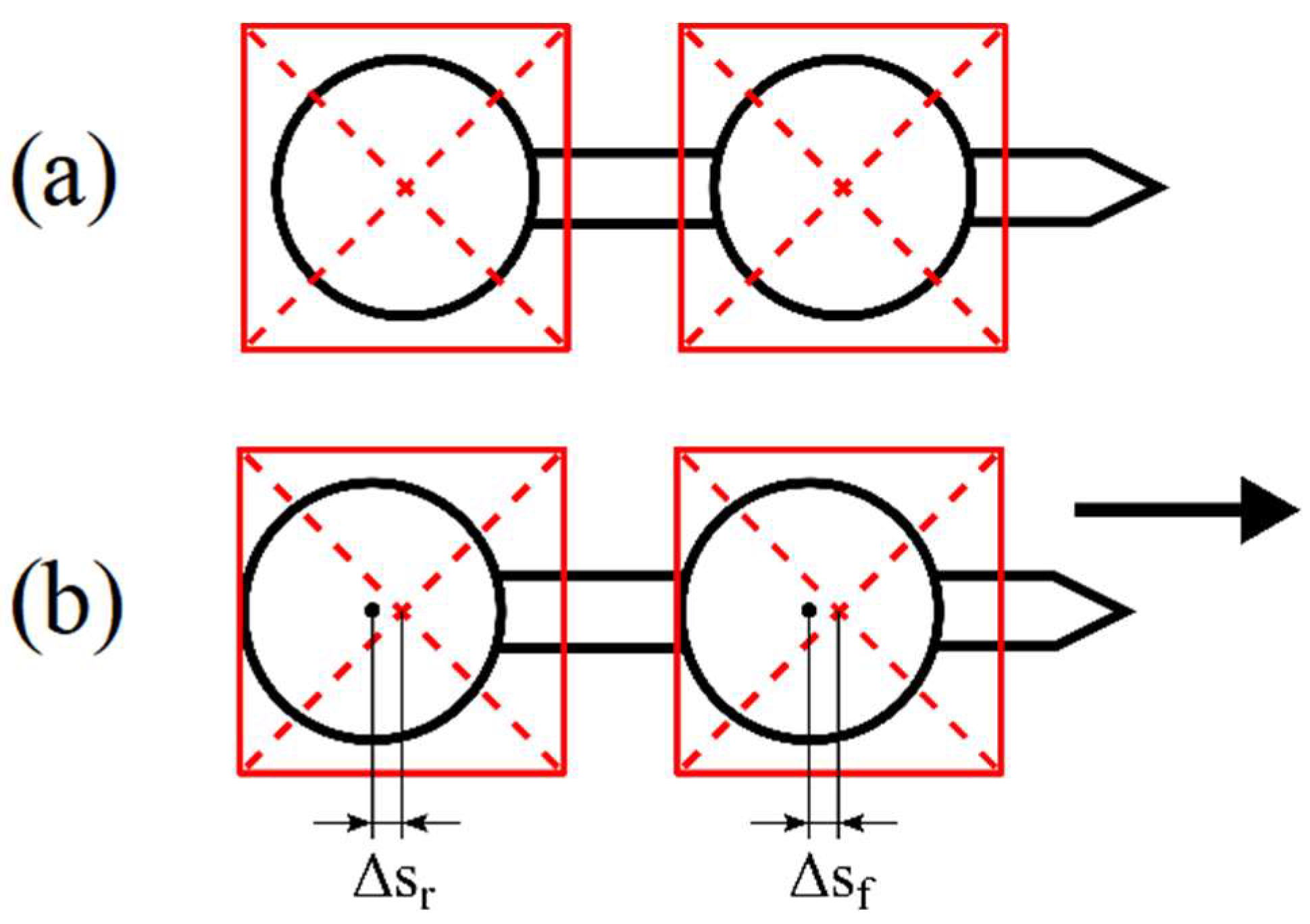
3.3. Vibrations Detection and Measurements
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lamperska, W.; Masajada, J.; Drobczyński, S.; Gusin, P. Two-Laser Optical Tweezers with a Blinking Beam. Opt. Lasers Eng. 2017, 94, 82–89. [Google Scholar] [CrossRef]
- Frączkowska, K.; Bacia, M.; Przybyło, M.; Drabik, D.; Kaczorowska, A.; Rybka, J.; Stefanko, E.; Drobczyński, S.; Masajada, J.; Podbielska, H.; et al. Alterations of Biomechanics in Cancer and Normal Cells Induced by Doxorubicin. Biomed. Pharmacother. 2018, 97, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, K.; Schmidt, C.F.; Schnapp, B.J.; Block, S.M. Direct Observation of Kinesin Stepping by Optical Trapping interferometry. Nature 1993, 365, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Lim, T.W.; Son, Y.; Yang, D.-Y.; Kong, H.-J.; Lee, K.-S. Proportional Enlargement of Movement by Using an Optically Driven Multi-Link System with an Elastic Joint. Opt. Express 2010, 18, 13745–13753. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Lee, Y.-H.; Lin, C.-T.; Liu, Y.-J.; Hwang, J.-L.; Chung, T.-T.; Baldeck, P.L. Multiplying Optical Tweezers Force Using a Micro-Lever. Opt. Express 2011, 19, 20604–20609. [Google Scholar] [CrossRef] [PubMed]
- Villangca, M.J.; Palima, D.; Bañas, A.R.; Glückstad, J. Light-Driven Micro-Tool Equipped with a Syringe Function. Light Sci. Appl. 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Phillips, D.; Gibson, G.; Bowman, R.; Padgett, M.; Hanna, S.; Carberry, D.; Miles, M.; Simpson, S. An Optically Actuated Surface Scanning Probe. Opt. Express 2012, 20, 29679–29693. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.B.; Simpson, S.H.; Grieve, J.A.; Bowman, R.; Gibson, G.M.; Padgett, M.J.; Rarity, J.G.; Hanna, S.; Miles, M.J.; Carberry, D.M. Force Sensing with a Shaped Dielectric Micro-Tool. EPL 2012, 99, 58004. [Google Scholar] [CrossRef]
- Simpson, S.H.; Hanna, S. Thermal Motion of a Holographically Trapped SPM-Like Probe. Nanotechnology 2009, 20, 395710. [Google Scholar] [CrossRef] [PubMed]
- Daily, B.I.L.L.; Elson, E.L.; Zahalak, G.I. Cell Poking. Determination of the Elastic Area Compressibility Modulus of the Erythrocyte Membrane. Biophys. J. 1984, 45, 671–682. [Google Scholar] [CrossRef]
- Evans, E.; La Celle, P. Intrinsic Material Properties of the Erythrocyte Membrane Indicated by Mechanical Analysis of Deformation. Blood 1975, 45, 29–43. [Google Scholar] [PubMed]
- Ostendorf, A.; Chichkov, B.N. Two-Photon Polymerization: A New Approach to Micromachining. Photonics Spectra 2006, 40, 72. [Google Scholar]
- Nanoscribe GmbH. 3D Printing on the Micrometer Scale. Available online: https://www.nanoscribe.de/en/ (accessed on 20 April 2018).
