3D Multi-Microchannel Helical Mixer Fabricated by Femtosecond Laser inside Fused Silica
Abstract
:1. Introduction
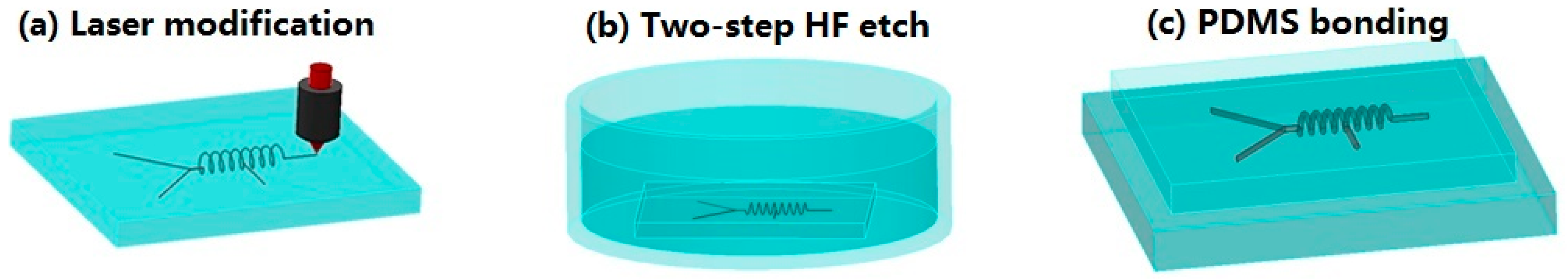
2. Materials and Methods
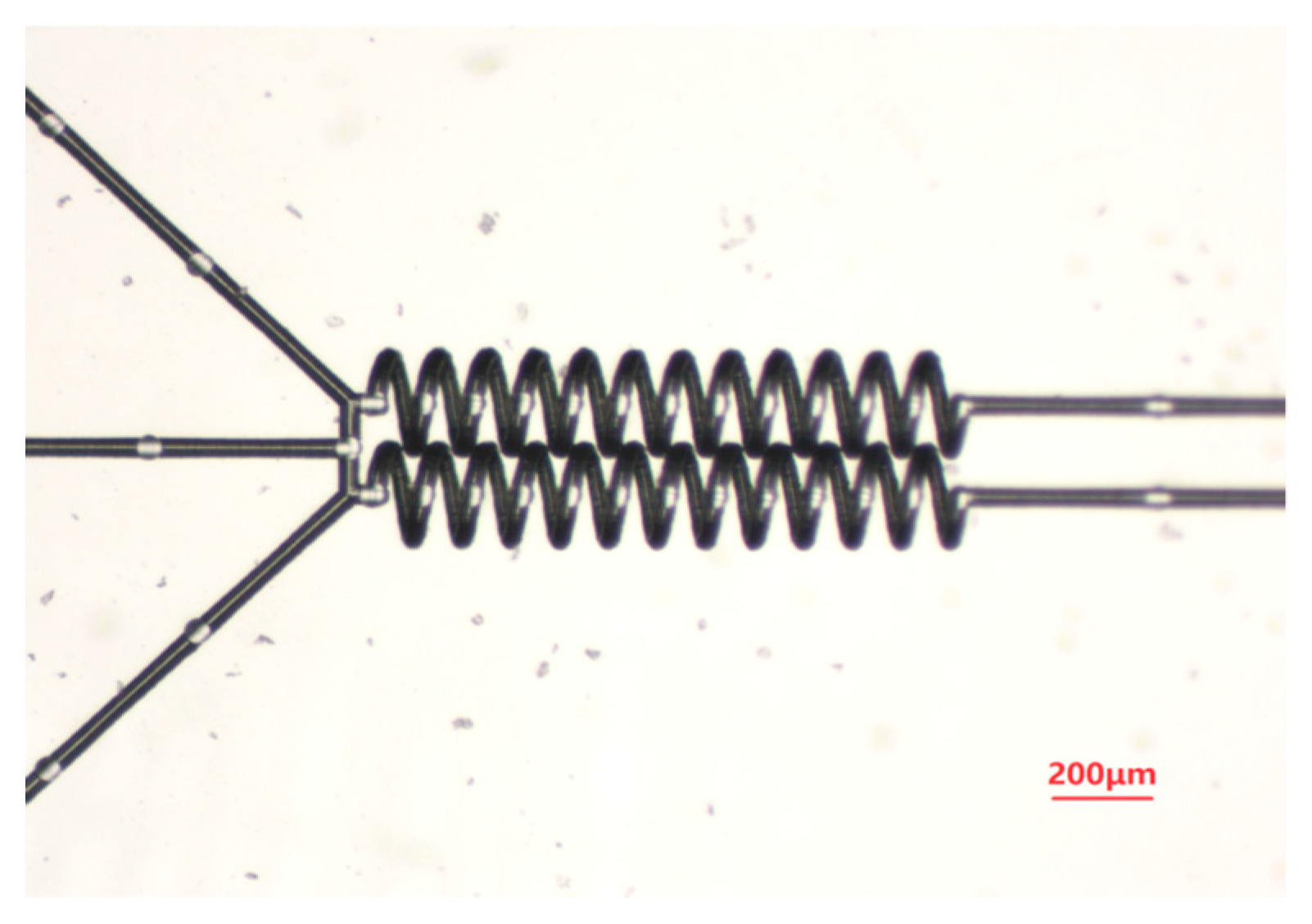
3. Results
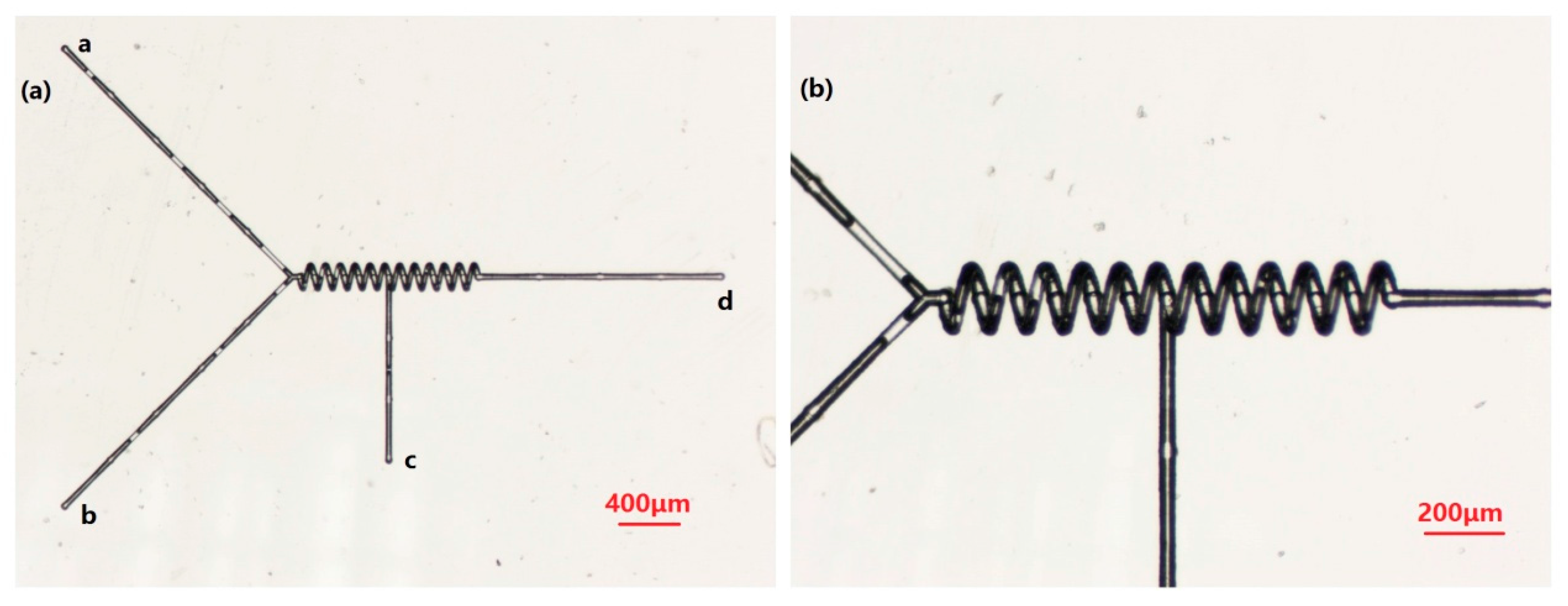
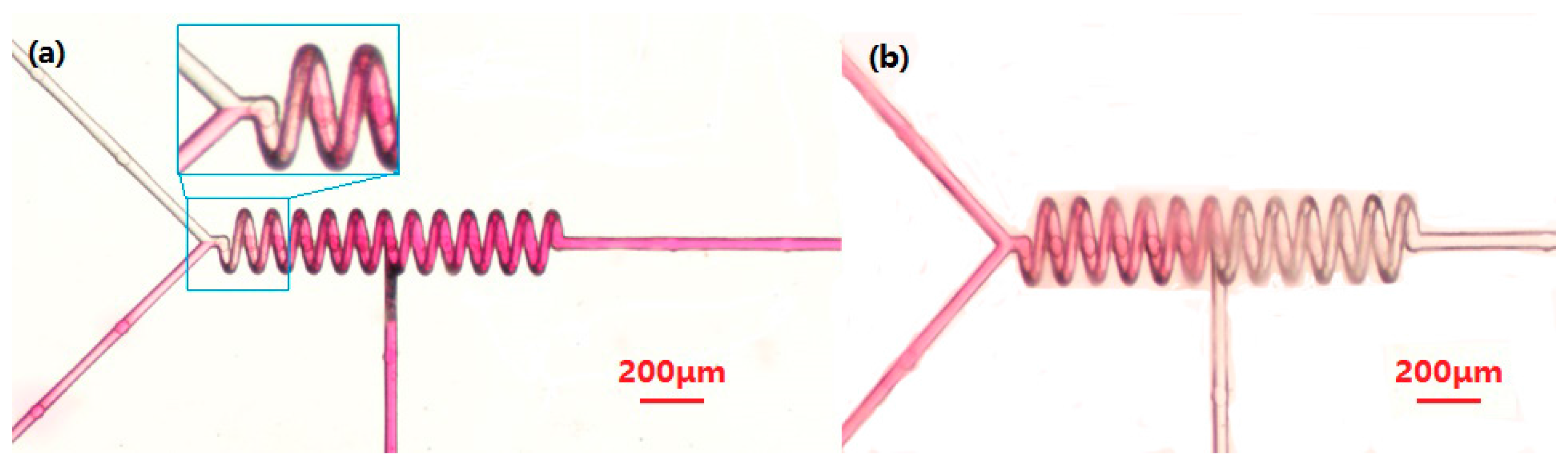
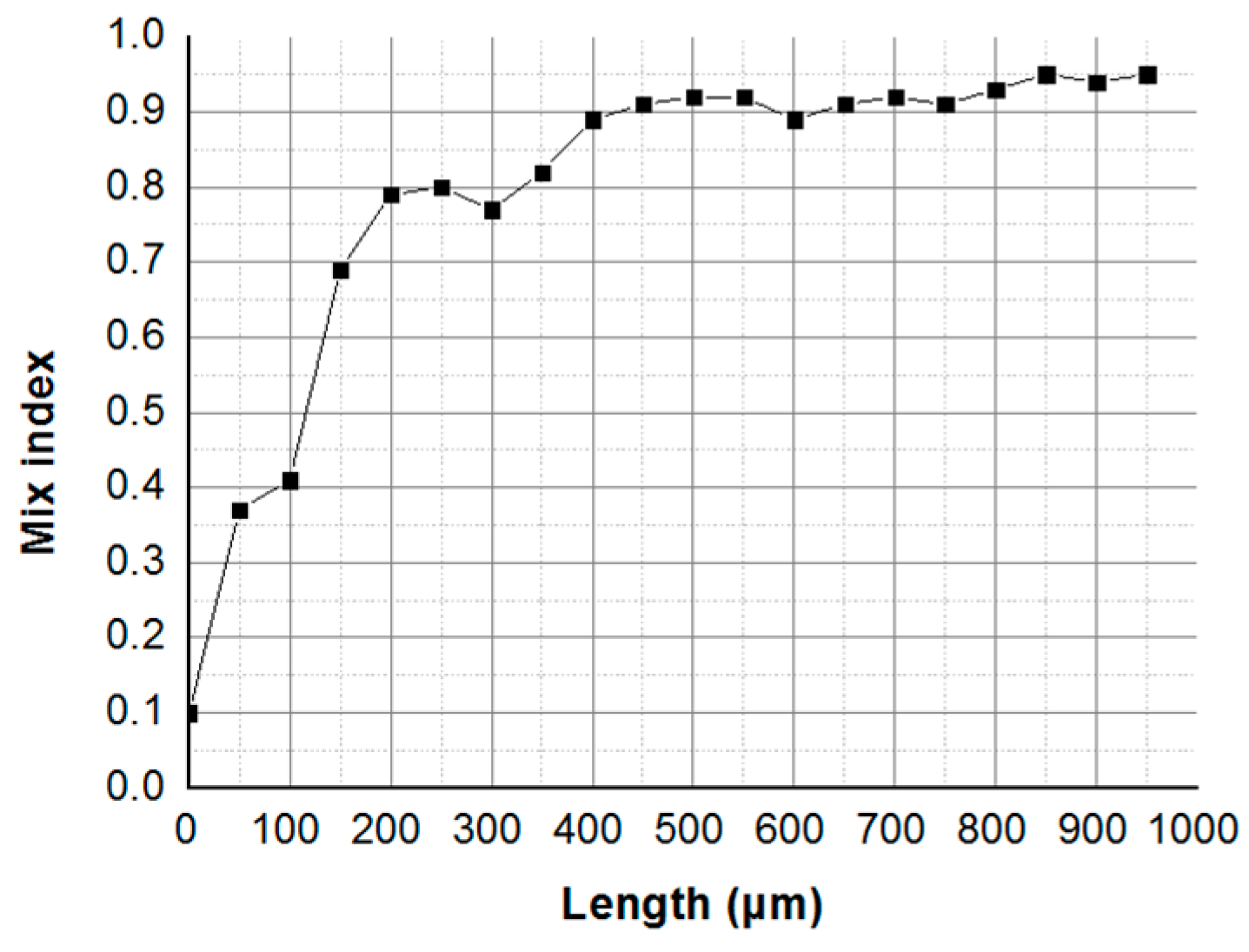
Parallel Mixer
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chin, C.D.; Laksanasopin, T.; Cheung, Y.K. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat. Med. 2011, 17, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.S.; Chung, S.; Kim, C.B. Applications of micromixing technology. Analyst 2010, 135, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Blazej, R.G.; Kumaresan, P.; Mathies, R.A. Microfabricated bioprocessor for integrated nanoliter-scale sanger DNA sequencing. Proc. Natl. Acad. Sci. USA 2006, 103, 7240–7245. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Demello, A.J. Control and detection of chemical reactions in microfluidic systems. Nature 2006, 442, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.F.; Yang, J.T. A novel microreactor with 3D rotating flow to boost fluid reaction and mixing of viscous fluids. Sens. Actuators B-Chem. 2009, 140, 629–642. [Google Scholar] [CrossRef]
- Sadabadi, H.; Packirisamy, M.; Wuthrich, R. High performance cascaded PDMS micromixer based on split-and-recombination flows for lab-on-a-chip applications. RSC Adv. 2013, 3, 7296–7305. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, S.S. Rotation effect in split and recombination micromixing. Sens. Actuators B-Chem. 2008, 129, 364–371. [Google Scholar] [CrossRef]
- Nimafar, M.; Viktorov, V.; Martinelli, M. Experimental investigation of split and recombination micromixer in confront with basic T- and O- type micromixers. Int. J. Mech. Appl. 2012, 2, 61–69. [Google Scholar] [CrossRef]
- Lee, K.; Kim, C.; Shin, K.S. Fabrication of round channels using the surface tension of PDMS and its application to a 3D serpentine mixer. J. Micromech. Microeng. 2007, 17, 1533–1541. [Google Scholar] [CrossRef]
- Lee, N.Y.; Yamada, M.; Seki, M. Development of a passive micromixer based on repeated fluid twisting and flattening, and its application to DNA purification. Anal. Bioanal. Chem. 2005, 383, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.K.S.; Ganneboyina, S.R.; Vinayak Rakshith, R.; Ghatak, A. Three-dimensional multi helical microfluidic mixers for rapid mixing of liquids. Langmuir 2008, 24, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Jani, J.M.; Wessling, M.; Lammertink, R.G.H. Geometrical influence on mixing in helical porous membrane microcontactors. J. Membr. Sci. 2011, 378, 351–358. [Google Scholar] [CrossRef]
- Osellame, R.; Hoekstra, H.J.W.M.; Cerullo, G. Femtosecond laser microstructuring: An enabling tool for optofluidic lab-on-chips. Laser Photonics Rev. 2011, 5, 442–463. [Google Scholar] [CrossRef]
- Chung, C.K.; Shih, T.R.; Chen, T.C. Mixing behavior of the rhombic micromixers over a wide Reynolds number range using Taguchi method and 3D numerical simulations. Biomed. Microdevices 2008, 10, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.M.; Shu, C.; Wan, S.Y.M. Influence of the Reynolds number on chaotic mixing in a spatially periodic micromixer and its characterization using dynamical system techniques. J. Micromech. Microeng. 