- Curtis, J.E.; Koss, B.A.; Grier, D.G. Dynamic Holographic Optical Tweezers. Opt. Commun. 2002, 207, 169–175. [Google Scholar] [CrossRef]
- Zhang, Z.; You, Z.; Chu, D. Fundamentals of Phase-only Liquid Crystal on Silicon (LCOS) devices. Light Sci. Appl. 2014, 3, e213. [Google Scholar] [CrossRef]
- Stigwall, J. Optimization of a Spatial Light Modulator for Beam Steering and Tracking Applications. Ph.D. Thesis, Department of Physics and Measurement Technology, Linköping University, Linköping, Sweden, 2002. [Google Scholar]
- Drobczyński, S.; Ślęzak, J. Time-Series Methods in Analysis of the Optical Tweezers Recordings. Appl. Opt. 2015, 54, 7106–7114. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Käll, M. Light-Driven Rotation of Plasmonic Nanomotors. Adv. Funct. Mater. 2018, 1706272. [Google Scholar] [CrossRef]
- Cheng, N.S. Formula for the Viscosity of a Glycerol–Water Mixture. Ind. Eng. Chem. Res. 2008, 47, 3285–3288. [Google Scholar] [CrossRef]
- Klug, U.; Boyle, M.; Friederich, F.; Kling, R.; Ostendorf, A. Laser beam shaping for micromaterial processing using a liquid crystal display. Proc. SPIE 2008, 6882, 688207. [Google Scholar] [CrossRef]
- Serati, S.; Harriman, J. Spatial light modulator considerations for beam control in optical manipulation applications. Proc. SPIE 2006, 6326, 63262W. [Google Scholar] [CrossRef]
- Wright, W.H.; Sonek, G.J.; Berns, M.W. Parametric study of the forces on microspheres held by optical tweezers. Appl. Opt. 1994, 33, 1735–1748. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.M.; Leach, J.; Keen, S.; Wright, A.J.; Padgett, M.J. Measuring the Accuracy of Particle Position and Force in Optical Tweezers Using High-Speed Video Microscopy. Opt. Express 2008, 16, 14561–14570. [Google Scholar] [CrossRef] [PubMed]
- Drobczyński, S.; Duś-Szachniewicz, K.; Symonowicz, K.; Głogocka, D. Spectral Analysis by a Video Camera in a Holographic Optical Tweezers Setup. Opt. Appl. 2013, 43, 739–746. [Google Scholar]
- Song, L.; Sjollema, J.; Sharma, P.K.; Kaper, H.J.; van der Mei, H.C.; Busscher, H.J. Nanoscopic Vibrations of Bacteria with Different Cell-Wall Properties Adhering to Surfaces under Flow and Static Conditions. ACS Nano 2014, 8, 8457–8467. [Google Scholar] [CrossRef] [PubMed]
- Pelling, A.E.; Sehati, S.; Gralla, E.B.; Valentine, J.S.; Gimzewski, J.K. Local Nanomechanical Motion of the Cell Wall of Saccharomyces Cerevisiae. Science 2004, 305, 1147–1150. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamperska, W.; Drobczyński, S.; Nawrot, M.; Wasylczyk, P.; Masajada, J. Micro-Dumbbells—A Versatile Tool for Optical Tweezers. Micromachines 2018, 9, 277. https://doi.org/10.3390/mi9060277
Lamperska W, Drobczyński S, Nawrot M, Wasylczyk P, Masajada J. Micro-Dumbbells—A Versatile Tool for Optical Tweezers. Micromachines. 2018; 9(6):277. https://doi.org/10.3390/mi9060277
Chicago/Turabian StyleLamperska, Weronika, Sławomir Drobczyński, Michał Nawrot, Piotr Wasylczyk, and Jan Masajada. 2018. "Micro-Dumbbells—A Versatile Tool for Optical Tweezers" Micromachines 9, no. 6: 277. https://doi.org/10.3390/mi9060277
APA StyleLamperska, W., Drobczyński, S., Nawrot, M., Wasylczyk, P., & Masajada, J. (2018). Micro-Dumbbells—A Versatile Tool for Optical Tweezers. Micromachines, 9(6), 277. https://doi.org/10.3390/mi9060277