2006, 16, 53–61. [Google Scholar] [CrossRef]
- Sugioka, K.; Xu, J.; Wu, D. Femtosecond laser 3D micromachining: A powerful tool for the fabrication of microfluidic, optofluidic, and electrofluidic devices based on glass. Lab Chip 2014, 14, 3447–3458. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Shan, C.; Liu, K. Process for the fabrication of complex three-dimensional microcoils in fused silica. Opt. Lett. 2013, 38, 2911–2914. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Chen, F.; Liu, K.; Yang, Q. Fabrication of three-dimensional helical microchannels with arbitrary length and uniform diameter inside fused silica. Opt. Lett. 2012, 37, 3825–3827. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liao, Y.; He, F.; Zeng, B.; Cheng, Y. Tuning etch selectivity of fused silica irradiated by femtosecond laser pulses by controlling polarization of the writing pulses. J. Appl. Phys. 2011, 109, 053114. [Google Scholar] [CrossRef]
- Bian, H.; Shan, C.; Chen, F. Miniaturized 3-D solenoid-type micro-heaters in coordination with 3-D microfluidics. J. Microelectromech. Syst. 2017, 26, 588–592. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, C.; Chen, F.; Yang, Q.; Jiang, Z.; Hou, X. 3D Multi-Microchannel Helical Mixer Fabricated by Femtosecond Laser inside Fused Silica. Micromachines 2018, 9, 29. https://doi.org/10.3390/mi9010029
Shan C, Chen F, Yang Q, Jiang Z, Hou X. 3D Multi-Microchannel Helical Mixer Fabricated by Femtosecond Laser inside Fused Silica. Micromachines. 2018; 9(1):29. https://doi.org/10.3390/mi9010029
Chicago/Turabian StyleShan, Chao, Feng Chen, Qing Yang, Zhuangde Jiang, and Xun Hou. 2018. "3D Multi-Microchannel Helical Mixer Fabricated by Femtosecond Laser inside Fused Silica" Micromachines 9, no. 1: 29. https://doi.org/10.3390/mi9010029
APA StyleShan, C., Chen, F., Yang, Q., Jiang, Z., & Hou, X. (2018). 3D Multi-Microchannel Helical Mixer Fabricated by Femtosecond Laser inside Fused Silica. Micromachines, 9(1), 29. https://doi.org/10.3390/mi9010029





